Abstract
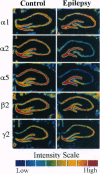
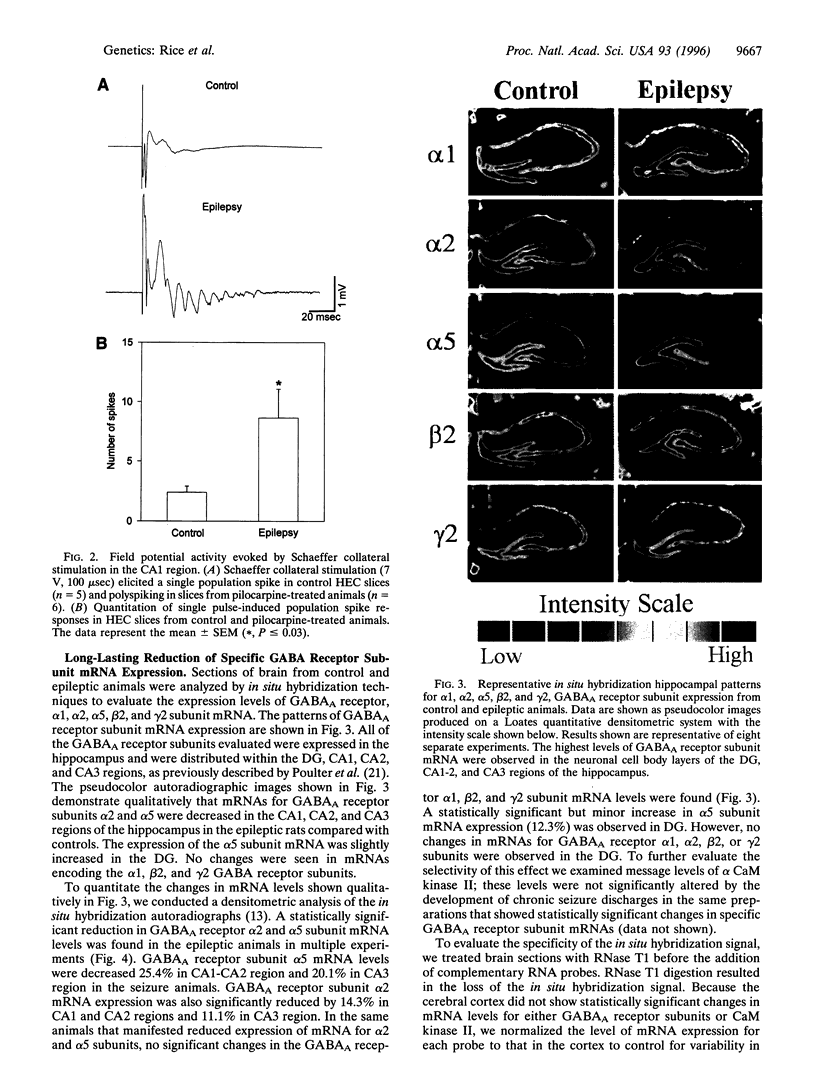
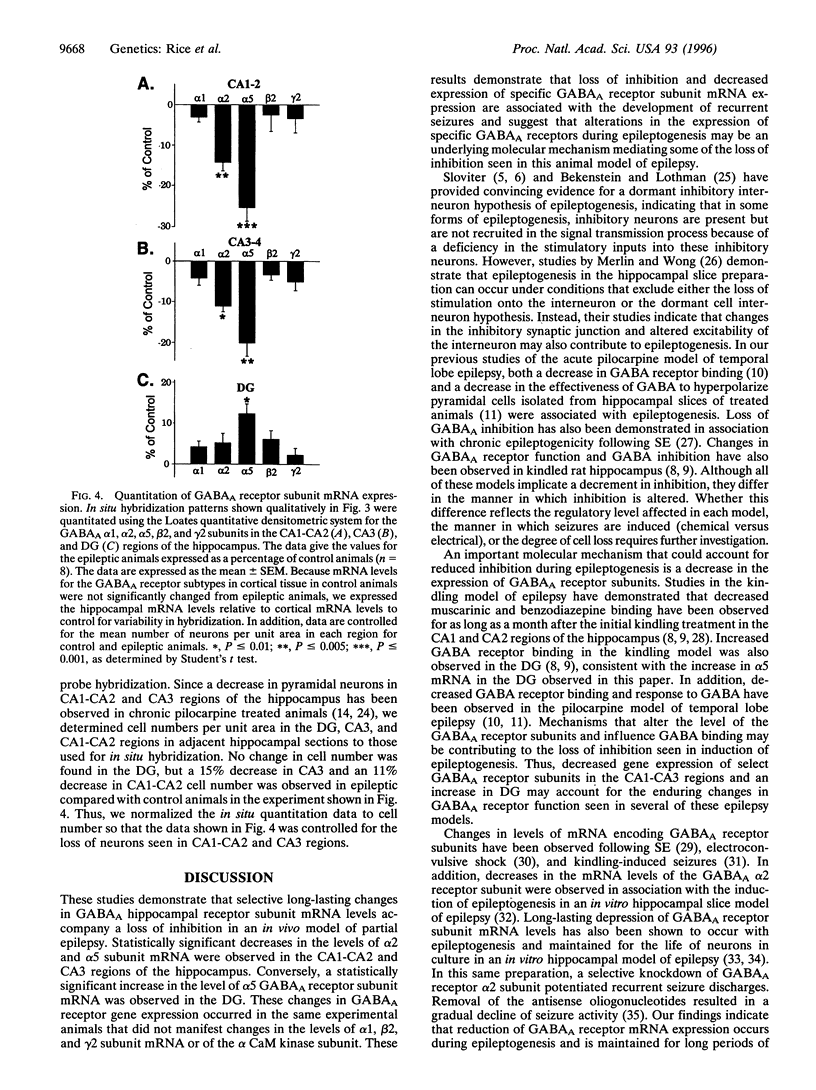
This study evaluated hippocampal inhibitory function and the level of expression of gamma-aminobutyric acid type A (GABAA) receptor mRNA in an in vivo model of epilepsy. Chronic recurrent limbic seizures were induced in rats using injections of pilocarpine. Electrophysiological studies performed on hippocampal slices prepared from control and epileptic animals 1 to 2 months after pilocarpine injections demonstrated a significant hyperexcitability in the epileptic animals. Reduced levels of mRNA expression for the alpha 2 and alpha 5 subunits of the GABAA receptors were evident in the CA1, CA2, and CA3 regions of the hippocampus of epileptic animals. No decrease in mRNA encoding alpha 1, beta 2, or gamma 2 GABAA receptor subunits was observed. In addition, no change in the mRNA levels of alpha CaM kinase II was seen. Selective decreases in mRNA expression did not correlate with neuronal cell loss. The results indicate that selective, long-lasting reduction of GABAA subunit mRNA expression and increased excitability, possibly reflecting loss of GABAergic inhibition, occur in an in vivo model of partial complex epilepsy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekenstein J. W., Lothman E. W. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993 Jan 1;259(5091):97–100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- Burgin K. E., Waxham M. N., Rickling S., Westgate S. A., Mobley W. C., Kelly P. T. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990 Jun;10(6):1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. R., Kamatchi G. L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991 Nov;5(14):2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Cavalheiro E. A., Leite J. P., Bortolotto Z. A., Turski W. A., Ikonomidou C., Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991 Nov-Dec;32(6):778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- De Deyn P. P., Marescau B., MacDonald R. L. Epilepsy and the GABA-hypothesis a brief review and some examples. Acta Neurol Belg. 1990;90(2):65–81. [PubMed] [Google Scholar]
- Friedman L. K., Pellegrini-Giampietro D. E., Sperber E. F., Bennett M. V., Moshé S. L., Zukin R. S. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in situ hybridization study. J Neurosci. 1994 May;14(5 Pt 1):2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoi E. R., Sombati S., Gerwin C., DeLorenzo R. J. Excitatory amino acid receptor activation produces a selective and long-lasting modulation of gene expression in hippocampal neurons. Brain Res. 1992 Jun 12;582(2):282–290. doi: 10.1016/0006-8993(92)90145-y. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Heinemann U. Synaptic and intrinsic responses of medical entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol. 1988 May;59(5):1476–1496. doi: 10.1152/jn.1988.59.5.1476. [DOI] [PubMed] [Google Scholar]
- Kapur J., Lothman E. W., DeLorenzo R. J. Loss of GABAA receptors during partial status epilepticus. Neurology. 1994 Dec;44(12):2407–2408. doi: 10.1212/wnl.44.12.2407. [DOI] [PubMed] [Google Scholar]
- Kokaia M., Pratt G. D., Elmér E., Bengzon J., Fritschy J. M., Kokaia Z., Lindvall O., Mohler H. Biphasic differential changes of GABAA receptor subunit mRNA levels in dentate gyrus granule cells following recurrent kindling-induced seizures. Brain Res Mol Brain Res. 1994 Jun;23(4):323–332. doi: 10.1016/0169-328x(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Lloyd K. G., Bossi L., Morselli P. L., Munari C., Rougier M., Loiseau H. Alterations of GABA-mediated synaptic transmission in human epilepsy. Adv Neurol. 1986;44:1033–1044. [PubMed] [Google Scholar]
- Lothman E. W., Bertram E. H., 3rd, Stringer J. L. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37(1):1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mello L. E., Cavalheiro E. A., Tan A. M., Kupfer W. R., Pretorius J. K., Babb T. L., Finch D. M. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993 Nov-Dec;34(6):985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Merlin L. R., Wong R. K. Synaptic modifications accompanying epileptogenesis in vitro: long-term depression of GABA-mediated inhibition. Brain Res. 1993 Nov 12;627(2):330–340. doi: 10.1016/0006-8993(93)90338-n. [DOI] [PubMed] [Google Scholar]
- Perlin J. B., Gerwin C. M., Panchision D. M., Vick R. S., Jakoi E. R., DeLorenzo R. J. Kindling produces long-lasting and selective changes in gene expression of hippocampal neurons. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1741–1745. doi: 10.1073/pnas.90.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter M. O., Barker J. L., O'Carroll A. M., Lolait S. J., Mahan L. C. Differential and transient expression of GABAA receptor alpha-subunit mRNAs in the developing rat CNS. J Neurosci. 1992 Aug;12(8):2888–2900. doi: 10.1523/JNEUROSCI.12-08-02888.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J. S., Kang I., Bazan N. G., Miller L. G. Electroconvulsive shock alters GABAA receptor subunit mRNAs: use of quantitative PCR methodology. Brain Res Bull. 1993;30(5-6):691–693. doi: 10.1016/0361-9230(93)90101-g. [DOI] [PubMed] [Google Scholar]
- Racine R. J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972 Mar;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rafiq A., DeLorenzo R. J., Coulter D. A. Generation and propagation of epileptiform discharges in a combined entorhinal cortex/hippocampal slice. J Neurophysiol. 1993 Nov;70(5):1962–1974. doi: 10.1152/jn.1993.70.5.1962. [DOI] [PubMed] [Google Scholar]
- Rafiq A., Zhang Y. F., DeLorenzo R. J., Coulter D. A. Long-duration self-sustained epileptiform activity in the hippocampal-parahippocampal slice: a model of status epilepticus. J Neurophysiol. 1995 Nov;74(5):2028–2042. doi: 10.1152/jn.1995.74.5.2028. [DOI] [PubMed] [Google Scholar]
- Savic I., Persson A., Roland P., Pauli S., Sedvall G., Widén L. In-vivo demonstration of reduced benzodiazepine receptor binding in human epileptic foci. Lancet. 1988 Oct 15;2(8616):863–866. doi: 10.1016/s0140-6736(88)92468-3. [DOI] [PubMed] [Google Scholar]
- Shin C., Pedersen H. B., McNamara J. O. gamma-Aminobutyric acid and benzodiazepine receptors in the kindling model of epilepsy: a quantitative radiohistochemical study. J Neurosci. 1985 Oct;5(10):2696–2701. doi: 10.1523/JNEUROSCI.05-10-02696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka Y., Wasterlain C. G. Chronic epileptogenicity following focal status epilepticus. Brain Res. 1994 Aug 29;655(1-2):33–44. doi: 10.1016/0006-8993(94)91594-6. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987 Jan 2;235(4784):73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994 Jun;35(6):640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- Sombati S., Delorenzo R. J. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J Neurophysiol. 1995 Apr;73(4):1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Titulaer M. N., Ghijsen W. E., Kamphuis W., De Rijk T. C., Lopes da Silva F. H. Opposite changes in GABAA receptor function in the CA1-3 area and fascia dentata of kindled rat hippocampus. J Neurochem. 1995 Jun;64(6):2615–2621. doi: 10.1046/j.1471-4159.1995.64062615.x. [DOI] [PubMed] [Google Scholar]
- Titulaer M. N., Kamphuis W., Pool C. W., van Heerikhuize J. J., Lopes da Silva F. H. Kindling induces time-dependent and regional specific changes in the [3H]muscimol binding in the rat hippocampus: a quantitative autoradiographic study. Neuroscience. 1994 Apr;59(4):817–826. doi: 10.1016/0306-4522(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Turski L., Ikonomidou C., Turski W. A., Bortolotto Z. A., Cavalheiro E. A. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3(2):154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Vick R. S., Rafiq A., Coulter D. A., Jakoi E. R., DeLorenzo R. J. GABAA alpha 2 mRNA levels are decreased following induction of spontaneous epileptiform discharges in hippocampal-entorhinal cortical slices. Brain Res. 1996 May 20;721(1-2):111–119. doi: 10.1016/0006-8993(96)00060-1. [DOI] [PubMed] [Google Scholar]