Abstract
BACKGROUND
Average real variability (ARV) is a recently proposed index for short-term blood pressure (BP) variability. We aimed to determine the minimum number of BP readings required to compute ARV without loss of prognostic information.
METHODS
ARV was calculated from a discovery dataset that included 24-hour ambulatory BP measurements for 1,254 residents (mean age = 56.6 years; 43.5% women) of Copenhagen, Denmark. Concordance between ARV from full (≥80 BP readings) and randomly reduced 24-hour BP recordings was examined, as was prognostic accuracy. A test dataset that included 5,353 subjects (mean age = 54.0 years; 45.6% women) with at least 48 BP measurements from 11 randomly recruited population cohorts was used to validate the results.
RESULTS
In the discovery dataset, a minimum of 48 BP readings allowed an accurate assessment of the association between cardiovascular risk and ARV. In the test dataset, over 10.2 years (median), 806 participants died (335 cardiovascular deaths, 206 cardiac deaths) and 696 experienced a major fatal or nonfatal cardiovascular event. Standardized multivariable-adjusted hazard ratios (HRs) were computed for associations between outcome and BP variability. Higher diastolic ARV in 24-hour ambulatory BP recordings predicted (P < 0.01) total (HR = 1.12), cardiovascular (HR = 1.19), and cardiac (HR = 1.19) mortality and fatal combined with nonfatal cerebrovascular events (HR = 1.16). Higher systolic ARV in 24-hour ambulatory BP recordings predicted (P < 0.01) total (HR = 1.12), cardiovascular (HR = 1.17), and cardiac (HR = 1.24) mortality.
CONCLUSIONS
Forty-eight BP readings over 24 hours were observed to be adequate to compute ARV without meaningful loss of prognostic information.
Keywords: ambulatory blood pressure, blood pressure, blood pressure variability, epidemiology, hypertension, population science, risk factors.
Average real variability (ARV) is a recently proposed index to represent short-term, reading-to-reading, within-subject variability in blood pressure (BP).1 ARV attempts to correct for the limitations of the commonly used standard deviation (SD), which accounts only for the dispersion of values around the mean, and not for the order of the BP readings.1–3
Several recent studies reported on the association between cardiovascular outcome and BP variability as assessed by 24-hour ambulatory BP monitoring.1,2,4–6 Most of these studies included a small (n < 350) general1,6 or hypertensive population5 with a high percentage (>70%) of valid BP readings obtained at intervals ranging from 151,5 to 304,6 minutes during daytime and from 301,5 to 604,6 minutes during nighttime. Participants with fewer than 32,6 57,1 or 595 BP readings during the 24-hour monitoring period were excluded from these studies. The study with the largest number of participants (n = 8,938), taken from the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes (IDACO),7 excluded subjects that had less than 10 daytime or 5 nighttime BP readings or missing BP measurements during 3 consecutive hours.2 However, none of the aforementioned studies considered the minimum number of BP readings required to estimate BP variability in an accurate manner. The aim of our study was to use a dataset of 1,254 IDACO subjects with at least 80 ambulatory BP readings to determine an adequate number of BP readings needed to calculate ARV. We took advantage of the prospective design of IDACO to determine such number based on outcome data. The results were then validated using a test dataset with a larger number of subjects.
METHODS
Study population
At the time of writing this report, the IDACO database included 12,722 participants from 12 randomly recruited population cohorts.8–17 The Copenhagen cohort,8,18,19 for which ambulatory BP was recorded at 15-minute intervals from 7:00 am to 11:00 pm, and at 30-minute intervals from 11:00 pm to 7:00 am, was selected as the discovery dataset for this study. The Copenhagen cohort included 2,311 subjects equally distributed among the 2 sexes and among 4 age groups (41, 51, 61, and 71 years). Subjects with incomplete ambulatory BP recordings (<80 readings during 24 hours) were excluded (n = 1,057), leaving 1,254 subjects for the discovery analysis. The results were tested for prognostic accuracy, using a larger sample of IDACO participants (test dataset). The test dataset included 5,353 IDACO subjects, who (i) were at least 18 years old, (ii) had at least 10 daytime readings, 5 nighttime readings, and 48 readings over 24 hours, and (iii) were not included in the discovery dataset.
BP measurement
A detailed description of the methods employed for conventional and ambulatory BP monitoring is provided in the Supplementary Data. Hypertension was defined as a conventional BP of at least 140mm Hg systolic or 90mm Hg diastolic, or the use of antihypertensive drugs.20 The devices used in IDACO all passed validation and were programmed to obtain readings at 30-minute intervals throughout the whole day, or at intervals ranging 15–30 minutes during daytime and 30–60 minutes at night. Within individual subjects, the means of the ambulatory BP were weighted by the interval between readings. ARV over 24 hours was calculated using the following formula:
 |
where n is the number of BP readings and wk is the time interval between BPk and BPk-1.1,2
Ascertainment of events
We ascertained vital status and the incidence of fatal and nonfatal diseases from the appropriate sources in each country, as described in previous publications.1,7,9,12,13,15 Fatal and nonfatal stroke did not include transient ischemic attacks. Cardiac events encompassed death from ischemic heart disease, sudden death, fatal and nonfatal heart failure, nonfatal myocardial infarction, and coronary revascularization. The composite cardiovascular endpoint included all aforementioned endpoints plus cardiovascular mortality. In all outcome analyses, we only considered the first event within each category. The cardiovascular endpoints and the International Classification of Disease code numbers used to differentiate the events are available in Supplementary Table S1.
Statistical analysis
For database management and statistical analyses, we used SAS software, version 9.1.3 (SAS Institute, North Carolina, USA) and MATLAB software, version R2009a (MathWorks, Massachusetts, USA). Statistical significance was α < 0.05 on 2-tailed tests. For comparison of means and proportions, we applied the large-sample z test and the χ2statistic, respectively. In the discovery dataset, we randomly excluded 8, 16, 24, 32, 40, and 48 BP readings from each full ambulatory recording, generating reduced recordings with 72, 64, 56, 48, 40, and 32 readings. Each reduction was based on the original full recording and used a different random seed. In an attempt to approximate the effect of missed readings, we did not eliminate BP measurements during the first or last hour of the ambulatory readings, and we did not eliminate more than 4 consecutive daytime or 2 consecutive nighttime readings. We quantified concordance between ARV calculated from full and reduced recordings using Pearson’s correlation coefficient, repeatability, and relative repeatability coefficients.21–23 The repeatability coefficient was defined as twice the SD of the within-subject difference between ARV calculated from full and reduced recordings. The relative repeatability coefficient was the repeatability coefficient expressed as a percentage of the maximal variability (4 times the SD of ARV averaged across the full and reduced recordings). Higher values of repeatability and relative repeatability coefficients indicate lower concordance.
We used the Cox proportional hazard regression model to compute standardized hazard ratios (HRs), which express the risk for an increase by 1 SD in the independent variables. The HRs in the discovery dataset were initially computed adjusting only for sex and age, the most significant independent cardiovascular predictors for this cohort (P < 0.001), because the original Copenhagen Cohort was sampled with stratifying for sex and 4 age groups. The prognostic information contributed by ARV based on reduced recordings was considered accurate if the standardized HR remained significant (P ≤ 0.05) and ≥1.10. We plotted the 10-year risk (expressed as a percentage) of all-cause mortality and the composite cardiovascular endpoint in relation to ARV calculated from full and reduced recordings, standardized to the mean distribution of sex and age in the discovery dataset. In the test dataset, further adjustments were applied for cohort, body mass index, serum cholesterol, smoking status, alcohol intake, history of cardiovascular disease, and treatment with antihypertensive drugs. Additionally, in fully adjusted models, we accounted for 24-hour BP. Finally, we calculated the generalized R 2, which is a measure for the refinement of the risk prediction by adding covariables to the Cox model.24
RESULTS
Discovery dataset
The Copenhagen sample of 1,254 subjects included 545 (43.5%) women and 569 (45.4%) individuals with hypertension on conventional BP measurements, of whom 192 (33.7%) were taking antihypertensive medication (Table 1). Mean age (±SD) was 56.6±10.4 years, and body mass index averaged 25.8±3.7kg/m2. At enrollment, 75 (6.0%) participants had a history of cardiovascular disease, 47 (3.8%) had diabetes mellitus, 539 (43.0%) were smokers, and 1,081 (86.2%) reported intake of alcohol. Mean 24-hour BP was 128.9±12.8mm Hg systolic and 75.9±8.4mm Hg diastolic.
Table 1.
Baseline characteristics of participants enrolled in the discovery and test datbases
| Discovery cohort | Test cohort | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Number of subjects, no. (%) | 545 | 709 | 2,440 | 2,913 |
| Smokers | 221 (40.6) | 318 (44.9) | 499 (20.6) | 963 (33.5) |
| Use of alcohol | 419 (77.7) | 662 (93.6) | 855 (36.0) | 1,756 (71.2) |
| Hypertension | 231 (42.4) | 338 (47.7) | 1,040 (42.6) | 1,502 (51.6) |
| On antihypertensive treatment | 97 (17.8) | 95 (13.4) | 500 (20.6) | 632 (21.8) |
| Diabetes mellitus | 15 (2.8) | 32 (4.5) | 156 (6.4) | 234 (8.0) |
| Cardiovascular disorders | 23 (4.2) | 52 (7.3) | 431 (17.7) | 436 (15.0) |
| Mean characteristic (SD) | ||||
| Age, y | 56.4 (10.3) | 56.7 (10.5) | 51.2 (15.5) | 56.4 (16.2) |
| Body mass index, kg/m2 | 25.2 (4.0) | 26.3 (3.4) | 25.3 (4.9) | 25.8 (3.9) |
| Conventional blood pressure | ||||
| Systolic, mm Hg | 130.1 (19.7) | 133.4 (19.2) | 132.7 (27.5) | 138.1 (22.9) |
| Diastolic, mm Hg | 81.8 (10.7) | 84.8 (11.1) | 78.9 (12.1) | 82.3 (11.5) |
| 24-h blood pressure | ||||
| Systolic, mm Hg | 126.4 (12.8) | 130.7 (12.4) | 121.5 (13.8) | 128.1 (14.4) |
| Diastolic, mm Hg | 73.2 (8.0) | 77.9 (8.1) | 71.8 (8.1) | 75.5 (8.4) |
| 24-h average real variability | ||||
| Systolic, mm Hg | 11.7 (2.4) | 11.8 (2.5) | 10.5 (3.2) | 11.4 (3.4) |
| Diastolic, mm Hg | 7.6 (1.3) | 7.9 (1.4) | 8.0 (2.4) | 8.8 (2.8) |
| 24-h heart rate, beats/min | 74.4 (8.0) | 71.0 (8.8) | 73.6 (8.4) | 70.0 (9.4) |
| Serum cholesterol, mg/dl | 6.3 (1.1) | 6.1 (1.1) | 5.6 (1.2) | 5.6 (1.2) |
Hypertension was a conventional blood pressure of at least 140mm Hg systolic or 90mm Hg diastolic or use of antihypertensive drugs. All between-sex differences were significant (P < 0.05) with the exception of smoking (P = 0.13), age (P = 0.63), prevalence of hypertension (P = 0.07) and diabetes mellitus (P = 0.13), and 24-hour systolic average real variability (P = 0.32) in the discovery dataset and serum cholesterol (P = 0.33) and prevalence of antihypertensive drug intake (P = 0.3) in the test dataset.
Median follow-up time for the Copenhagen cohort was 12.4 years. During 14,246 person-years of follow-up, 235 participants died (16.5 per 1,000 person-years), and 171 subjects experienced a fatal or nonfatal cardiovascular complication (12.4 per 1,000 person-years). Mortality included 76 cardiovascular deaths, 152 noncardiovascular deaths, 2 renal deaths, and 5 deaths of unknown cause. The first occurring cardiovascular events consisted of 7 fatal strokes, 36 nonfatal strokes, 46 nonfatal myocardial infarctions, 1 coronary revascularization, 15 deaths from ischemic heart disease, 5 sudden deaths, 46 nonfatal heart failures, 8 fatal heart failures, 4 deaths from peripheral arterial disease, and 3 unspecified cardiovascular deaths.
The median number of BP readings in the discovery dataset was 81 (5th–95th percentile = 80–84). In general, mean ARV increased with decreasing number of BP measurements. In addition, the repeatability and relative repeatability coefficients increased, and Pearson correlation coefficients decreased with fewer BP readings, indicating reduced concordance with ARV based on full recordings as more readings were excluded (Table 2).
Table 2.
Concordance between average real variability computed from full and reduced recordings in the discovery database
| Minimum number of blood pressure readings | Systolic blood pressure | Diastolic blood pressure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ARV (SD) | Delta, mm Hg | RC, mm Hg | RRC, % | r | Mean ARV (SD) | Delta, mm Hg | RC, mm Hg | RRC, % | r | |
| 80 | 11.74 (2.45) | NA | NA | NA | NA | 7.79 (1.36) | NA | NA | NA | NA |
| 72 | 11.95 (2.55) | 0.20 | 1.58 | 16.0 | 0.95 | 7.97 (1.42) | 0.18 | 1.07 | 19.6 | 0.93 |
| 64 | 12.21 (2.66) | 0.47 | 2.33 | 23.4 | 0.90 | 8.16 (1.50) | 0.37 | 1.54 | 28.0 | 0.86 |
| 56 | 12.54 (2.90) | 0.80 | 3.04 | 29.5 | 0.85 | 8.42 (1.62) | 0.63 | 1.90 | 33.5 | 0.81 |
| 48 | 12.84 (3.00) | 1.09 | 3.70 | 35.9 | 0.79 | 8.67 (1.72) | 0.89 | 2.36 | 41.2 | 0.73 |
| 40 | 13.12 (3.21) | 1.37 | 4.45 | 42.2 | 0.72 | 8.93 (1.86) | 1.14 | 2.81 | 47.8 | 0.66 |
| 32 | 13.48 (3.40) | 1.74 | 7.28 | 77.6 | 0.26 | 9.23 (2.01) | 1.45 | 4.41 | 84.1 | 0.18 |
Abbreviations: ARV, average real variability over 24 hours; Delta, ARV based on reduced recording minus ARV based on full recording (at least 80 measurements); NA, not applicable; RC, repeatability coefficient for the correspondence between the reduced and full recordings; RRC, relative repeatability coefficient for the correspondence between the full and reduced recordings.
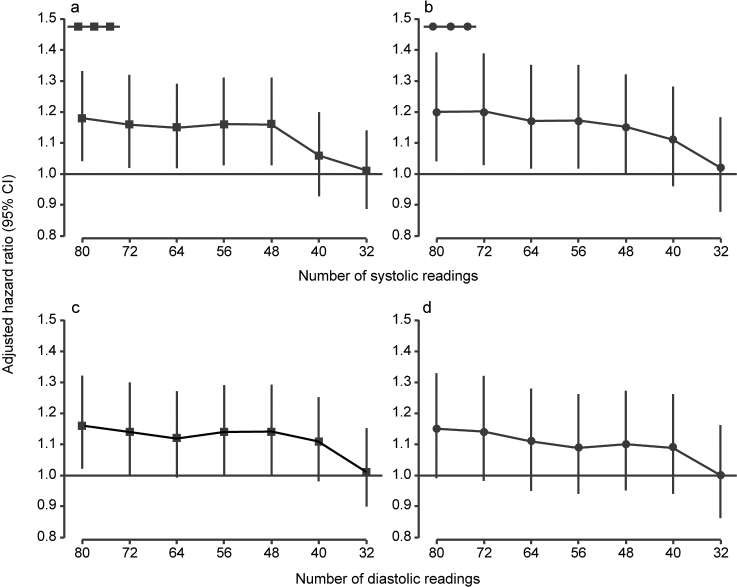
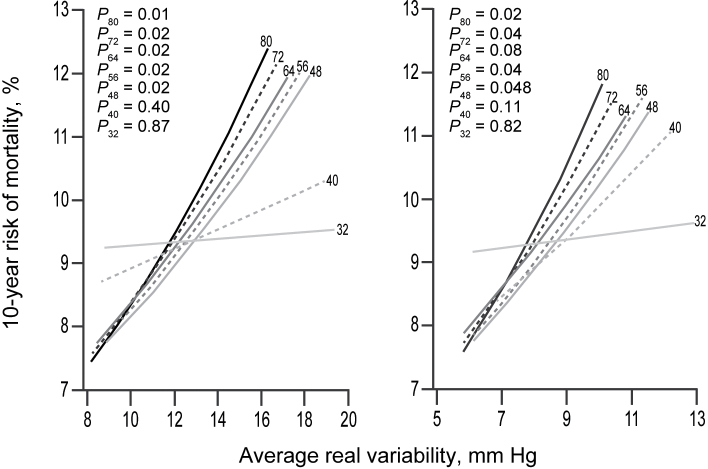
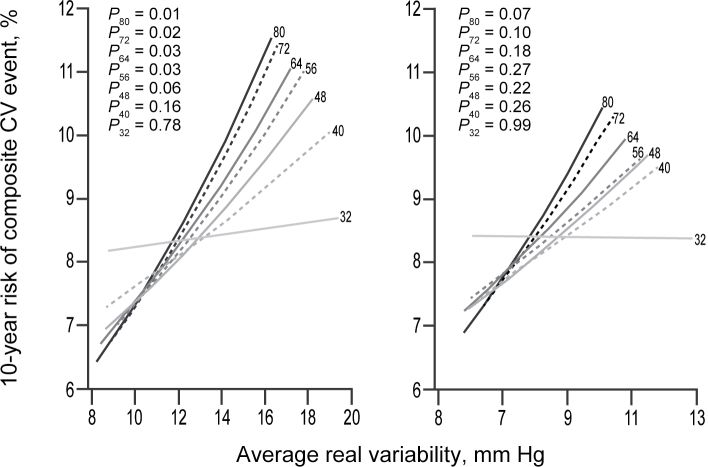
When the full recordings were used, the sex- and age-adjusted HRs associated with an increase in systolic ARV by 1 SD were 1.18 (95% confidence interval (CI) = 1.04–1.33; P = 0.01) for total mortality and 1.20 (95% CI = 1.04–1.39; P = 0.01) for the composite cardiovascular endpoint. For diastolic BP, these HRs were 1.16 (95% CI = 1.02–1.32; P = 0.02) and 1.15 (95% CI = 0.99–1.33; P = 0.07). In general, reducing the number of BP readings led to smaller HRs (Tables 3 and 4; Figure 1) and, consequently, a weaker association between the 10-year risk and ARV (Figures 2 and 3). However, the HRs remained fairly constant down to 40–48 BP readings. For total mortality, the HRs associated with both systolic and diastolic ARV were significant (P < 0.05) and >10% when ARV was calculated with at least 48 BP readings. Similarly, for the composite cardiovascular endpoint, reduced recordings composed of at least 48 readings generally yielded HRs ≥1.10 for both systolic and diastolic ARV. However, for systolic ARV statistical significance was lost when <56 readings were used. For diastolic ARV, the HRs were not significant either for the full or reduced recordings.
Table 3.
Standardized hazard ratios for all-cause mortality associated with mean 24-hour blood pressure and average real variability calculated from full and reduced recordings in the discovery dataset
| Minimum number of BP readings | Systolic BP | Diastolic BP | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean 24-h BP | Average real variability | Mean 24-h BP | Average real variability | |||||
| Hazard ratio (95% CI) | Delta, % | Hazard ratio (95% CI) | Delta, % | Hazard ratio (95% CI) | Delta, % | Hazard ratio (95% CI) | Delta, % | |
| 80 | 1.17 (1.04–1.33)** | NA | 1.18 (1.04–1.33)** | NA | 1.18 (1.04–1.34)** | NA | 1.16 (1.02–1.32)** | NA |
| 72 | 1.17 (1.03–1.32)** | −0.3 | 1.16 (1.02–1.32)** | −1.3 | 1.18 (1.04–1.35)** | 0.3 | 1.14 (1.00–1.30)** | −1.5 |
| 64 | 1.17 (1.04–1.33)** | 0.0 | 1.15 (1.02–1.29)** | −2.5 | 1.18 (1.03–1.34)** | −0.2 | 1.12 (0.99–1.27)*** | −3.3 |
| 56 | 1.16 (1.03–1.32)** | −0.9 | 1.16 (1.03–1.31)** | −1.6 | 1.16 (1.02–1.32)** | −1.9 | 1.14 (1.00–1.29)** | −1.9 |
| 48 | 1.17 (1.03–1.32)** | −0.6 | 1.16 (1.03–1.31)** | −1.6 | 1.18 (1.04–1.35)** | 0.2 | 1.14 (1.00–1.29)** | −2.0 |
| 40 | 1.18 (1.04–1.33)* | 0.2 | 1.06 (0.93–1.20) | −10.2 | 1.18 (1.04–1.35)** | 0.4 | 1.11 (0.98–1.25)*** | −4.5 |
| 32 | 1.06 (0.94–1.21) | −9.2 | 1.01 (0.89–1.14) | −14.2 | 0.98 (0.86–1.12) | −17.0 | 1.01 (0.90–1.15) | −12.5 |
Standardized hazard ratios (95% confidence intervals (CIs)) express the risk in all-cause mortality associated with 1 SD increase in the 24-hour blood pressure (BP) or the 24-hour average real variability. The SD of the 24-hour systolic BP was 12.77mm Hg for the full recordings (at least 80 readings) and 12.79, 12.81,12.82,1 2.90, 12.92, and 12.83mm Hg for the reduced recordings including at least 72, 64, 56, 48, 40, and 32 readings, respectively. For systolic average real variability, these SDs were 2.45, 2.55, 2.66, 2.45, 3.00, 3.21, and 3.40mm Hg, respectively. The corresponding SDs for the 24-hour diastolic BP and the diastolic average real variability were 8.38, 8.38, 8.42, 8.43, 8.46, 8.51, and 8.46mm Hg; and 1.36,1.42, 1.50, 1.62, 1.72, 1.86, and 2.01mm Hg, respectively. All hazard ratios were computed by Cox regression and were adjusted for sex and age. Delta is the percent difference between the hazard ratio calculated from the reduced recording and the hazard ratio calculated from the full recording.
Abbreviation: NA, not applicable.
Significance of the hazard ratios: *P < 0.01; **P < 0.05; ***0.05 ≤ P < 0.10.
Table 4.
Standardized hazard ratios for the composite cardiovascular endpoint associated with mean 24-hour blood pressure and average real variability calculated from full and reduced recordings in the discovery dataset
| Minimum number of BP readings | Systolic BP | Diastolic BP | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean 24-h BP | Average real variability | Mean 24-h BP | Average real variability | |||||
| Hazard ratio (95% CI) | Delta, % | Hazard ratio (95% CI) | Delta, % | Hazard ratio (95% CI) | Delta, % | Hazard ratio (95% CI) | Delta, % | |
| 80 | 1.39 (1.21–1.60)* | NA | 1.20 (1.04–1.39)*** | NA | 1.30 (1.12–1.52)** | NA | 1.15 (0.99–1.33)**** | NA |
| 72 | 1.40 (1.21–1.61)* | 0.3 | 1.20 (1.03–1.39)*** | −0.4 | 1.30 (1.12–1.51)** | −0.2 | 1.14 (0.98–1.32)**** | −0.9 |
| 64 | 1.40 (1.22–1.61)* | 0.6 | 1.17 (1.02–1.35)*** | −2.7 | 1.30 (1.12–1.51)** | −0.2 | 1.11 (0.95–1.28) | −3.6 |
| 56 | 1.38 (1.20–1.60)* | −0.6 | 1.17 (1.02–1.35)*** | −2.6 | 1.29 (1.12–1.50)** | −0.8 | 1.09 (0.94–1.26) | −5.2 |
| 48 | 1.38 (1.20–1.60)* | −0.6 | 1.15 (1.00–1.32)**** | −4.6 | 1.30 (1.12–1.51)** | −0.4 | 1.10 (0.95–1.27) | −4.3 |
| 40 | 1.41 (1.23–1.63)* | 1.4 | 1.11 (0.96–1.28) | −7.8 | 1.29 (1.11–1.50)** | −1.0 | 1.09 (0.94–1.26) | −5.2 |
| 32 | 1.11 (0.96–1.29) | −20.3 | 1.02 (0.88–1.18) | −15.1 | 1.04 (0.90–1.21) | −20.1 | 1.00 (0.86–1.16) | −12.9 |
Standardized hazard ratios (95% confidence intervals (CIs)) express the risk in all-cause mortality associated with 1 SD increase in the 24-hour blood pressure (BP) or the 24-hour average real variability. The SD of the 24-hour systolic BP was 12.77mm Hg for the full recordings (at least 80 readings) and 12.79, 12.81,12.82,1 2.90, 12.92, and 12.83mm Hg for the reduced recordings including at least 72, 64, 56, 48, 40, and 32 readings, respectively. For systolic average real variability, these SDs were 2.45, 2.55, 2.66, 2.45, 3.00, 3.21, and 3.40mm Hg, respectively. The corresponding SDs for the 24-hour diastolic BP and the diastolic average real variability were 8.38, 8.38, 8.42, 8.43, 8.46, 8.51, and 8.46mm Hg; and 1.36,1.42, 1.50, 1.62, 1.72, 1.86, and 2.01mm Hg, respectively. All hazard ratios were computed by Cox regression and were adjusted for sex and age. Delta is the percent difference between the hazard ratio calculated from the reduced recording and the hazard ratio calculated from the full recording.
Abbreviation: NA, not applicable.
Significance of the hazard ratios: *P < 0.0001; **P < 0.001; ***P < 0.05; ****0.05 ≤ P < 0.10.
Figure 1.
Standardized hazard ratios (95% confidence intervals (CI)) relating all-cause mortality (a and c) and all fatal combined with nonfatal cardiovascular events (b and d) to systolic (a and b) and diastolic (c and d) average real variability over 24 hours calculated from full (at least 80 readings) and reduced recordings with a minimum of 72, 64, 56, 48, 40, or 32 readings. Hazard ratios were adjusted for sex and age and express the risk per SD increase in average real variability in the 1,254 subjects included in the discovery dataset.
Figure 2.
Ten-year risk (%) of death in relation to systolic (left panel) and diastolic (right panel) average real variability over 24 hours (ARV) calculated from full (at least 80 readings) and reduced recordings with a minimum of 72, 64, 56, 48, 40, or 32 readings in the discovery cohort. The analyses were standardized to the midpoint (mean or ratio) of the distributions in the discovery cohort of sex and age. P values are for the independent effect of ARV calculated from full (P 80) and reduced (P 72, P 64, P 56, P 48, P 40, and P 32) recordings.
Figure 3.
Ten-year risk (%) of a fatal or nonfatal cardiovascular (CV) event in relation to systolic (left panel) and diastolic (right panel) average real variability over 24 hours (ARV) calculated from full (at least 80 readings) and reduced recordings with a minimum of 72, 64, 56, 48, 40, or 32 readings in the discovery cohort. The analyses were standardized to the midpoint (mean or ratio) of the distributions in the discovery cohort of sex and age. P values are for the independent effect of ARV calculated from full (P 80) and reduced (P 72, P 64, P 56, P 48, P 40, and P 32) recordings.
Test dataset
The results based on artificially reduced ambulatory BP recordings were confirmed using a larger sample of 5,353 subjects, whose ambulatory BP recordings included at least 48 BP readings. The test dataset included 879 residents of Copenhagen, Denmark, who were not included in the discovery dataset;8 954 subjects from Noorderkempen, Belgium;17 925 older men from Uppsala, Sweden;9 242 subjects from Novosibirsk, the Russian Federation;10,11 422 inhabitants of Ohasama, Japan;15 344 villagers from JingNing County, China;12 161 subjects from Pilsen, the Czech Republic;11 265 subjects from Dublin, Ireland;14 310 subjects from Padova, Italy;11 308 subjects from Kraków, Poland;11 and 543 older subjects from Maracaibo, Venezuela.13 Compared with the discovery dataset, the 2,440 women and 2,913 men included in the test dataset were younger (54.0±16.1 years) and had lower body mass index (25.6±4.1kg/m2) and lower 24-hour ambulatory BP (125.1±14.5mm Hg systolic, 73.8±8.4mm Hg diastolic). The test dataset included a similar proportion of hypertensive patients (47.5%) but more subjects receiving antihypertensive drug treatment (21.3%). The test dataset included smaller proportions of smokers (27.6%) and subjects reporting alcohol intake (54.0%) and a higher proportion of subjects with a history of cardiovascular disease (16.2%) or diabetes mellitus (7.3%). The median number of BP readings per recording in the test dataset was 64 (5th–95th percentile = 48–79).
Median follow-up for participants in the test dataset was 10.2 years (5th–95th percentile = 2.5–18.4 years), ranging from 2.5 years (5th–95th percentile = 2.3–2.6 years) in JinNing to 17.6 years (5th–95 percentile = 6.4–20.4 years) in Noorderkempen. During 55,856 person-years of follow-up, 806 participants died (14.4 per 1,000 person-years), and 696 experienced a fatal or nonfatal cardiovascular event (12.9 per 1,000 person-years). The cause of death was cardiovascular in 335 participants. Considering cause-specific first cardiovascular events, the incidences of fatal and nonfatal stroke were 39 and 200, respectively. Cardiac events consisted of 172 fatal and 265 nonfatal events, including 143 nonfatal cases of acute myocardial infarction, 155 deaths from ischemic heart diseases, 6 sudden deaths, 11 fatal and 84 nonfatal cases of heart failure, and 38 cases of surgical or percutaneous coronary revascularization.
The standardized HRs relating outcome to ARV, adjusted for cohort, sex, age, body mass index, smoking status, alcohol intake, serum cholesterol, history of cardiovascular disease, and treatment with antihypertensive drugs, showed that systolic and diastolic ARV significantly (P < 0.0005) predicted total, cardiovascular, and cardiac mortality and fatal plus nonfatal cardiovascular and cerebrovascular events (Table 5). Systolic but not diastolic ARV also significantly (P = 0.003) predicted cardiac events. However, after additional adjustment for 24-hour mean BP, systolic and diastolic ARV significantly (P < 0.01) predicted only total, cardiovascular, and cardiac deaths. In addition, higher diastolic ARV was significantly (P = 0.009) related to the incidence of fatal plus nonfatal cerebrovascular outcomes. The R 2 statistics for adding ARV to BP level and other covariables as combined predictors of the composite cardiovascular endpoint were 0.035% and 0.031% for systolic and diastolic ARV, respectively (Supplementary Table S2)
Table 5.
Multivariable-adjusted standardized hazard ratios associated with average real variability in the test dataset
| Outcome (number of events) | 24-h average real variability | |
|---|---|---|
| Systolic blood pressure, HR (95% CI) | Diastolic blood pressure, HR (95% CI) | |
| Mortality | ||
| Total (n = 806) | ||
| Adjusted | 1.21 (1.12–1.30)* | 1.16 (1.08–1.23)* |
| Fully adjusted | 1.12 (1.03–1.21)*** | 1.12 (1.04–1.20)*** |
| Cardiovascular (n = 335) | ||
| Adjusted | 1.33 (1.20–1.48)* | 1.27 (1.16–1.38)* |
| Fully adjusted | 1.17 (1.04–1.31)*** | 1.19 (1.08–1.30)** |
| Cardiac (n = 335) | ||
| Adjusted | 1.38 (1.20–1.59)* | 1.24 (1.10–1.40)** |
| Fully adjusted | 1.24 (1.06–1.45)*** | 1.19 (1.05–1.35)*** |
| Fatal and nonfatal events combined | ||
| All cardiovascular (n = 696) | ||
| Adjusted | 1.19 (1.10–1.29)* | 1.13 (1.06–1.21)** |
| Fully adjusted | 1.06 (0.98–1.15) | 1.05 (0.98–1.13) |
| Cardiac (n = 437) | ||
| Adjusted | 1.17 (1.06–1.29)*** | 1.04 (0.95–1.14) |
| Fully adjusted | 1.05 (0.94–1.17) | 0.98 (0.89–1.09) |
| Cerebrovascular (n = 239) | ||
| Adjusted | 1.26 (1.11–1.43)** | 1.29 (1.16–1.42)* |
| Fully adjusted | 1.08 (0.95–1.23) | 1.16 (1.04–1.29)*** |
Values are standardized hazard ratios (HRs) (95% confidence intervals (CIs)) associated with 1 SD increase in average real variability (ARV) calculated using at least 48 readings. The SD of the ARV was 3.32mm Hg for systolic blood pressure and 2.66mm Hg for diastolic blood pressure. All HRs were computed by Cox regression, stratified for cohort, and adjusted for sex, age, body mass index, smoking status, alcohol intake, serum cholesterol, history of cardiovascular disease, and treatment with antihypertensive drugs. Fully adjusted models were additionally adjusted for the 24-hour blood pressure level.
Significance of the hazard ratios: *P < 0.0001; **P < 0.001; ***P < 0.01.
DISCUSSION
The technique of noninvasive ambulatory BP monitoring has rapidly expanded during the past 30 years, both as an instrument in clinical research and as a diagnostic tool in clinical practice. Several studies have examined the effect of intermittent readings on the accurate assessment of true 24-hour average BP.23,25–27 Despite numerous studies that have reported on the predictive value of short-term BP variability,1,2,5,28,29 the frequency and/or number of BP readings necessary to accurately estimate variability of 24-hour BP has not been assessed before. Di Rienzo et al. 25 demonstrated that accurate estimation of 24-hour average BP could be achieved at intervals as great as 30–60 minutes; however, short-term BP variability at sampling intervals of ≥15 minutes, as assessed by within-subject SD, deviated considerably from beat-to-beat analysis.
Several studies found that both short-term reading-to-reading23,25–27 and long-term visit-to-visit30–33 BP variability, estimated by ambulatory BP monitoring, are poorly reproducible, which could explain the rather diverse findings regarding its clinical value as a predictor of cardiovascular outcomes.34 Several factors are potentiality responsible for the poor reproducibility of BP variability. First, it could be influenced by day-to-day variation in the subject’s activities. Second, predictions could have been based on insufficient numbers of BP readings; a small sample size increases the potential error in estimating the true variance.25 Finally, although the SD is a convenient measure, it might not be the most accurate method to assess BP variability.1–3,5,30 To address the latter 2 problems, in the present IDACO study, BP variability was assessed using ARV,1,2 and the number of BP readings needed to make accurate risk predictions was formally tested.
The most important observation was that the number of BP readings used to calculate ARV affected both its reproducibility and its strength as a predictor of mortality and cardiovascular outcomes. Concordance between ARV values based on full and randomly reduced BP measurements and predictive value declined significantly as more BP readings were excluded. A possible explanation is that within-subject ARV might increase when calculated from fewer BP readings, masking differences between subjects that actually have high or low BP variability.
In a discovery dataset that included >1,000 randomly recruited subjects with a median of 12.4 years of follow-up, a minimum of approximately 48 BP readings was necessary to accurately estimate ARV. This conclusion was supported using a test dataset with >5,000 subjects, randomly recruited from 11 populations, with a median of 10.2 years of follow-up. Using this minimum number of BP readings, both systolic and diastolic ARV were significant predictors of mortality and cardiovascular endpoints in models adjusted for 24-hour BP level and other covariables. These results are in partial agreement with those of Hansen et al.,2 who computed ARV with a less restrictive minimum number of BP readings (at least 10 daytime and 5 nighttime BP readings). They found that both systolic ARV and diastolic ARV were significant and independent predictors of total and cardiovascular mortality and of fatal combined with nonfatal cardiovascular events. However, this study did not find that ARV was a significant and independent predictor of cardiac mortality in a fully adjusted model.2
Although prognostic value1,2,5 and reproducibility30 are better for ARV than for other variability indexes, such as SD or weighted SD,35 ARV computed with at least 48 BP readings accounted for <0.1% of risk of a composite cardiovascular event, beyond the proportion explained by 24-hour BP level. Furthermore, although the precision of ARV could be improved by increasing the number of measurements, increasing the number of BP readings beyond 48 readings in clinical practice seems inconvenient, because this makes 24-hour ambulatory BP monitoring less comfortable. Therefore, accurate assessment of BP variability might have greater relevance for research than for clinical purposes. It could produce unconfounded information on mechanisms of homeostatic control of BP under physiological and pathological conditions, such as essential and secondary hypertension, congestive heart failure, diabetes, and renal insufficiency. A reliable assessment of BP variability, with an adequate minimum number of BP readings could also be used to design studies to improve the diagnosis and prognosis of hypertension by providing better information on end-point organ damage associated with high BP values and could be used to assess the efficacy of antihypertensive agents.36 Finally, accurate calculation of ARV could be useful in the development of therapeutic drugs to reduce BP variability, which might represent a new strategy for the treatment of hypertension.37
Despite the statistical power and the consideration of fatal and nonfatal events, this study had several potential limitations. First, although the test dataset was composed of 11 population-based cohorts from 3 continents, our results might not be applicable to all ethnic groups, particularly to Africans of black ancestry or African Americans, although Veerabhadrappa et al. 6 showed that ARV was significantly correlated with target organ damage in African Americans. Second, we and most other investigators used intermittent techniques of ambulatory BP monitoring, which are less precise than continuous BP monitoring for measuring short-term BP variability. Unfortunately, intra-arterial recordings or continuous recordings of the arterial signal at the finger are difficult, if not impossible, to implement in large epidemiological studies. Third, because the goal was to estimate an appropriate minimum range of BP readings for calculating ARV in a reproducible manner, relatively large numbers of BP measurements were omitted, ultimately producing low concordances. However, Kikuya et al. 22 excluded fewer BP readings and found that the parameter under study lost prognostic significance only when ≥16 readings were randomly excluded.
In summary, 48 BP readings over 24 hours is sufficient to compute ARV without meaningfull loss of prognostic information. This number of BP readings should be considered for research purposes when trying to assess the effects of BP variability on outcome variables or the effects of an intervention on BP variability using ARV as an estimate. Usefulness of ARV in the clinical setting remains to be determined, but ARV needs to be computed with enough measurements to retain its prognostic value.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of Eber Orozco, Vannesa Félix, and Armando Cervantes (Universidad Politécnica de Sinaloa, Mazatlán, México); Jesús Melgarejo (Laboratorio de Neurociencias, Universidad del Zulia, Maracaibo, Venezuela); José Aizpurua (Instituto Regional de Investigación y Estudios de Enfermedades Cardiovasculares Universidad del Zulia, Maracaibo, Venezuela); and Sandra Covens and Annick De Soete (Studies Coordinating Centre, Leuven, Belgium).
The Secretaria de Educación Pública, México DF, México (PROMEP/103–5/11/4145 and PROMEP/103–5/11/6951) gave support for this investigation. The European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006–037093 InGenious HyperCare, HEALTH-F4-2007–201550 HyperGenes, HEALTH-F7-2011- 278249 EU-MASCARA, and the European Research Council Advanced Research Grant 294713 EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0734.09) supported the Studies Coordinating Centre (Leuven, Belgium). The European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550) also supported the research groups in Shanghai, Kraków, Padova, and Novosibirsk. The Danish Heart Foundation (grant 01-2-9-9A-22914), and the Lundbeck Fonden (grant R32-A2740) supported the studies in Copenhagen. The Ohasama study received support via Grant-in-Aid for Scientific Research (22590767, 22790556, 23249036, 23390171, and 23790242) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Health Labour Sciences Research Grant (H23-Junkankitou [Seishuu]-Ippan-005) from the Ministry of Health, Labour and Welfare, Japan; Japan Arteriosclerosis Prevention Fund; and a grant from the Central Miso Research Institute, Tokyo, Japan. The National Natural Science Foundation of China (grants 30871360 and 30871081), Beijing, China, and the Shanghai Commissions of Science and Technology (grant 07JC14047 and the “Rising Star” program 06QA14043) and Education (grant 07ZZ32 and the “Dawn” project) supported the JingNing study in China. The Comisión Sectorial de Investigación Científica de la Universidad de la República (Grant I+D GEFA-HT-UY) and the Agencia Nacional de Innovación e Investigación supported research in Uruguay. Grant 1R01AG036469-01A1 from the National Institute on Aging and Fogarty International Center, grants G-97000726 and 98000726 from FONACIT, and grant LOCTI/008-2008 from FundaConciencia supported the Maracaibo Aging Study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any other funding agency.
REFERENCES
- 1. Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 2005; 23:505–511 [DOI] [PubMed] [Google Scholar]
- 2. Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Pedersen CT, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA, for the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome Investigators Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057 [DOI] [PubMed] [Google Scholar]
- 3. Parati G, Rizzoni D. Assessing the prognostic relevance of blood pressure variability: discrepant information from different indices. J Hum Hypertens 2005; 23:483–486 [DOI] [PubMed] [Google Scholar]
- 4. Eguchi K, Hoshi H, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens 2012; 25:962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, Cuccurullo F. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens 2009; 22:842–847 [DOI] [PubMed] [Google Scholar]
- 6. Veerabhadrappa P, Diaz KM, Feairheller DL, Sturgeon KM, Williamson S, Crabbe DL, Kashem A, Ahrensfield D, Brown MD. Enhanced blood pressure variability in a high cardiovascular risk group of African Americans: FIT4life Study. J Am Soc Hypertens 2010; 4:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Li Y, Dolan E, Tikhonoff V, Sleidlerová J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang JG, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, on behalf of the IDACO Investigators The International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit 2007; 12:255–262 [DOI] [PubMed] [Google Scholar]
- 8. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure and mortality: a population based study. Hypertension 2005; 45:499–504 [DOI] [PubMed] [Google Scholar]
- 9. Ingelsson E, Björklund K, Lind L, Ärnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA 2006; 295:2859–2866 [DOI] [PubMed] [Google Scholar]
- 10. Kuznetsova T, Malyutina S, Pello E, Thijs L, Nikitin Y, Staessen JA. Ambulatory blood pressure of adults in Novosibirsk, Russia: interim report on a population study. Blood Press Monit 2000; 5:291–296 [DOI] [PubMed] [Google Scholar]
- 11. Kuznetsova T, Staessen JA, Kawecka-Jaszcz K, Babeanu S, Casiglia E, Filipovský J, Nachev C, Nikitin Y, Peleská J, O’Brien E, on behalf of the EPOGH Investigators Quality control of the blood pressure phenotype in the European Project on Genes in Hypertension. Blood Press Monit 2002; 7:215–224 [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Wang JG, Gao HF, Nawrot T, Wang GL, Qian YS, Staessen JA, Zhu DL. Are published characteristics of the ambulatory blood pressure generalizable to rural Chinese? The JingNing population study. Blood Press Monit 2005; 10:125–134 [DOI] [PubMed] [Google Scholar]
- 13. Maestre GE, Pino-Ramírez G, Molero AE, Silva ER, Zambrano R, Falque L, Gamero MP, Sulbarán TA. The Maracaibo Aging Study: population and methodological issues. Neuroepidemiology 2002; 21:194–201 [DOI] [PubMed] [Google Scholar]
- 14. O’Brien E, Murphy J, Tyndall A, Atkins N, Mee F, McCarthy G, Staessen J, Cox J, O’Malley K. Twenty-four-hour ambulatory blood pressure in men and women aged 17 to 80 years: the Allied Irish Bank Study. J Hypertens 1991; 9:355–360 [DOI] [PubMed] [Google Scholar]
- 15. Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20:2183–2189 [DOI] [PubMed] [Google Scholar]
- 16. Schettini C, Bianchi M, Nieto F, Sandoya E, Senra H, Hypertension Working Group Ambulatory blood pressure. Normality and comparison with other measurements. Hypertension 1999; 34:818–825 [DOI] [PubMed] [Google Scholar]
- 17. Staessen JA, Bieniaszewski L, O’Brien ET, Imai Y, Fagard R. An epidemiological approach to ambulatory blood pressure monitoring: the Belgian population study. Blood Press Monit 1996; 1:13–26 [PubMed] [Google Scholar]
- 18. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens 2006; 19:243–250 [DOI] [PubMed] [Google Scholar]
- 19. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Relation between insulin and aortic stiffness: a population-based study. J Hum Hypertens 2004; 18:1–7 [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker-Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Manolis A, Nilsson PM, Redon J, Struijker-Boudier HA, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, O’Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. 2007 guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007; 28:1462–1536 [DOI] [PubMed] [Google Scholar]
- 21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–310 [PubMed] [Google Scholar]
- 22. Kikuya M, Staessen JA, Ohkubo T, Thijs L, Asayama K, Satoh M, Hashimoto T, Hirose T, Metoki H, Obara T, Inoue R, Li Y, Dolan E, Hoshi H, Totsune K, Satoh H, Wang JG, O’Brien E, Imai Y. How many measurements are needed to provide reliable information in terms of the ambulatory arterial stiffness index? The Ohasama study. Hypertens Res 2011; 34:314–318 [DOI] [PubMed] [Google Scholar]
- 23. Thijs L, Staessen J, Fagard R, Zachariah P, Amery A. Number of measurements required for the analysis of diurnal blood pressure profile. J Hum Hypertens 1994; 8:239–244 [PubMed] [Google Scholar]
- 24. Atkinson AC. A note on the generalized information criterion for choice of a model. Biometrika 1980; 67:413–418 [Google Scholar]
- 25. Di Rienzo M, Grassi G, Pedotti A, Mancia G. Continuous vs intermittent blood pressure measurements in estimating 24-hour average blood pressure. Hypertension 1983; 5:264–269 [DOI] [PubMed] [Google Scholar]
- 26. Mancia G, Parati G, Pomidossi G, Di Rienzo M. Validity and usefulness of non-invasive ambulatory blood pressure monitoring. J Hypertens Suppl 1985; 3:S5–S11 [PubMed] [Google Scholar]
- 27. Palatini P, Mormino P, Canali C, Santonastaso M, De Venuto G, Zanata G, Pessina AC. Factors affecting ambulatory blood pressure reproducibility. Results of the HARVEST Trial. Hypertension 1994; 23:211–216 [DOI] [PubMed] [Google Scholar]
- 28. Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 2000; 36:901–906 [DOI] [PubMed] [Google Scholar]
- 29. Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH, on behalf of the Syst-Eur Investigators Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens 2003; 21:2251–2257 [DOI] [PubMed] [Google Scholar]
- 30. Eguchi K, Hoshide S, Hoshide Y, Ishikawa S, Shimada K, Kario K. Reproducibility of ambulatory blood pressure in treated and untreated hypertensive patients. J Hypertens 2010; 28:918–924 [DOI] [PubMed] [Google Scholar]
- 31. Stenehjem AE, Os I. Reproducibility of blood pressure variability, white-coat effect and dipping pattern in untreated, uncomplicated and newly dagnosed essential hypertension. Blood Press 2004; 13:214–224 [DOI] [PubMed] [Google Scholar]
- 32. Trazzi S, Mutti E, Frattola A, Imholz B, Parati G, Mancia G. Reproducibility of non-invasive and intra-arterial blood pressure monitoring: implications for studies on antihypertensive treatment. J Hypertens 1991; 9:115–119 [DOI] [PubMed] [Google Scholar]
- 33. van der Steen MS, Lenders JWM, Graafsma SJ, den Arend J, Thien T. Reproducibility of ambulatory blood pressure monitoring in daily practice. J Hum Hypertens 1999; 13:303–308 [DOI] [PubMed] [Google Scholar]
- 34. Hansen TW, Li Y, Staessen JA. Blood pressure variability remains an elusive predictor of cardiovascular outcome. Am J Hypertens 2009; 22:3–4 [DOI] [PubMed] [Google Scholar]
- 35. Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, Mancia G, Parati G. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens 2007; 25:2058–2066 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Agnoletti D, Safar ME, Blacher J. Effect of antihypertensive agents on blood pressure variability: the Natrillix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X-CELLENT) study. Hypertension 2011; 58:155–160 [DOI] [PubMed] [Google Scholar]
- 37. Su DF, Miao CY. Reduction of blood pressure variability: a new strategy for the treatment of hypertension. Trends Pharmacol Sci 2005; 26:388–390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.