Abstract
BACKGROUND
In response to high salt intake, transcription factor hypoxia-inducible factor (HIF) 1α activates many antihypertensive genes, such as heme oxygenase 1 (HO-1) 1 and cyclooxygenase 2 (COX-2) in the renal medulla, which is an important molecular adaptation to promote extra sodium excretion. We recently showed that high salt inhibited the expression of HIF prolyl-hydroxylase 2 (PHD2), an enzyme that promotes the degradation of HIF-1α, thereby upregulating HIF-1α, and that high salt–induced inhibition in PHD2 and subsequent activation of HIF-1α in the renal medulla was blunted in Dahl salt-sensitive hypertensive rats. This study tested the hypothesis that silencing the PHD2 gene to increase HIF-1α levels in the renal medulla attenuates salt-sensitive hypertension in Dahl S rats.
METHODS
PHD2 short hairpin RNA (shRNA) plasmids were transfected into the renal medulla in uninephrectomized Dahl S rats. Renal function and blood pressure were then measured.
RESULTS
PHD2 shRNA reduced PHD2 levels by >60% and significantly increased HIF-1α protein levels and the expression of HIF-1α target genes HO-1 and COX-2 by >3-fold in the renal medulla. Functionally, pressure natriuresis was remarkably enhanced, urinary sodium excretion was doubled after acute intravenous sodium loading, and chronic high salt-induced sodium retention was remarkably decreased, and as a result, salt-sensitive hypertension was significantly attenuated in PHD2 shRNA rats compared with control rats.
CONCLUSIONS
Impaired PHD2 response to high salt intake in the renal medulla may represent a novel mechanism for hypertension in Dahl S rats, and inhibition of PHD2 in the renal medulla could be a therapeutic approach for salt-sensitive hypertension.
Keywords: blood pressure, cyclooxygenase 2, heme oxygenase 1, hypertension, hypoxia-inducible factor, pressure natriuresis, sodium excretion.
Salt-sensitive hypertension accounts for 50% of hypertension cases1 and exhibits a much higher risk for development of organ damage than salt-resistant hypertension.2,3 The mechanism regulating salt sensitivity of blood pressure is not very clear. Renal medullary function is well known to play a critical role in the regulation of sodium excretion and blood pressure, and it is known that dysfunction in the renal medulla is involved in salt-sensitive hypertension.4,5 We have recently shown that transcription factor hypoxia-inducible factor (HIF) 1α–mediated activation of antihypertensive genes in the renal medulla enhances the production of a variety of protective factors in the renal medulla, which promotes the excretion of extra sodium load and regulates the renal adaptation to high salt intake.6
HIF-1α and many HIF-1α target genes, such as hemeoxygenase 1 (HO-1), cyclooxygenase 2 (COX-2), nitric oxide synthase 2 (NOS-2), and endothelin 1, are highly expressed in the renal medulla and significantly upregulated in response to high salt intake.7–13 The products of these HIF-1α target genes importantly participate in the regulation of blood flow and/or tubular activity in the renal medulla and play critical roles in sodium balance and long-term control of arterial blood pressure as well as salt sensitivity of blood pressure.7,8,11–15 We have demonstrated that high salt diet upregulates HIF-1α levels in the renal medulla6,16 and that blockade of HIF-1α function to inhibit the expression of its target genes in the renal medulla induces sodium retention after high-salt challenge, producing a salt-sensitive hypertension.6 These results suggest that HIF-1α–mediated gene activation in the renal medulla represents an important molecular adaptive mechanism to maintain sodium balance in response to high salt intake.
Interestingly, it has been shown that the above protective genes regulated by HIF-1α are defective in Dahl salt-sensitive hypertensive (Dahl S) rats12,13,16–18 and that the deficiencies of these HIF-1α target genes in the renal medulla are considered to be responsible for the development of hypertension in this animal model.12,13,17 We recently showed that upregulation of renal medullary HIF-1α levels in response to high salt intake was blunted in Dahl S rats,16,19 indicating that the abnormal responses of the above protective genes may be due to a defect of HIF-1α in the renal medulla and that impairment in HIF-1α–mediated gene activation in the renal medulla may be responsible for salt-sensitive hypertension in Dahl S rats. Indeed, correction of HIF-1α deficiency in the renal medulla increased the expression of antihypertensive genes in the renal medulla, enhanced the urinary sodium excretion, reduced sodium retention, and consequently, attenuated salt-sensitive hypertension in Dahl S rats.19
Furthermore, it has been demonstrated that HIF prolyl-hydroxylase 2 (PHD2), an enzyme that promotes the degradation of HIF-1α, is the most abundant isoform of PHDs in the kidneys20,21 and is highly expressed in the renal medulla.16,20,21 We have shown that high salt intake suppresses the expression of PHD2 in the renal medulla and that this high salt–induced inhibition of PHD2 is an upstream signal that increases HIF-1α–mediated gene expression in the renal medulla in response to high-salt challenge.16 Notably, the high salt–induced inhibition in PHD2 in the renal medulla is also defective in Dahl S rats.16 This study sought to test the hypothesis that deficiency in PHD2/HIF-1α–mediated molecular adaptation in response to high salt intake in the renal medulla may be the pathogenic mechanism responsible for salt-sensitive hypertension and that silencing the PHD2 gene to increase the levels of HIF-1α and its target genes in the renal medulla enhances the sodium excretion and attenuates salt-sensitive hypertension in Dahl S rats. We first transfected PHD2 short hairpin RNA (shRNA) plasmids into the renal medulla and then detected the pressure natriuresis, the renal sodium excretion after sodium overload, and the arterial blood pressure after high-salt challenge in Dahl S rats. Our data showed that correction of the defect in PHD2 response to high salt intake attenuated salt-sensitive hypertension in Dahl S rats.
METHODS
Animals
Male Dahl S rats (Charles River, Wilmington, MA) that weighed 250–350g were used. Rats were kept on a low-salt diet (0.4% NaCl), with some fed a high-salt diet (4% NaCl) as indicated in the Results section. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Transfection of plasmids expressing rat PHD2 shRNA
The right kidney was removed 1 week before transfection, and the renal medulla of the left kidney was transfected with designated plasmids using the transfection reagent in vivo-jetPEI (Polyplus Transfection, New York, NY), a polyethylenimine derivative, as previously described.6,19,22 In brief, 50 µg of DNA was mixed with 8 µl of in vivo-jetPEI in 5% glucose (600 µl) and then infused into the renal medulla (20 µl/min). In the middle of and after the infusion, an ultrasound transducer (Sonitron 2000, Rich-Mar, Inola, OK) was applied to the kidneys (1 MHz, 10% output) for a total of 60 seconds with 30-second intervals on each side of the kidney. Fluorescent imaging confirmed the localization of transfected plasmids within the renal medulla using red fluorescent protein plasmids in our previous pilot study.
Rat PHD2 small interfering RNA (siRNA) sequences (sense: GUG UGA CAU GUA UAU AUU A; antisense: UAA UAU AUA CAU GUC ACA C) were designed and synthesized by QIAGEN. The target sequence was ATG TGT GAC ATG TAT ATA TTA (accession No.: NM_178334). After the confirmation of effective knocking down of PHD2 genes by these siRNAs in cultured renal cells, the sequences were constructed into a pRNA-CMV3.2 vector (Genscript, Piscataway, NJ) to produce shRNA. The effective gene silencing of renal PHD2 by shRNA in vivo was also verified in previous experiments.16 Plasmids expressing luciferase were used in control rats.
Measurement of pressure natriuresis in response to the elevations of renal perfusion pressure
After transfection of plasmids expressing PHD2 shRNA or control plasmids, animals were maintained on low-salt diet for 10 days and then subjected to the measurement of pressure natriuresis, as previously described.11,23 In brief, after equilibration, renal perfusion pressure (RPP) was acutely increased by tying off the celiac and mesenteric arteries, and the RPP was set to 160, 120, and 80 mmHg, respectively, by an adjustable clamp placed on the aorta above renal arteries. At each RPP level, after a 10-minute equilibration period, urine samples were collected during a 20-minute clearance period. Urinary volume and sodium excretion were measured and factored per gram of kidney weight. Renal cortical and medullary blood flows in response to the elevations of RPP were also measured using a laser Doppler flow meter, as previously described.6
Measurement of urinary sodium excretion in response to acute sodium loading
Additional rats were transfected with PHD2 shRNA or control plasmids and maintained on a low-salt diet for 10 days as above. Urinary volume and sodium excretion after acute sodium loading were measured as previously described.6 In brief, after surgical preparation and equilibration, two 10-minute control-period urine samples were collected and then a 5% body weight isotonic saline load was administered intravenously within 30 minutes. Three 10-minute samples were collected over 30 minutes and three more 10-minute postcontrol samples were taken. Urinary volume and sodium excretion were measured and factored per gram of kidney weight.
Measurement of daily sodium balance
Additional rats were treated the same as above and then housed in metabolic cages. Daily sodium balance was calculated by subtracting sodium excretion from sodium intake. After 1 day of control measurements, rats were fed with 2% NaCl water, and daily sodium balance was measured for 3 more days.6
Chronic monitoring of arterial blood pressure in conscious rats
Blood pressure was measured using a telemetry system as previously described.6,11,19,22 Three-day baseline mean arterial pressure (MAP) was recorded when the animals remained on low-salt diet. Then rats were fed with a high-salt diet (Dyets, Bethlehem, PA), and MAP was recorded for an additional 2 weeks. Rats were divided into 3 groups: (i) low-salt diet + control plasmids; (ii) high-salt diet + control plasmids; and (iii) high-salt diet + PHD2 shRNA plasmids. After MAP recording, kidneys were removed and saved for the isolation of protein and RNA later.
Preparation of tissue homogenate and nuclear extracts and Western blot analyses for protein levels of PHD2 and HIF-1α
Renal tissue homogenates and nuclear proteins from the renal medulla were extracted as previously described20,24 and then subjected (50 µg) to Western blot analysis. Primary antibodies were from Novus Biologicals (Littleton, CO): antirat PHD2 (rabbit polyclonal, 1:300) and HIF-1α (monoclonal, 1:300). The intensities of the blots were analyzed using ImageJ software (http://rsbweb.nih.gov/ij/). The levels of β-tubulin were used as internal control.
RNA extraction and quantitative reverse-transcription polymerase chain reaction analysis of PHD2, HO-1 and COX-2 mRNA
Total RNA was extracted using TRIzol solution (Life Technologies, Rockville, MD) and then reverse-transcribed (cDNA Synthesis Kit; Bio-Rad, Hercules, CA). The reverse-transcription products were amplified using TaqMan Gene Expression Assays kits (Applied Biosystems, Grand Island, NY). The level of 18S ribosomal RNA was used as a control. The relative mRNA levels were calculated in accordance with the ΔΔCt method and expressed by the values of 2-ΔΔCt.
Statistical analysis
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an analysis of variance followed by a Duncan multiple range test. Student t test was used to evaluate statistical significance of differences between 2 groups. P < 0.05 was considered statistically significant.
RESULTS
Effect of PHD2 shRNA on HIF-1α levels and the expression of HIF-1α target genes in the renal medulla
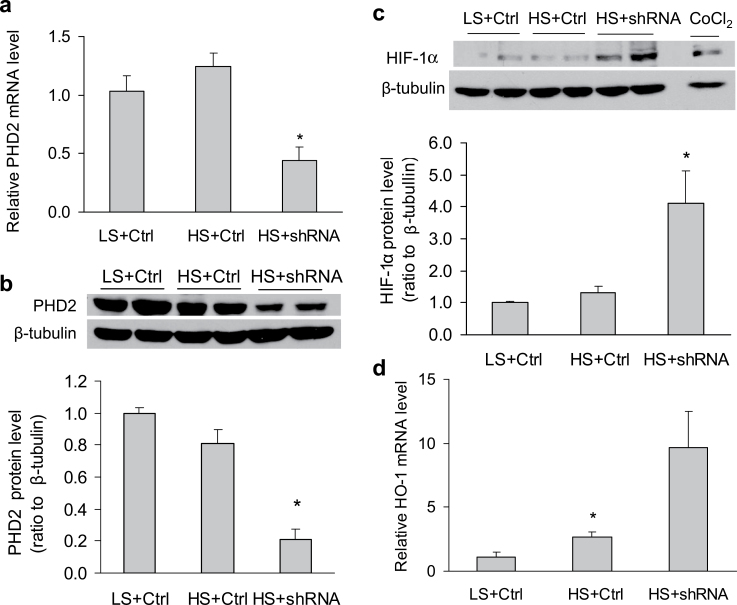
High-salt challenge did not significantly reduce PHD2 mRNA and protein levels and did not increase HIF-1α protein levels in the renal medulla in control animals (Figure 1), which was consistent with our previous studies.16,19 In animals treated with PHD2 shRNA to reduce PHD2 levels (Figure 1a,b), however, the protein levels of HIF-1α were significantly upregulated after high salt intake (Figure 1c), which was accompanied by a significant increase in the transcription of HIF-1α target gene HO-1 in the renal medulla (Figure 1d). The mRNA levels of another HIF-1α target gene, COX-2, were also similarly increased by >3-fold (data not shown). These results verified a successful inhibition in PHD2 levels and activation in HIF-1α–mediated gene regulation in the renal medulla by PHD2 shRNA.
Figure 1.
Effects of prolyl-hydroxylase 2 (PHD2) short hairpin RNA (shRNA) transfection into the renal medulla on the levels of PHD2, hypoxia-inducible factor 1α (HIF-1α), and HIF-1α target genes heme oxygenase 1 (HO-1) 1 and cyclooxygenase 2 (COX-2) in the renal medulla. (a) PHD2 mRNA levels. (b) Representative Enhanced chemiluminescence (ECL) gel documents of Western blot analyses depicting the protein levels of PHD2 and summarized intensities of PHD2 blots (ratio to β-tubulin). (c) Representative ECL gel documents of Western blot analyses depicting the protein levels of HIF-1α and summarized intensities of the HIF-1α blots (ratio to β-tubulin). (d) mRNA levels of HO-1. *P < 0.05 vs. other groups (n = 5–7). Abbreviations: CoCl2, sample from cells treated with CoCl2 as positive control; Ctrl, control vectors expressing luciferase; HS, high salt; LS, low salt; shRNA, PHD2 shRNA vectors.
Effects of PHD2 shRNA on sodium excretion and renal blood flow in response to renal perfusion pressure
The urine flow (U·V) and sodium excretion (UNa·V) were increased in response to the elevation of RPP. However, these pressure diuretic and natriuretic responses were remarkably improved in PHD2 shRNA–treated rats compared with control rats (Figure 2a,b). There was no difference in renal cortical blood flows between the 2 groups, whereas RPP-induced increase in renal medullary blood flow was significantly enhanced in shRNA-treated rats (Figure 2c,d), which was consistent with our previous study that showed that the HIF-1α–mediated pathway is of importance in determining the RPP-induced elevation of renal medullary blood flow.6 Because renal medullary blood flow is one of the important determinants to pressure natriuresis, enhanced renal medullary blood flow may contribute to improved natriuresis in response to RPP.
Figure 2.
Effects of prolyl-hydroxylase 2 (PHD2) short hairpin RNA (shRNA) transfection into the renal medulla on pressure natriuresis. (a) Urine flow rates (U·V) in response to the elevations of renal perfusion pressure (RPP). (b) Urinary sodium excretion rates (UNa·V) in response to the elevations of RPP. *P < 0.05 vs. control (n = 5). Abbreviations: Ctrl, control plasmids; kwt, kidney weight; CBF, cortical blood flow; MBF, medullary blood flow.
Effects of PHD2 shRNA on urinary sodium excretion after acute sodium loading
Acute sodium loading increased U·V and UNa·V. These increases in U·V and UNa·V were significantly enhanced in PHD2 shRNA–treated rats compared with control rats (Figure 3a,b).
Figure 3.
Effects of prolyl-hydroxylase 2 (PHD2) short hairpin RNA (shRNA) transfection into the renal medulla on urinary sodium excretion in response to acute sodium loading and on salt balances after chronic sodium loading. (a and b) Urinary volume (U·V) and urinary sodium excretion (UNa·V) after acute sodium loading. C, control; P, post sodium loading; S, sodium loading. (c and d) Daily sodium balance and cumulative sodium balance. (e and f) Representative enhanced chemiluminescence gel documents of Western blot analyses depicting the protein levels of hypoxia-inducible factor 1α (HIF-1α) and summarized intensities of the HIF-1α blots (ratio to β-tubulin). Abbreviations: CoCl2, sample from cells treated with CoCl2 as positive control; Ctrl, control vectors expressing luciferase; HS, high salt; LS, low salt; shRNA, PHD2 shRNA vectors. *P < 0.05 vs. controls (n = 5).
Effects of PHD2 shRNA on salt balance after chronic sodium loading
High salt intake produced a positive daily and cumulative salt balance. The daily positive salt balances were progressively increased in the first 2 days and decreased on the third day of high salt intake. The positive salt balances were significantly attenuated in PHD2 shRNA–treated rats compared with control rats (Figure 3c,d). Western blot analysis confirmed the upregulation of HIF-1α in PHD2 shRNA–treated rats (Figure 3e,f).
Effects of PHD2 shRNA on high salt–induced hypertension
The baseline MAPs were not different among control and PHD2 shRNA–treated rats when the rats were on a low-salt diet. After the rats were fed with a high-salt diet for 2 weeks, the MAPs were significantly increased in both control and PHD2 shRNA groups. However, the high salt–induced increase in MAP was substantially attenuated in PHD2 shRNA–treated rats compared with control rats by the end of the experiment (Figure 4).
Figure 4.
Effects of prolyl-hydroxylase 2 (PHD2) short hairpin RNA (shRNA) transfection into the renal medulla on mean arterial pressure (MAP) after 2 weeks of a high-salt diet. *P < 0.05 vs. others (n = 5–9). Abbreviations: Ctrl, control plasmids; HS, high salt; LS, low salt; shRNA, PHD2 shRNA plasmids.
DISCUSSION
Our results demonstrated that silencing of PHD2 gene expression induced HIF-1α–mediated gene activation, which stimulated the expression of antihypertensive genes in the renal medulla and consequently enhanced the urinary sodium excretion in response to the elevations of RPP and sodium overloading, reduced sodium retention, and as a result, attenuated salt-sensitive hypertension in Dahl S rats.
Our results showed that local delivery of PHD2 shRNA plasmids to silence PHD2 gene expression substantially upregulated the levels of HIF-1α and enhanced the transcription of its target genes in the renal medulla, which validated the manipulation of HIF-1α–mediated gene expression by PHD2 in the renal medulla and allowed us to evaluate the contribution of PHD2 in HIF-1α–mediated gene activation as well as in the development of hypertension in response to high salt intake in Dahl S rats.
We first determined the effects of PHD2 gene silencing to upregulate HIF-1α–mediated gene activation on pressure natriuresis. Several HIF-1α target genes, such as HO-1, COX-2 and NOS-2, have been reported to play an important role in the regulation of renal medullary function, including pressure natriuresis.5,25–29 Blunted pressure natriuresis has been shown to be one of the mechanisms for Dahl S rats to develop hypertension.30–33 It has been reported that expression of the protective genes regulated by HIF-1α are much lower in the renal medulla17,34,35 and high salt–induced upregulation in the expression of these protective genes is diminished13,17,18,36 in Dahl S rats compared with normal rats. Furthermore, high salt–induced inhibition of PHD2 expression and subsequent activation of HIF-1α is also diminished in Dahl S rats.16 Therefore, silencing PHD2 gene expression to increase HIF-1α levels and subsequently stimulate the expression of the above HIF-1α target genes in the renal medulla would be expected to improve the pressure natriuresis relationship. Our data showed that silencing of PHD2 gene expression in the renal medulla significantly enhanced the pressure natriuresis, suggesting that impaired PHD2/HIF-1α pathway may be responsible for the renal medullary dysfunction in Dahl S rats. Because the products of the protective genes regulated by HIF-1α have been shown to exhibit vascular and/or tubular actions,5,25,26,37 the effect of PHD2/HIF-1α–mediated pathway on pressure natriuresis may be through regulating both vascular and tubular function.
To further determine whether correction of the defects in the renal medullary PHD2/HIF-1α pathway would improve the salt handling in Dahl S rats, we examined the urinary sodium excretion in response to acute sodium overload and the sodium balance after chronic high-salt challenge. Because the high salt–induced inhibition in PHD2 and the consequent activation of HIF-1α in the renal medulla is blunted in Dahl S rats,16 the deficiencies of those antihypertensive factors, such as COX-2 and HO-1, in the renal medulla are probably caused by the abnormal function of the PHD2/HIF-1α pathway in this animal model. Correction of the abnormal response in PHD2 after high-salt challenge in the renal medulla would induce HIF-1α–mediated gene activation and improve the sodium excretion in Dahl S rats. The results from these sodium overloading experiments showed that restoration of high salt–induced inhibition in PHD2 expression in the renal medulla recovered the upregulation in HIF-1α levels and remarkably improved the renal capability of removing extra sodium load, thereby reducing sodium retention. These results additionally indicate that impaired sodium excretion may be attributed to the defects in the PHD2/HIF-1α pathway in the renal medulla of Dahl S rats.
Because renal medullary function, including pressure natriuresis, is a key determinant to the long-term regulation of blood pressure,5,25,26,38,39 improvement in sodium excretions in responses to elevation of RPP and sodium overloading by PHD2 shRNA would eventually attenuate high salt–induced hypertension in Dahl S rats. Indeed, our results showed that high salt–induced increase in MAP was significantly reduced in PHD2 shRNA–treated rats. It has been shown that activation of HIF-1α–mediated gene regulation after high salt intake is considered an important molecular adaptation in the renal medulla, which induces the production of various protective factors and promotes the excretion of extra sodium loading.6,16 Therefore, abnormal response of PHD2 facing high-salt challenge may cause deficiency in HIF-1α–mediated gene transcription, reduce the production of various protective factors, impair renal medullary function, and damage the capability of the kidneys to remove sodium overloading, which consequently produces a salt-sensitive hypertension in Dahl S rats. This defect in the PHD2/HIF-1α pathway may represent an important mechanism in the pathogenesis of salt-sensitive hypertension. To inhibit PHD2 in the renal medulla may restore the impaired molecular adaptation facing high-salt challenge and induce the production of different protective or antihypertensive factors in the renal medulla, thereby attenuating salt-sensitive hypertension.
This study focused on the improvement of impaired renal capacity to remove extra sodium loading in Dahl S rats. On the other hand, overproduction of those natriuretic factors in the renal medulla may increase sodium excretion more than the desired amount and damage renal capacity to retain sodium when on a low-salt diet. Therefore, the role of renal medullary PHD2/HIF-1α in sodium retaining and how to properly manipulate the PHD2/HIF-1α pathway under different situations need to be determined and detailed in future studies.
In summary, our results demonstrated that silencing PHD2 gene expression to upregulate HIF-1α levels in the renal medulla activated the transcription of antihypertensive genes in the renal medulla and corrected the impairment in PHD2/HIF-1α–mediated molecular adaptation in response to high salt intake. Consequently, this correction enhanced urinary sodium excretion, reduced sodium retention, and attenuated salt-sensitive hypertension in Dahl S rats. It is concluded that defects in the PHD2/HIF-1α pathway in the renal medulla may be responsible for the development of salt-sensitive hypertension in Dahl S rats and inhibition of PHD2 in the renal medulla may be a useful therapeutic strategy for salt-sensitive hypertension.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
Qing Zhu and Junping Hu are co–first authors of this article. This work was supported by the National Institutes of Health (grants HL089563 and HL106042).
REFERENCES
- 1. Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986; 8:II127–II134 [DOI] [PubMed] [Google Scholar]
- 2. Campese V. Salt sensitivity in hypertension. Renal and cardiovascular implications [clinical conference]. Hypertension 1994; 23:531–550 [DOI] [PubMed] [Google Scholar]
- 3. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001; 37:429–432 [DOI] [PubMed] [Google Scholar]
- 4. Cowley AW, Jr, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension 1995; 25:663–673 [DOI] [PubMed] [Google Scholar]
- 5. Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 2003; 284:R13–R27 [DOI] [PubMed] [Google Scholar]
- 6. Li N, Chen L, Yi F, Xia M, Li P-L. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1{alpha} in the renal medulla. Circ Res 2008; 102:1101–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension 1996; 27:688–692 [DOI] [PubMed] [Google Scholar]
- 8. Zewde T, Mattson DL. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension 2004; 44:424–428 [DOI] [PubMed] [Google Scholar]
- 9. Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 1998; 274:F481–F489 [DOI] [PubMed] [Google Scholar]
- 10. Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol 2001; 281:F1–F11 [DOI] [PubMed] [Google Scholar]
- 11. Li N, Yi F, dos Santos EA, Donley DK, Li P-L. Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure. Hypertension 2007; 49:148–154 [DOI] [PubMed] [Google Scholar]
- 12. Nakano D, Pollock D. New concepts in endothelin control of sodium balance. Clin Exp Pharmacol Physiol 2012; 39:104–110 [DOI] [PubMed] [Google Scholar]
- 13. Tan DY, Meng S, Cason GW, Manning RD., Jr Mechanisms of salt-sensitive hypertension: role of inducible nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 2000; 279:R2297–R2303 [DOI] [PubMed] [Google Scholar]
- 14. Stec DE, Drummond HA, Gousette MU, Storm MV, Abraham NG, Csongradi E. Expression of heme oxygenase-1 in thick ascending loop of henle attenuates angiotensin ii-dependent hypertension. J Am Soc Nephrol 2012; 23:834–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakanishi K, Hara N, Nagai Y. Salt-sensitive hypertension in conscious rats induced by chronic nitric oxide blockade. Am J Hypertens 2002; 15:150–156 [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension 2010; 55:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szentivanyi M, Jr., Zou A-P, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW., Jr Renal medullary nitric oxide deficit of dahl s rats enhances hypertensive actions of angiotensin ii. Am J Physiol Regul Integr Comp Physiol 2002; 283:R266–R272 [DOI] [PubMed] [Google Scholar]
- 18. Yoshihara F, Suga S, Yasui N, Horio T, Tokudome T, Nishikimi T, Kawano Y, Kangawa K. Chronic administration of adrenomedullin attenuates the hypertension and increases renal nitric oxide synthase in dahl salt-sensitive rats. Regul Pept 2005; 128:7–13 [DOI] [PubMed] [Google Scholar]
- 19. Zhu Q, Wang Z, Xia M, Li P-L, Zhang F, Li N. Overexpression of HIF-1α transgene in the renal medulla attenuated salt sensitive hypertension in Dahl S rats. Biochim Biophys Acta 2012; 1822:936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li P-L. Expression and actions of hif prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol 2007; 292:F207–216 [DOI] [PubMed] [Google Scholar]
- 21. Schodel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt K-U, Willam C. HIF-prolyl hydroxylases in the rat kidney: physiologic expression patterns and regulation in acute kidney injury. Am J Pathol 2009; 174:1663–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu Q, Liu M, Han WQ, Li PL, Wang Z, Li N. Overexpression of HIF prolyl-hydoxylase-2 transgene in the renal medulla induced a salt sensitive hypertension. J Cell Mol Med 2012; 16:2701–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, Roman RJ. Inhibition of the formation of EETS and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol Regul Integr Comp Physiol 2004; 287:R58–R68 [DOI] [PubMed] [Google Scholar]
- 24. Zhu Q, Wang Z, Xia M, Li PL, Van Tassell BW, Abbate A, Dhaduk R, Li N. Silencing of hypoxia-inducible factor-1{alpha} gene attenuated angiotensin ii-induced renal injury in sprague-dawley rats. Hypertension 2011; 58:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Granger JP, Alexander BT, Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep 2002; 4:152–159 [DOI] [PubMed] [Google Scholar]
- 26. Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol 2005; 32:400–409 [DOI] [PubMed] [Google Scholar]
- 27. Gross JM, Dwyer JE, Knox FG. Natriuretic response to increased pressure is preserved with cox-2 inhibitors. Hypertension 1999; 34:1163–1167 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez F, Kemp R, Balazy M, Nasjletti A. Effects of exogenous heme on renal function: role of heme oxygenase and cyclooxygenase. Hypertension 2003; 42:680–684 [DOI] [PubMed] [Google Scholar]
- 29. Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens 2001; 14:74S–82S [DOI] [PubMed] [Google Scholar]
- 30. Roman RJ. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 1986; 251:F57–F65 [DOI] [PubMed] [Google Scholar]
- 31. Takenaka T, Suzuki H, Sakamaki Y, Itaya Y, Saruta T. Contribution of prostaglandins to pressure natriuresis in dahl salt-sensitive rats. Am J Hypertens 1991; 4:489–493 [DOI] [PubMed] [Google Scholar]
- 32. Patel A, Layne S, Watts D, Kirchner KA. L-arginine administration normalizes pressure natriuresis in hypertensive dahl rats. Hypertension 1993; 22:863–869 [DOI] [PubMed] [Google Scholar]
- 33. Kirchner KA, Crosby BA, Patel AR, Granger JP. Segmental chloride transport in the dahl-s rat kidney during l-arginine administration. J Am Soc Nephrol 1995; 5:1567–1572 [DOI] [PubMed] [Google Scholar]
- 34. Ikeda Y, Saito K, Kim J-I, Yokoyama M. Nitric oxide synthase isoform activities in kidney of dahl salt-sensitive rats. Hypertension 1995; 26:1030–1034 [DOI] [PubMed] [Google Scholar]
- 35. Cowley AW, Jr., Mori T, Mattson D, Zou A-P. Role of renal no production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol 2003; 284:R1355–R1369 [DOI] [PubMed] [Google Scholar]
- 36. Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J Hypertens 2005; 23:165–174 [DOI] [PubMed] [Google Scholar]
- 37. Wang T, Sterling H, Shao WA, Yan Q, Bailey MA, Giebisch G, Wang W-H. Inhibition of heme oxygenase decreases sodium and fluid absorption in the loop of henle. Am J Physiol Renal Physiol 2003; 285:F484–F490 [DOI] [PubMed] [Google Scholar]
- 38. Hall JE. The kidney, hypertension, and obesity. Hypertension 2003; 41:625–633 [DOI] [PubMed] [Google Scholar]
- 39. Bergstrom G, Evans RG. Mechanisms underlying the antihypertensive functions of the renal medulla. Acta Physiologica Scandinavica 2004; 181:475–486 [DOI] [PubMed] [Google Scholar]