Abstract
Objective: To evaluate treating epidermal melasma using a 4% hydroquinone skin care system plus tretinoin 0.05% cream. Design: Multicenter open-label study with all patients receiving above-mentioned treatment for up to 24 weeks. Setting: Private dermatology and plastic surgery clinics and clinical research facilities. Participants: Thirty-seven adult females with moderate or marked epidermal melasma, melasma pigmentation of mild-to-marked intensity and Fitzpatrick skin type III to VI. Measurements: Melasma severity melasma pigmentation intensity melasma improvement, patient satisfaction, quality-of-life measures, erythema, dryness, peeling, burning/stinging. Results: No patient discontinued due to lack of efficacy or treatment-related adverse events. Treatment was associated with a significant reduction from baseline in melasma severity and melasma pigmentation intensity from Week 4 onward (P≤0.001), and 100 percent of patients showed improvement from Week 8 onward. At Week 24, 100 percent of patients were “satisfied” or “very satisfied” with the overall effectiveness of their treatment. Patients’ quality of life also improved (e.g., the proportion of patients feeling embarrassed or self-conscious about their skin “a lot” or “very much” declined from 78 percent at baseline to four percent at Week 24). Mean and median scores for erythema, dryness, peeling, and burning/stinging did not exceed trace levels. Conclusion: Treating moderate-to-severe melasma using the 4% hydroquinone skin care system plus 0.05% tretinoin can significantly reduce the severity of melasma and the intensity of melasma pigmentation within four weeks. Treatment was generally well tolerated and associated with an improved quality of life and high levels of patient satisfaction.
Despite the potential of melasma for causing considerable negative effects on emotional wellbeing and quality of life,1 it has been reported that the condition may be undertreated.2 Hydroquinone is well known as being effective in reducing hyperpigmentation in a variety of conditions and it is commonly used in conjunction with tretinoin in the treatment of melasma. Triple combination therapy, which contains a corticosteroid as well as hydroquinone and tretinoin, may also be used. However, the presence of the steroid can limit the clinical usefulness of such therapy as the only triple therapy combination product approved by the United States Food and Drug Administration (FDA) is indicated for short-term use only (up to 8 weeks).3 As melasma is prone to relapse, it may be easier and safer to manage the condition using a steroid-free treatment that is suitable for longer term treatment.
When any dermatological treatment is recommended, it is prudent to consider how easy it will be for patients to incorporate the new regimen into their existing skin care routine because if patients become confused juggling different regimens, this may result in poor compliance and suboptimal dinical outcomes. This is especially important in conditions such as melasma where the majority of patients are female4 and where long-term treatment may be needed. By offering an easy-to-follow regimen that provides both overall skin care and treatment for a specific skin condition, skin care systems have proven very popular with patients.
A skin care system is available that incorporates a 4% formulation of hydroquinone into a comprehensive skin care regimen providing cleansing, toning, exfoliation, and photoprotection. Photoprotection is always essential to achieve and maintain efficacy in melasma and the proprietary cleanser, toner, and exfoliant are each designed to facilitate the penetration of other ingredients in the system into the skin. In this way, the system aims to achieve superior clinical outcomes than might be achieved with the uncoordinated use of standard products. The authors conducted a multicenter study to evaluate treating epidermal melasma with this 4% hydroquinone skin care system in conjunction with tretinoin 0.05% cream for up to 24 weeks.
METHODS
Study design. In this multicenter open-label study, all patients received the same treatment. The protocol (MEL004) was approved by the relevant institutional review boards, and the study was conducted according to the principles of the 2004 version of the Declaration of Helsinki. All patients signed informed consent.
Main inclusion criteria. Patients were eligible for enrollment into the study if they were healthy females with epidermal melasma of moderate or marked severity and melasma pigmentation of mild-to-marked intensity. The cutaneous melanosis was required to have remained stable for the last three months with the diagnosis of epidermal melasma being confirmed by Wood’s lamp examination. Patients were required to be 25 to 65 years of age and to have a Fitzpatrick skin type of III to VI. They were also required to be willing to refrain from the following during the study: facial use of non-study topical products including medicated make-up (oil-free non-comedogenic make-up was allowed); use of tanning booths; use of facials and facial procedures (including those involving chemical peels, microdermabrasion, laser resurfacing, botulinum toxin type A, dermal fillers, and hair removal with the exception of tweezer plucking of eyebrows); and nonablative laser, light, or radiofrequency treatment. Patients taking oral contraceptives or hormone replacement therapy could be enrolled if they agreed not to alter their regimen during the study.
Main exclusion criteria. Exclusion criteria included the following: history or presence of any facial skin condition that might interfere with diagnosis or evaluation in the study; irritation of exposed skin (e.g., from ultraviolet [UV] light); facial sunburn at the baseline visit; known allergy or hypersensitivity to sulfites or study product ingredients including parabens or aloe; presence of dermal melasma, combination dermal and epidermal melasma, postinflammatory hyperpigmentation, or vitiligo; history of Nevus of Ota; history of increased pigmentation and/or contact dermatitis with previous use of hydroquinone or tretinoin; depressed or atrophic macular lesions; anticipated need for other drugs that might enhance pigmentation or facial use of other medicated products; participation in activities involving prolonged exposure to the sun without protective clothing; any uncontrolled systemic disease; and being pregnant, breastfeeding, or planning a pregnancy during the study.
Washout periods included one week for the use of medicated facial cleansers and facial hair removal procedures. There was also a 30-day washout period for sunbathing/UV light therapy, use of topical prescription treatments or photosensitizing medications or procedures, facial use of bleaching products and other topical medications (including corticosteroids, hydroquinone, alpha-hydroxy acids, beta-hydroxy acids, kojic acid, retinoic acid/tretinoin, retinol, salicylic acid, and vitamin С and vitamin D products or derivatives), use of any drag with a known potential for major organ toxicity, and participation in another investigational study. Other washout periods were six weeks for the use of facial microdermabrasion, 12 weeks for the use of systemic steroids, and six months for the use of systemic retinoids, methotrexate, laser resurfacing procedures, deep skin peels, dermal fillers, botulinum toxin type A, and photoallergy, phototoxic, and photosensitizing drags.
Treatment regimen. Patients were treated with the 4% hydroquinone skin care system (Obagi Nu-Derm®, OMP, Inc., Long Beach, California) and 0.05% tretinoin cream for 12 weeks and, optionally, could continue in an extension to receive an additional 12 weeks of treatment. The hydroquinone skin care system involved applying the Mbwing proprietary products: foaming gel cleanser (twice daily), toner (twice daily), 4% hydroquinone (twice daily), exfoliant containing alpha hydroxy acids (each morning), and sunscreen containing micronized zinc oxide and octinoxate (each morning). Tretinoin 0.05% cream was applied each evening mixed 1:1 with 4% hydroquinone. Patients were also allowed to use a provided moisturizer (Action®, OMP, Inc.) and/or 0.5% hydrocortisone (Tolereen, OMP, Inc.) as needed for dryness and other tolerability issues, respectively.
Outcome measures. The investigators evaluated melasma severity, melasma pigmentation intensity, melasma improvement, erythema, dryness, peeling, and burning/stinging (Table 1). Patients evaluated the effectiveness of their treatment compared with other treatments; the level of improvement they observed in skin texture/roughness, skin firmness, brown spots/discoloration, and fine lines and wrinkles; their level of satisfaction with the overall effectiveness of study treatment; and the ease of application of the study treatment (Table 2). They also evaluated several parameters giving an indication of their quality of life—how embarrassed or self-conscious they had been because of their skin, how much their skin discoloration had made them feel unattractive to others, how much effort they had put into hiding their skin discoloration from others, how much others had focused on their skin discoloration rather than on what they were saying or doing, and how much their skin had affected any of their social and leisure activities (Table 3).5 The above-mentioned parameters were evaluated at baseline and Weeks 4, 8, 12, 18, and 24, except melasma improvement (which was not evaluated at baseline) and erythema, dryness, peeling, and burning/stinging (which were evaluated up to Week 12 only).
TABLE 1.
Scales used for investigator evaluations
| MELASMA SEVERITY | MELASMA PIGMENTATION INTENSITY | MELASMA IMPROVEMENT | ERYTHEMA | DRYNESS | PEELING | BURNING/STINGING |
|---|---|---|---|---|---|---|
| None (0) No noticeable lesion area | None (0) No noticeable lesion area | Worse | None—no erythema present | None | None | Normal, no discomfort |
| Minimal/trace (1) Melasma covering 1-10% of face | Minimal (1) Localized deposits of pigment | Unchanged—no detectable improvement from baseline evaluation | Trace erythema | Slight flaking | Trace, localized peeling | Trace, awareness without discomfort |
| Mild (2 or 3) Melasma covering 11-25% of face | Mild (2 or 3) Mild, diffuse deposits of pigment | Slight improvement (1-10%) | Mild erythema | Mild flaking | Mild diffuse peeling | Mild, noticeable discomfort causing intermittent awareness |
| Moderate (4 or 5) Melasma covering 26-40% of face | Moderate (4 or 5) Moderate, diffuse deposits of pigment | Mild improvement (11-25%) | Moderate confluent erythema | Moderate flaking/scaling | Moderate—definitely noticeable peeling | Moderate, noticeable discomfort causing continuous awareness |
| Marked (6 or 7) Melasma covering 41-50% of face | Marked (6 or 7) Marked, dense deposits of pigment | Moderate improvement (26-50%) | Marked erythema, slight edema | Marked scaling, slight fissuring | Marked—dense extensive peeling | Marked, definite discomfort that occasionally interferes with normal daily activities |
| Severe (8) Melasma covering >50% of face | Severe (8) Severe, dense deposits of pigment | Marked improvement (51-75%) | Severe erythema, edema, flare, possible erosion | Severe scaling, fissuring | Severe—extensive peeling | Severe, marked, continuous discomfort that interferes with normal daily activities |
| – | – | Almost complete clearing (76-99% improvement) | – | – | – | – |
| – | – | Complete clearing, no signs of hyperpigmentation (100% improvement) | – | – | – | – |
TABLE 2.
Scales used for patient evaluations of efficacy, satisfaction, and ease of application
| COMPARED WITH OTHER TREATMENTS, HOW EFFECTIVE IS YOUR CURRENT STUDY TREATMENT? | IMPROVEMENT IN SKIN TEXTURE/ROUGHNESS, SKIN FIRMNESS, BROWN SPOTS/ DISCOLORATION, AND FINE LINES AND WRINKLES | HOW SATISFIED ARE YOU WITH THE OVERALL EFFECTIVENESS OF YOUR STUDY TREATMENT? | HOW EASY WAS APPLYING YOUR STUDY TREATMENT? |
|---|---|---|---|
| Much more effective | Poor/no change | Very satisfied | Very easy |
| More effective | Fair (>25%) | Satisfied | Easy |
| Same | Good (>50%) | Indifferent | Average |
| Less effective | Very good (>75%) | Dissatisfied | Difficult |
| Much less effective | Excellent (>90%) | Very dissatisfied | Very difficult |
TABLE 3.
Scales used for evaluating parameters giving an indication of quality of life5
| HOW EMBARRASSED OR SELF-CONSCIOUS HAVE YOU BEEN BECAUSE OF YOUR SKIN? | HOW MUCH HAS YOUR SKIN DISCOLORATION MADE YOU FEEL UNATTRACTIVE TO OTHERS? | HOW MUCH EFFORT HAVE YOU PUT INTO HIDING YOUR SKIN DISCOLORATION FROM OTHERS? | HOW MUCH HAVE PEOPLE FOCUSED ON YOUR SKIN DISCOLORATION RATHER THAN ON WHAT YOU ARE SAYING OR DOING? | HOW MUCH HAS YOUR SKIN AFFECTED ANY OF YOUR SOCIAL OR LEISURE ACTIVITIES? |
|---|---|---|---|---|
| Very much | Very much | Very much | Very much | Very much |
| A lot | A lot | A lot | A lot | A lot |
| A little | A little | A little | A little | A little |
| Not at all | Not at all | Not at all | Not at all | Not at all |
Statistical analyses. Changes from baseline in melasma severity, melasma pigmentation intensity, erythema, dryness, peeling, and burning/stinging were evaluated using a paired t-test or Wilcoxon signed-rank test. An α of ≤0.05 was considered statistically significant.
RESULTS
The study was conducted between May 2010 and February 2011.
Patients. Among 37 patients enrolled in the initial study, 34 (92%) completed the study. Of these, 27 also enrolled in the extension study and 25 (93%) completed the extension study. No patient discontinued due to lack of efficacy or treatment-related adverse events. Overall, five patients discontinued—as a result of an unrelated adverse event (1), noncompliance (1), voluntary withdrawal (1), being lost to follow-up (1), and other reason (1).
Patients were a mean of 46 years old. Overall, 60 percent were Caucasian, 35 percent were African American, and five percent were Asian. They had a Fitzpatrick skin type of III (27%), IV (51%), V (11%), or VI (11%), and their melasma was centrofacial in 65 percent, malar in 27 percent, and mandibular in eight percent.
At baseline, melasma severity was moderate in 76 percent of patients and marked in 24 percent. Melasma pigmentation intensity was mild in 27 percent of patients, moderate in 54 percent, and marked in 19 percent.
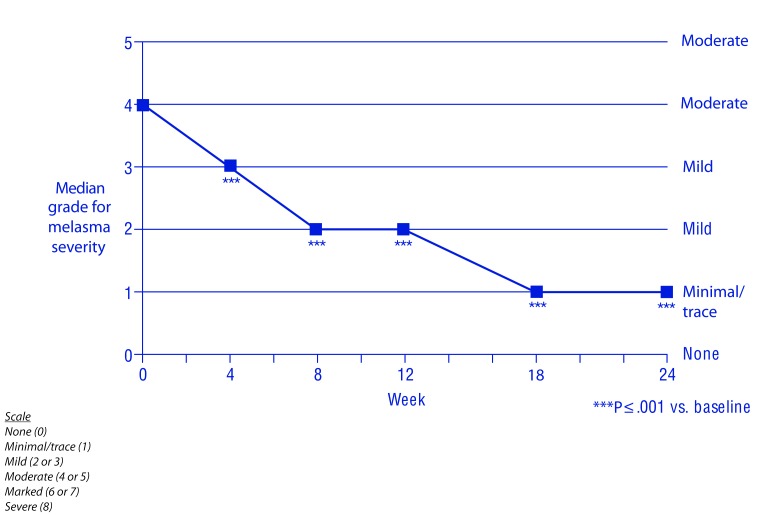
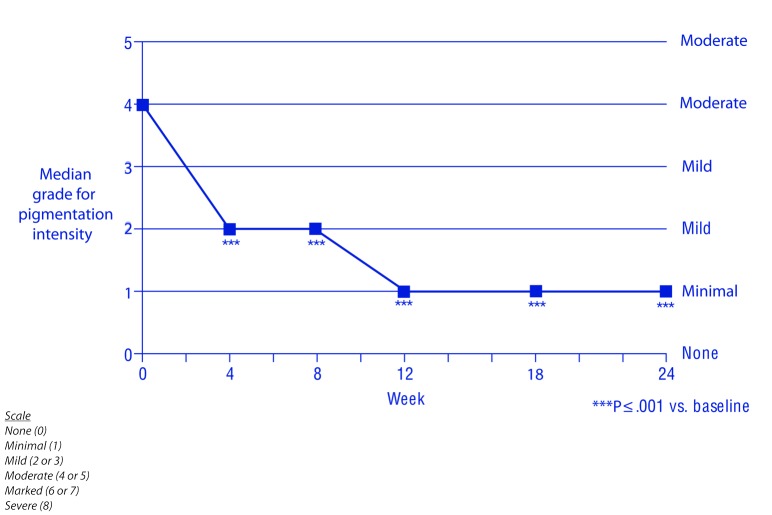
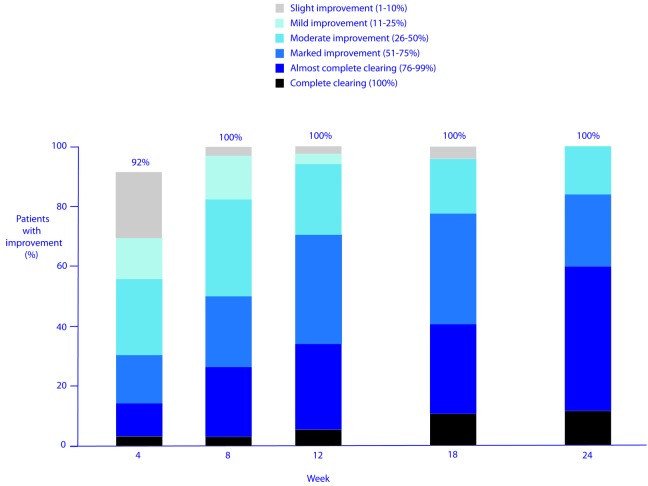
Efficacy. The hydroquinone system plus tretinoin regimen was associated with a significant reduction from baseline in melasma severity and melasma pigmentation intensity from Week 4 onward (P≤0.001) (Figures 1–3). The investigators reported improvement in melasma in 100 percent of patients from Week 8 onward, and a marked (≥ 51%) improvement in 31, 71, and 84 percent of patients at Weeks 4, 12, and 24, respectively (Figure 4). The proportion of patients whose melasma was at least moderate in severity dedined from 100 percent at baseline to 39, 17, and 12 percent at Weeks 4, 12, and 24, respectively. And, the proportion of patients whose melasma pigmentation intensity was at least moderate in severity dedined from 73 percent at baseline to 28, 9, and 8 percent at Weeks 4, 12, and 24, respectively.
Figure 2.
Melasma severity
Figure 1.
Improvements in melasma
Figure 3.
Melasma pigmentation intensity
Figure 4.
Degree of improvement in melasma
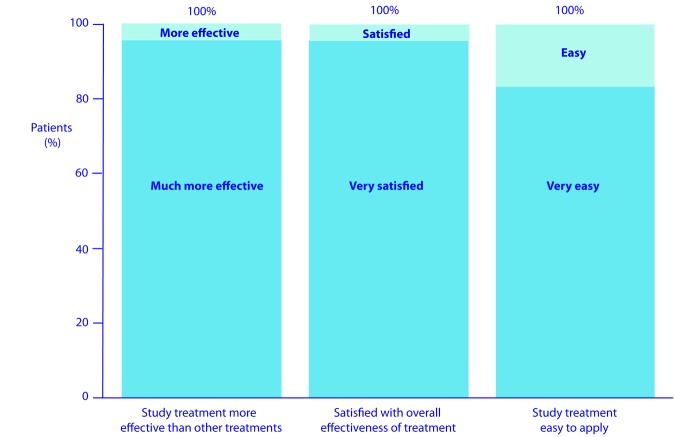
At Week 24, 100 percent of patients considered their study treatment was “more effective” or “much more effective” than other treatments, 100 percent were “satisfied” or “very satisfied” with the overall effectiveness of their treatment, and 100 percent considered the study treatment was “easy” or “very easy” to apply (Figure 5).
Figure 5.
Patient perceptions at Week 24
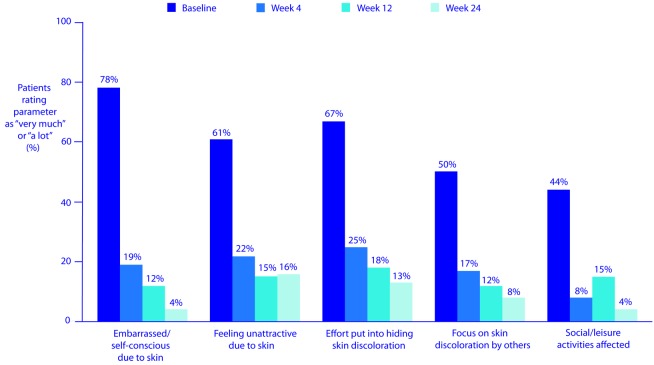
In addition, patients’ quality of life improved. For example, between baseline and Week 24, the proportion of patients feeling embarrassed or self-conscious about their skin “a lot” or “very much” dedined from 78 to four percent, the proportion who believed their skin discoloration made them feel unattractive “a lot” or “very much” dedined from 61 to 16 percent, and the proportion who put “a lot” or “very much” effort into hiding their skin discoloration dedined from 67 to 13 percent (Figure 6). Furthermore, the proportion who felt others focused “a lot” or “very much” on their skin discoloration rather than on what they were saying or doing dedined from 50 to eight percent, and the proportion whose skin affected their social/leisure activities “a lot” or “very much” dedined from 44 to four percent.
Figure 6.
Improvement in quality-of-life parameters
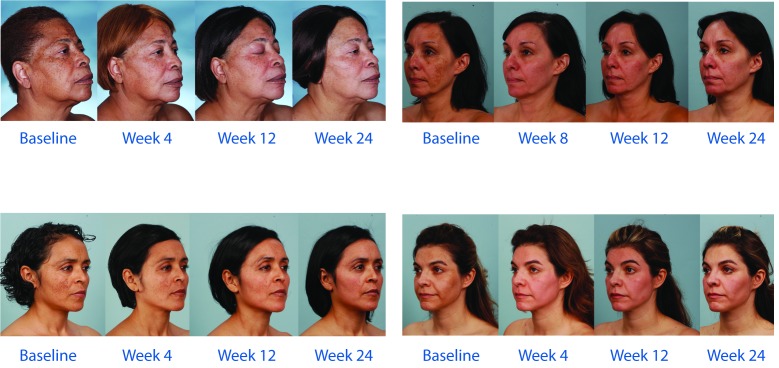
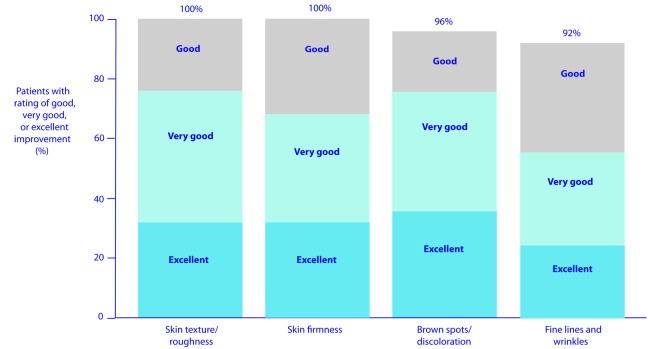
The majority of patients also reported improvements in other skin characteristics. A “good”, “very good”, or “excellent” improvement (reduction) was reported at Weeks 12 and 24, respectively, in 91 and 100 percent of patients for skin texture/ roughness, 78 and 100 percent of patients for skin firmness, 100 and 96 percent of patients for brown spots/discoloration, and 81 and 92 percent of patients for fine lines and wrinkles (Figure 7).
Figure 7.
Patient-reported improvements at Week 24
Tolerability. Erythema, dryness, peeling, and burning/stinging were assessed up to Week 12. Levels of each of these parameters were statistically significantly higher than baseline at all visits. However, mean and median scores did not exceed “trace” levels suggesting that these changes were not clinically significant. Four patients (11%) had adverse events probably or definitely related to treatment (moderate erythema, moderate vesicles/erythema, mild dryness/ tightness/soreness, and mild acne). Overall, 26/37 (70%) patients used the moisturizer provided, including 17 who used it as a preventive measure. In addition, 16/37 (43%) used the hydrocortisone, including eight who used it preventatively.
DISCUSSION
The results of this study demonstrate that treatment of epidermal melasma with the 4% hydroquinone skin care system plus 0.05% tretinoin can achieve significant reductions in melasma severity and melasma pigmentation intensity from Week 4 onward. All patients showed some improvement in melasma from Week 8 onward. Furthermore, at Week 24, all patients were satisfied or very satisfied with the overall effectiveness of their treatment, and all patients considered their treatment to be more effective or much more effective than other treatments, and easy or very easy to apply. Patient ratings showed that the study treatment was also associated with improvements in all quality-of-life parameters evaluated as well as in skin characteristics, such as texture/roughness, firmness, brown spots/discoloration, and fine lines and wrinkles. Treatment was generally well tolerated with mean and median scores for erythema, dryness, peeling, and burning/stinging not exceeding trace levels.
This study was performed in females with Fitzpatrick skin type III to VI. This population was selected because melasma occurs more commonly in females than in males4 and has a propensity for darker skin.6 Some research suggests that there may be differences in the histopathology of melasma between males and females,7 but as this study enrolled females only, there was no potential for any influences due to gender. However, for the same reason, only further research can confirm whether or not the results obtained in females are representative of those in males as well.
The results from this study are consistent with those from others that have evaluated treating melasma using the 4% hydroquinone skin care system in conjunction with other concentrations of tretinoin (0.025%8 or 0.1%9). However, various factors, including differences in inclusion criteria and patient characteristics, preclude meaningful direct comparisons of the results between these studies.
Overall, 22 percent (8/37) of patients used hydrocortisone in response to a tolerability issue. The study regimen is therefore preferable to combination products containing a steroid as it avoids steroid exposure completely in the majority of patients and facilitates intermittent use as needed without affecting the other components of treatment. It also means that the duration of therapy is not automatically restricted because of repeated, and possibly unnecessary, steroid exposure.
Although this study was open label, the results are consistent with those from similar studies that involved masking—a randomized investigator-masked study that evaluated the reduction in hyperpigmentation with a 4% hydroquinone system plus 0.05% tretinoin regimen versus a standard skin care regimen in patients undergoing facial rejuvenation with botulinum toxin type A10 and a randomized observer-masked study that evaluated the reduction in hyperpigmentation with a 4% hydroquinone system plus 0. 05. tretinoin regimen versus placebo products in patients receiving intense pulsed light therapy.11
CONCLUSION
Using the 4% skin care system plus 0.05% tretinoin to treat moderate or marked melasma can significantly reduce the severity of melasma and the intensity of melasma pigmentation within four weeks. An improved quality of life and high levels of patient satisfaction were reported, and the treatment was generally well tolerated. Although this study was open label, and therefore potentially at risk of investigator and patient bias, the results are consistent with those from masked studies.
ACKNOWLEDGMENT
The authors thank Gill Shears, PhD, who provided medical writing services on behalf of Gill Shears, Inc.
Footnotes
DISCLOSURE:This study was funded by OMP, Inc. Dr. Gold has been a consultant, investigator, and speaker for OMP, Inc. Dr. Rendon has been an investigator for OMP, Inc. Dr. DiBernardo has been a consultant and investigator for OMP, Inc. Dr. Bruce has been a consultant and investigator for OMP, Inc. Dr. Lucas-Anthony has been an investigator for OMP, Inc. At the time of the study, Dr. Watson was an employee of, and held stock and stock options in, OMP, Inc.
REFERENCES
- 1.Pawaskar MD, Parikh P, Markowski T, et al. Melasma and its impact on health-related quality of life in Hispanic women. J Dermatobg Treat. 2007;18:5–9. doi: 10.1080/09546630601028778. [DOI] [PubMed] [Google Scholar]
- 2.Rendon MI. Utilizing combination therapy to optimize melasma outcomes. J Drugs Dermatol. 2004;3(5 Suppl):S27–S34. [PubMed] [Google Scholar]
- 3. [June 11, 2013]. http://ww.triluma.com/TRI-LUMA-PI-20071-0210.pdf Tri-Luma® Cream [package insert]. Fort Worth, TX: Galderma Laboratories, LP; revised February 2010.
- 4.Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380–382. doi: 10.4103/0019-5154.84722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkrishnan R, Kelly AP, McMichael A, Torok H. Improved quality of life with effective treatment of facial melasma: the pigment trial. J Drugs Dermatol. 2004;3:377–381. [PubMed] [Google Scholar]
- 6.Sehgal VN, Verma P, Srivastava G, et al. Melasma: treatment strategy. J Gosmet Laser Ther. 2011;13:265–279. doi: 10.3109/14764172.2011.630088. [DOI] [PubMed] [Google Scholar]
- 7.Jang YH, Sim JH, Kang HY, et al. The histopathological characteristics of male melasma: comparison with female melasma and lentigo. J Am Acad Dermatol. 2012;66:642–649. doi: 10.1016/j.jaad.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Grimes P, Watson J. [June 11, 2013]. http://www.aad.org/Posters/Documents/AM2012/Poster/4581/4581.pdf Treatment of mild or moderate melasma in darker skin with a 4% hydroquinone skin care system plus 0.025% tretinoin cream. Poster presented at the 70th Annual Meeting of the American Academy of Dermatology, March 16-20, 2012, San Diego, CA.
- 9.Bruce S, Watson J. [June 11, 2013]. http://www.aad.org/Posters/Documents/AM2012/Poster/5131/5131.pdf Treatment of moderate or marked melasma with a 4% hydroquinone skin care system plus 0.1% tretinoin cream: comparison in Asian and Caucasian patients. Poster presented at the 70th Annual Meeting of the American Academy of Dermatology, March 16-20, 2012, San Diego, CA.
- 10.Schlessinger J, Kenkel J, Werschler P. Further enhancement of facial appearance with a hydroquinone skin care system plus tretinoin in patients previously treated with botulinum toxin Type A. Aesthet Surg J. 2011;31:529–539. doi: 10.1177/1090820X11411579. [DOI] [PubMed] [Google Scholar]
- 11.Woodhall KE, Goldman MP, Gold MH, Biron J. Benefits of using a hydroquinone/tretinoin skin care system in patients undergoing intense pulsed light therapy for photo-rejuvenation: a placebo-controlled study. J Drugs Dermatol. 2009;8:862–867. [PubMed] [Google Scholar]