Abstract
This three-part review presents what is currently known about the involvement and interdependency of the epidermal barrier and immune response in the etiopathogenesis of atopic dermatitis. Part 1 of this review depicted the role of filaggrin in atopic dermatitis while this article, Part 2, evaluates the role of serine proteases and specific lipids in the structural and functional integrity of the stratum corneum and its multiple barrier functions in atopic dermatitis. Upregulation of serine protease activity causes adverse structural changes of the stratum corneum due to degradation of certain stratum corneum proteins that are integral to epidermal structure and functions, interference with the formation of the stratum corneum intercellular lipid membrane, which normally regulates epidermal water flux and gradient, and induction of a TH2 pattern of inflammation, which is the hallmark profile of atopic skin. Alteration in lipid ratios and changes in lipid-directed enzymes may play a role in the impairment of barrier functions that are associated with atopic dermatitis. In Part 3, immune dysregulation, including upregulation of a TH2 inflammation pattern, augmented allergic sensitization, sustained wound healing inflammation, and impaired innate immunity are discussed. The roles of the stratum corneum permeability barrier, the immune defense barrier, and antimicrobial barrier in AD pathogenesis are explained in detail. With this explanation, the interdependence of the multitude of polymorphisms and dysregulations seen in AD skin will become clear. The condensing of these impaired and/or dysregulated functions and how they interact should provide further knowledge about the pathogenic mechanisms that cause atopic dermatitis, how they are clinically relevant, and how they may assist in developing more specific therapies directed at the pathogenesis of atopic dermatitis.
Introduction
Patients with atopic dermatitis (AD) exhibit impairment of certain stratum corneum (SC) barrier functions and dysregulated immune response. This review depicts our understanding of the complex interdependent role of both the physical integrity of the SC, its barrier functions, and the immune defense in the pathogenesis in AD. Further understanding of these complex polymorphisms and dysfunctions of the structure and function of the SC barrier and the immune system in AD will hopefully allow for a more targeted approach for prevention and treatment.
Stratum Corneum Barrier Function and Atopic Dermatitis
In Part 1 of this three-part review, we discussed the role of filaggrin and its breakdown products in the health and function of the SC permeabnity barrier and its role in the pathogenesis of AD. It was concluded that while filaggrin may play a significant role in the pathogenesis of AD, the structural and functional defects of the filaggrin alone are insufficient to induce or account for all of the abnormalities noted in AD.1
Dysregulation of several other known abnormalities of the SC barrier also appears to play a major integral role in disruption of the epidermal barrier resulting in mechanisms that are operative in the pathophysiology of AD. These other abnormalities of the SC barrier include increased serine protease activity1 and decreased ceramide fractions and total SC lipid levels.2,3
Serine Proteases and the SC Barrier in Atopic Dermatitis
Serine proteases (SPs) or serine endopeptidases are enzymes that structurally contain the amino acid serine in the active site of the enzyme, and functionally cut peptide bonds in proteins. The function of SPs is often to convert an inactive peptide with a longer chain into an active peptide form that can then induce specific physiological activities. SPs are an important part of normal skin function, and alterations in SP enzyme activities can lead to abnormalities in the SC.4,5
In AD, SP activity is increased.1 The increase in SP activity in AD may be attributable to changes in skin pH or genetic polymorphisms in the SP enzyme or one of its inhibitors. The pH of skin has a significant impact on SP activity because SP enzymes function optimally in the neutral to alkaline range.4,5 Therefore, as the pH of AD skin increases, SP activity increases as well.
There are two specific genetic polymorphisms that result in increased SP activity in AD patients: gain of function mutations in the SP gene KLK7 and loss of function mutations of the SP inhibitor gene SPINK 5. KLK7 is a gene that encodes the SP enzyme, kallikrein-related peptidase. Gain of function polymorphisms of the KLK7 gene render an SP enzyme, which is resistant to inhibition, hence increasing the activity of SPs in AD. Loss of function polymorphisms of the SPINK5 gene render its protein product LEKT1 (lymphoepithelial Kazal-type trypsin inhibitor) ineffective at inhibiting SP activity in AD. Therefore, mutations in the SP inhibitor LEKTI results in unopposed and increased SP enzymatic activity in AD.1,6
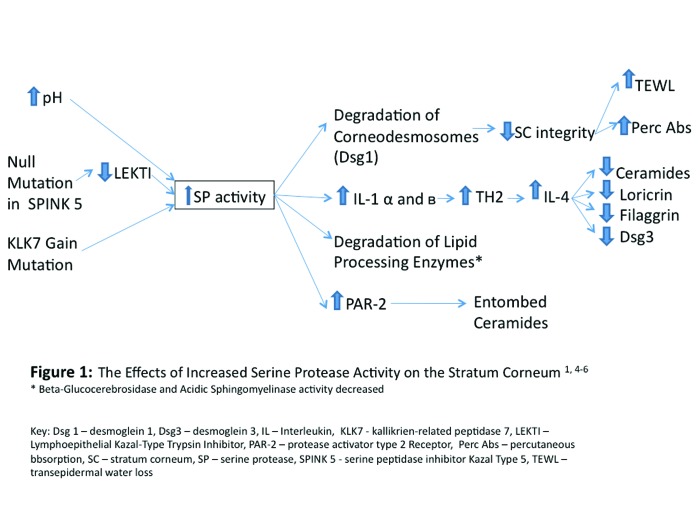
No matter the cause, increased SP activity induces adverse effects on some SC barrier functions because it leads to destruction of some proteins that are crucial to SC integrity, interferes with the construction of the intercellular lipid membrane, and increases TH2 inflammation. Increased SP activity decreases SC integrity by accelerating the degradation of corneodesmosomes via the degradation of desmoglein-1. The decreased SC integrity is evidenced by a measureable increase in TEWL and percutaneous absorption.5 Increased SP activities interfere with the construction of the intercellular lipid membrane by decreasing the total SC lipid content. SC lipid content is decreased secondary to the increased signaling of the protease activator type 2 receptor (PAR-2) and the degradation of two essential lipid processing enzymes beta-glucocerebrosidase and acidic sphingomyelinase. These lipid-processing enzymes downregulate the lamellar body secretion and the processing of the building blocks of the intercellular lipid membrane, respectively.1,4,5,7,8 Finally, increased SP activity increases TH2 inflammation by generating the cytokines IL-1 alpha and beta from their preforms, which stimulate the secretion of IL-4. The increase in TH2 inflammation and the secretion of IL-4 affects the SC barrier by decreasing ceramide synthesis, loricrin synthesis (a cornified envelope protein), filaggrin synthesis, and desmoglein-3 expression.1 Figure 1 summarizes the relationship between SP activity and the SC barrier compromise in AD.
Figure 1.
The effects of increased serine protease activity on the stratum corneum1,4-6.
*Beta-glucocerebrosidase and acidic sphingomyelinase activity decreased Dsg 1=desmoglein 1; Dsg3=desmoglein 3; IL=lnterleukin; KLK7=kallikrien-related peptidase 7; LEKTI=lymphoepithelial Kazal-type trypsin inhibitor; PAR-2=protease activator type 2 receptor; perc abs=percutaneous absorption; SC=stratum corneum; SP=serine protease; SPINK 5=serine peptidase inhibitor Kazal-type 5; TEWL=transepidermal water loss
Lipids and Stratum Comeum Function in Atopic Dermatitis
Lipids are an important component of the SC barrier. Many types of lipids must be produced, processed, and assembled to form the intercellular lipid membrane (the “mortar”) of the SC. The functions of the intercellular lipid membrane are to maintain SC cohesion/integrity, modulate epidermal water flux (inhibit the outward flux while allowing the inward flux of vital substances), and act as a barrier to infection.9
Glycosylceramides, sphingomyelin, and phospholipids are precursor lipids that are stored in lamellar bodies in the granular layer of the SC. Glycosylceramides, sphingomyelin, and phospholipids are converted by specific hydrolytic enzymes to ceramides 1-7, ceramides 2 and 5, and free fatty acids, respectively.9 Collectively, these hydrophobic lipids make up the inter cellular lipid membrane along with cholesterol, cholesterol sulfate, cholesterol esters, and nonpolar lipids.2,9,10 Ceramides are the main component of the lipid bilayer of the SC and they comprise approximately 50 percent of SC lipid content by weight. Cholesterol and its derivatives and free fatty acids account for 25 percent and 10 to 20 percent of SC lipid content by weight, respectively.11
In AD, there is reduced total lipid and ceramide content, alterations in subfractions of ceramides, an overall change in lipid composition in the intercellular lipid membrane, and possible altered activity of lipid-modulating enzymes involved in the production and processing of physiological SC lipids that are integral to SC structure and function, especially the permeability barrier.2,6,10,12,13 The major deficient lipid subclass in AD compared to healthy controls is ceramides.2,10,13-15 A decrease in SC ceramides has been shown in uninvolved plantar skin of patients with AD versus controls and in lesional and nonlesional forearm skin in AD patients.2,14,15 Among the ceramide subfractions, the greatest decrease in AD subjects has been noted with ceramide 1 (Cer-1) in both lesional and nonlesional skin; however, Cer-2 through Cer-6 were also markedly reduced in both lesional and nonlesional skin.2,10,14,15 In subjects with AD, a reduction in Cer-1 and in total ceramide content was also found in the SC of clinically xerotic skin that was otherwise devoid of dinical signs of eczematous dermatitis.2,16 Correlation of SC ceramide content with SC permeability barrier function in subjects with AD demonstrated a marked decrease in the amounts of Cer-1 and Cer-3, with an increase in TEWL correlating significantly with the reduction in Cer-3.7,10,17 Studies completed with different inhibitors of ceramide synthesis demonstrate the necessity of ceramides for normal (physiological) SC barrier function. Ultimately, the changes in SC lipid fractions observed in AD, as described above, lead to alteration of the relative ratio of SC lipids. The resultant effect in patients with AD is a fundamental disturbance in the balance of SC lipid composition in both lesional and nonlesional skin and disturbance in the SC barrier.18 The clinical correlation of these observations is that even when the skin of a patient with atopic dermatitis looks normal clinically there are innate SC abnormalities that are always present that impair SC barrier functions, especially the permeability barrier, predisposing to greater baseline TEWL and a lower threshold for initiation of cutaneous inflammation.2,3,18
To explain the SC ceramide abnormalities noted in AD, it has been hypothesized that abnormalities in the lipid-processing enzymes, alterations in skin pH resulting in increased SP activity, and/or a stress-induced increase in glucocorticoids may all play a pathophysiological role.
In normal skin, SC ceramide production and degradation involves four major lipid enzymes that operate in balance to produce an end result of proper ceramide types and quantities for normal SC integrity and function. These four enzymes are beta-glucocerebrosidase, sphingomyelinase, ceramidase, and serine palmitoyl transferase.2,3 Studies designed to quantify individual enzyme activity levels have been conflicting. Hachem et al4 has demonstrated decreased activity of beta-glucocerebrosidase and acidic sphingomyelinase in alkaline pH environments as well as the potential degradation of beta-glucocerebrosidase and acidic sphingomyelinase activity result of an alkaline pH-induced sustained serine protease (SP) activity in hairless mice models.4 Given the fact that AD patients have alkaline skin, increased SP activity, and decreased ceramide content, a degradation of lipid enzymes has been assumed. In addition, prosaposin, a sphingolipid activator protein, which promotes the enzymatic hydrolysis of sphingolipids such as glucosylceramides and sphingomyelin into ceramides and other “building blocks” of the intercellular lipid membrane, has been shown to be decreased in the epidermis of AD subjects.2,19 Cui et al20 evaluated expression of prosaposin in normal versus AD skin and found that the amount of prosaposin was 66 percent lower in AD skin than in normal control skin.20 It is thought that deficiencies in prosaposin may be associated specifically with diminished activation of beta-glucoscerebrosidase and acid sphingomyelinase and decreased ceramide production in AD.2,20 Yet, despite the above evidence supporting abnormal lipid processing enzyme activity in AD, Jin et al21 report that the activity levels of beta-glucocerebrosidase and sphingomyelinase were essentially normal in the skin of AD patients. This suggests that these enzymatic activities may not play a significant role in the lipid alterations seen in AD.21 Further experimentation is required to solidify the roles of beta-glucocerebrosidase and acidic sphingomyelinase in AD.
Higuchi et al22 discovered an enzyme involved in sphingomyelin hydrolysis, sphingomyelin deacylase (SMD), which may contribute to the decreased SC ceramide content seen in AD.22 Sphingomyelin in healthy skin can be metabolized in different ways. Two known pathways of sphingomyelin metabolism include acid and alkaline sphingomyelinase metabolism of sphingomyelin into different ceramide species or SMD hydrolysis of sphingomyelin at the acyl site to yield sphingosylphosphorylcholine and free fatty acids. Increased SMD activity has the potential to circumvent ceramide production, which results in decreased skin ceramide levels in the SC. Higuchi et al22 confirmed this suspicion by demonstrating an abnormally increased expression of a SMD enzyme, in both lesional and nonlesional AD skin.2,23 An eight-fold increase of SMD activity in lesional skin and a five-fold increase in SMD activity in nonlesional skin, have been documented.2,24 The discovery of abnormal expression and increased activity of SMD in AD suggests that a decrease in SC ceramide content and barrier impairment may be partially related to altered and accelerated sphingomyelin metabolism.7,22-24
In addition to the potential for SPs to affect lipid processing enzymes as described, the increased activity of SPs may also affect ceramide synthesis in two other ways. Increased SP activity increases PAR-2 signaling, which downregulates lamellar body secretion which deposits ceramide precursors into the intercellular space the SC and entombs these organelles within corneocytes. Failure of lamellar body secretion contributes to the overall decrease in SC lipids in AD.2 Secondly, SP activity increases the generation of interleukin-lalpha (IL-lalpha) and IL-lbeta whose proforms are stored in the cytosol of corneocytes. The generation of the active forms of these cytokines induces an inflammatory cascade in AD, which exhibits a TH2 cytokine and inflammatory cellular pattern. The TH2 inflammatory response is associated with increased secretion of IL-4, which leads to reduction in the synthesis of decreased ceramides, loricrin, filaggrin, and desmoglein-3.2 In a nutshell, the TH2 inflammatory pattern characteristic of AD correlates with impairment of both the integrity and several functions of the SC barrier.
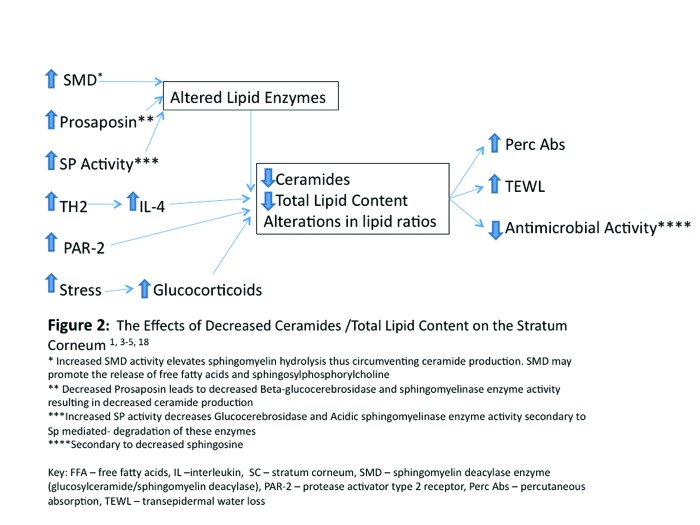
Increased and sustained psychological stress is very common in patients with AD. Stress is a well-known trigger for AD and can contribute adversely to exacerbation and response to therapy. There are data supporting the hypothesis that psychological stress in AD can lead to physical manifestations.25 In AD, it has been shown that psychological can decrease barrier function by increasing systemic levels of glucocorticoids, which has an inhibitory effect on the synthesis of three key epidermal lipids namely, ceramides, cholesterol, and free fatty acids, and stress has been shown to enhance skin sensitivity and SC permeability barrier function.2 Figure 2 summarizes the enzymatic alterations and physicochemical properties of the skin in AD, which lead to variations in ceramides and total lipid content in the SC.
Figure 2.
The effects of decreased ceramides/total lipid content on the stratum corneum1,3-5,18
*Increased SMD activity elevates sphingomyelin hydrolysis thus circumventing ceramide production. SMD may promote the release of free fatty acids and sphingosylphosphorylcholine
**Decreased prosaposin leads to decreased beta-glucocerebrosidase and sphingomyelinase enzyme activity resulting in decreased ceramide production
***lncreased Sp activity decreases glucocerebrosidase and acidic sphingomyelinase enzyme activity secondary to Sp-mediated degradation of these enzymes
*****Secondary to decreased sphingosine
FFA=free fatty acids; IL=interleukin; SC=stratum corneum; SMD=sphingomyelin deacylase enzyme (glucosylceramide/sphingomyelin deacylase); PAR-2=protease activator type 2 receptor; perc abs=percutaneous absorption; TEWL=transepidermal water loss
Conclusion
The “epidermal barrier” describes a collection of diverse functions, the majority of which occur within the SC, and include modulation of permeability and water balance, antimicrobial defense, immunological response, antioxidant reserve, and inherent photoprotection. In AD, innate impairments of the structural and functional integrity of the SC, such as decreased filaggrin, increased SP activity, and altered lipid ratios, predispose the patient to exacerbation of xerotic and eczematous skin changes. However, any of the mentioned epidermal dysfunctions alone are not solely responsible for the cascade of epidermal changes or the dinical manifestations of AD. Many of these structural and functional SC barrier abnormalities have been shown to exist both during and in between AD flares. However the net effects of a combination of these epidermal impairments are a reduced ability of the SC in atopic skin to self repair when challenged by exogenous insults, leading to prolonged signaling of inflammatory cascades. These cascades are initially set into motion for reparative changes, but instead lead to further impairment of skin functions and exacerbation of AD as they continue unchecked and amplify abnormalities with the skin that eventually become visible (xerosis, eczematous dermatitis, hyperkeratosis). In Part 3, the role of the inflammatory responses in the pathogenesis of AD will be discussed along with the relationship between structural and functional epidermal barrier dysfunctions and the inflammatory responses in AD.
Footnotes
Disclosure:Dr. Levin is an advisory board participant and consultant for Onset Dermatologies and Galderma Laboratories. Dr. Friedlander is a consultant, advisory board participant, clinical ivestigator, and/or speaker for Galderma, Top MD, Valeant, and Onset Dermatologies. Dr. Del Rosso is consultant, advisory board participant, clinical investigator, and/or speaker for Allergan, Bayer Healthcare, Eisai, Galderma, Medicis (a division of Valeant), Obagi Medical Products, Onset Dermatologies, PharmaDerm, Primus, Tromius, Quinnova, Ranbaxy, Taro Pharmaceuticals, TriaBeauty, Unilever, Valeant, and Warner-Chilcott.
References
- 1.Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9(5):437–446. doi: 10.1097/ACI.0b013e32832e7d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proksch E, Elias PM. Epidermal barrier in atopic dermatitis. In: Bieber T, Leung DYM, editors. Atopic Dermatitis. New York: Marcel Dekker; 2002. pp. 123–143. [Google Scholar]
- 3.Choi MJ, Maibach HI. Role of ceramides in barrier function of healthy and diseased skin. Am J Clin Dermatol. 2005;6(4):215–223. doi: 10.2165/00128071-200506040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hachem JP, Man MQ, Crumrine D, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125(3):510–520. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- 5.Hachem JP, Crumrine D, Fluhr J, et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121(2):345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Regan GM, Sandilands A, McLean WHI, et al. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Holleran WM, Takagi Y, Menon GK, et al. Permeability barrier requirements regulate epidermal beta-glucocerebrosidase. J Lipid Res. 1994;35(5):905–912. [PubMed] [Google Scholar]
- 8.Jensen JM, Schutze S, Fori M, et al. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J Clin Invest. 1999;104(12):1761–1770. doi: 10.1172/JCI5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias PM. Physiologic lipids for barrier repair in dermatology. In: Draelos ZD, editor. Cosmeceuticals, 1st ed. Philadelphia: Elsevier-Saunders; 2005. pp. 63–70. [Google Scholar]
- 10.Di Nardo A, Wetz PW. Atopic dermatitis. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization. New York: Marcel Dekker; 2002. pp. 165–178. [Google Scholar]
- 11.Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004;17(Suppl 1):6–15. doi: 10.1111/j.1396-0296.2004.04s1001.x. [DOI] [PubMed] [Google Scholar]
- 12.McLean I. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008;128:2117–2119. doi: 10.1038/jid.2008.29. [DOI] [PubMed] [Google Scholar]
- 13.Cork MJ, Danby SG, Vasilopoulos Y, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 14.Imokawa G. Ceramides as natural moisturizing factors and their efficacy in dry skin. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization, 1st ed. New York: Marcel Dekker; 2002. pp. 267–302. [Google Scholar]
- 15.Imokawa G, Abe A, Jin K, et al. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. :523–526. doi: 10.1111/1523-1747.ep12470233. 199196. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto A, Serizawa S, Ito M, et al. о Stratum corneum abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–223. doi: 10.1007/BF01106105. [DOI] [PubMed] [Google Scholar]
- 17.Di Nardo A, Wertz P, Giannetti A, et al. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 18.Del Rosso JQ. Repair and maintenance of the epidermal barrier in patients diagnosed with atopic dermatitis: an evaluation of the components of a body wash-moisturizer skin care regimen directed at management of atopic skin. J Clin Aestfuet Dermatol. 2011;4(6):18–28. [PMC free article] [PubMed] [Google Scholar]
- 19.Doering T, Holleran WM, Potratz A, et al. Sphingolipid activator proteins are required for epidermal permeability barrier formation. J Biol Chem. 1999;274:11038–11045. doi: 10.1074/jbc.274.16.11038. [DOI] [PubMed] [Google Scholar]
- 20.Cui CY, Kusuda S, Seguchi T, et al. Decreased level of prosaposin in atopic skin. J Invest Dermatol. 1997;109(3):319–323. doi: 10.1111/1523-1747.ep12335839. [DOI] [PubMed] [Google Scholar]
- 21.Jin K, Higaki Y, Takagi Y, et al. Analysis of beta-glucocerebrosidase and ceramidase activities in atopic and aged dry skin. Acta Derm Venereol. 1994;74(5):337–340. doi: 10.2340/0001555574337340. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi K, Hara J, Okamoto R, et al. The skin of atopic dermatitis patients contains a novel enzyme, glucosylceramide sphingomyelin deacylase, which cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. Biochem J. 2000;15(350):747–756. [PMC free article] [PubMed] [Google Scholar]
- 23.Hara J, Higuchi K, Okamoto R, et al. High-expression of sphingomyelindeacylase is an important determinant ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2000;115:406–413. doi: 10.1046/j.1523-1747.2000.00072.x. [DOI] [PubMed] [Google Scholar]
- 24.Murata Y, Ogata J, Higaki Y, et al. Abnormal expression of sphingomyelin acylase in atopic dermatitis: an etiologic factor for ceramide deficiency? J Invest Dermatol. 1996;106:1242–1249. doi: 10.1111/1523-1747.ep12348937. [DOI] [PubMed] [Google Scholar]