Abstract
The assessment of acute circulatory failure is a challenge in absence of solid gold standard. It is suggested that artifacts generated by lung ultrasound can be of help. The FALLS-protocol (Fluid Administration Limited by Lung Sonography) follows Weil’s classification of shocks. Firstly, it searches for pericardial fluid, then right heart enlargment, lastly abolished lung sliding. In this setting, the diagnoses of pericardial tamponade, pulmonary embolism and tension pneumothorax, i.e. obstructive shock, can be schematically ruled out. Moreover, the search of diffuse lung rockets (i.e. multiple B-lines, a comet-tail artifact) is performed. Its absence excludes pulmonary edema, that in clinical practice is left cardiogenic shock (most cases). At this step, the patient (defined FALLS-responder) receives fluid therapy. He/she has usually a normal sonographic lung surface, an A-profile. Any clinical improvement suggests hypovolemic shock. The absence of improvement generates continuation of fluid therapy, eventually yielding fluid overload. This condition results in the change from A-profile to B-profile. Lung ultrasound has the advantage to demonstrate this interstitial syndrome at an early and infraclinical stage (FALLS-endpoint). The change from horizontal A-lines to vertical B-lines can be considered as a direct marker of volemia in this use. By elimination, this change indicates schematically distributive shock, while in current practice septic shock. The major limitation is the B-profile on admission generated by an initial lung disorder. FALLS-protocol, which can be associated with no drawback with traditional hemodynamic tools, uses a simple machine (without Doppler) and a suitable microconvex probe allowing for heart, lung and vein assessment.
Keywords: acute circulatory failure, hemodynamic assessment, fluid responsiveness, lung ultrasound
Introduction
The FALLS-protocol (Fluid Administration Limited by Lung Sonography) is a tool proposed for the management of unexplained shock, mainly using lung ultrasound. Acute circulatory failure is one of the most familiar concerns of the intensivist, and echocardiography or transpulmonary thermodilution devices are among the most widely used tools at present [1]. They accurately measure changes in cardiac output. However, giving only an indirect idea of the mechanism of shock, they are not fully designed to provide a diagnosis. The FALLS-protocol was conceived based upon this consideration [2, 3]. It exploits the ability of ultrasound to detect interstitial syndrome, which will be considered as a direct marker of clinical volemia. The FALLS-protocol is the main product of the BLUE-protocol (Bedside Lung Ultrasound in Emergency).
Ultrasound is now a familiar tool in emergency fields. We had the privilege to define critical ultrasound some decades before the advent of the laptop revolution ( 1989 ) at François Jardin’s Intensive care unit, by means of a machine ironically faster (7”) and smaller (30 cm wide) than nowadays laptops. In the definition we provided, the lung was not the only target, as other dozens were studied, but it was certainly a priority to publish. The FALLS-protocol is integrated into a global approach, called Limited Investigation (considering hemodynamic therapy). It is designed for sequentially ruling out the main causes of shock, according to the Weil’s classification [4].
Obstructive shock
Our probe is first applied to the heart, immediately ruling out a pericardial tamponade. Afterwards, it searches for right heart enlargement suggestive of pulmonary embolism. In case of poor cardiac windows, the BLUE-protocol can be chosen instead, or be systematically associated. The BLUE-protocol exploits lung and venous ultrasounds, which provide a 81% sensitivity and a 99% specificity in the diagnosis of pulmonary embolism [5]. The following step in the FALLS-protocol is the positioning of the probe at the anterior lung area, immediately ruling out a pneumothorax, which constantly generates the A’-profile of the BLUE-protocol, namely lung sliding abolished plus a horizontal artificial repetition of the pleural line called A-line (Figure 1).
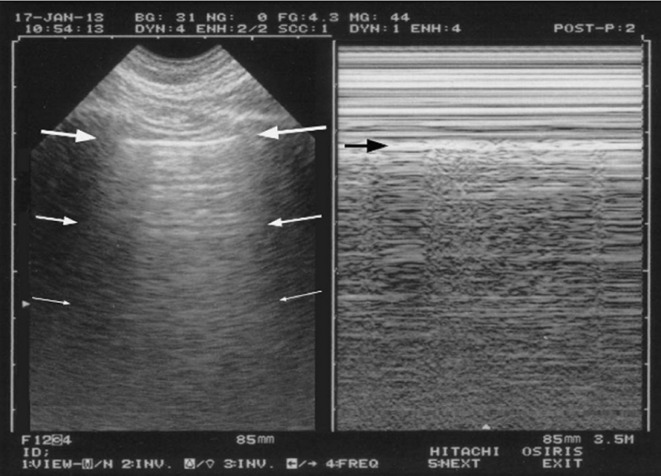
Figure 1.
The A-profile.
Left, from the pleural line (upper arrows), horizontal repetitions of the pleural line are displayed (lower arrows). These artifacts are called A-lines.
Right: M-mode indicates a sandy pattern homogeneously displayed exactly below the pleural line (arrow). This demonstrates the lung sliding.
Lung sliding associated to A-lines, at the anterior chest wall of a dyspneic patient, is called the A-profile (indicating normal lung surface).
Cardiogenic shock
If pericardial tamponade, pulmonary embolism and pneumothorax are ruled out, the diagnosis of obstructive shock can be ruled out as well, and the FALLS-protocol makes one step forward, searching for interstitial syndrome. At the ultrasound, the interstitial syndrome is characterized by the presence of lung rockets. Lung rockets are defined as three or more B-lines in a view between two ribs. The B-line is a particular comet-tail artifact whose updated properties are specified in Figure 2.
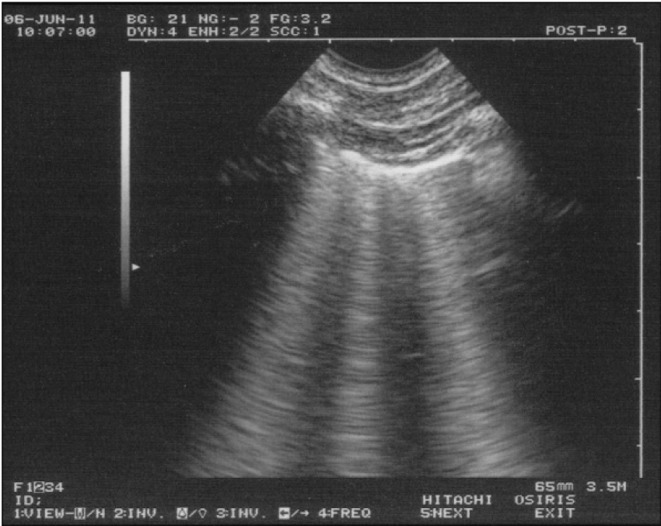
Figure 2.
Lung rockets.
The B-line includes 7 criteria. Three are constant: 1) This is a comet-tail, vertical artifact. 2) It arises from the pleural line. 3) It moves in concert with lung sliding. Four criteria are quite always present. 4) It does not fade, descends up to the edge of the screen. 5) It is well-defined, laser like. 6) It is hyperechoic, like the pleural line. 7) It obliterates the A-lines. All these criteria make it always possible to recognize B-lines from other comet-tail artifacts (E-lines, Z-lines...).
The B-line can be isolated, with little meaning. Multiple B-lines, like in this view (three being visible), are then called “lung rockets”, and indicate interstitial syndrome - usually interstitial edema when seen in acute settings.
In the BLUE-protocol, detection of an interstitial syndrome, anterior, bilateral, and associated with lung sliding is defined as the B-profile, and it is largely equivalent to the diagnosis of acute hemodynamic pulmonary edema, with a 97% sensitivity and a 95% specificity [5].
Pulmonary edema is associated with low cardiac output in cardiogenic shock from left origin, i.e., most cases (read below for the case of right cardiogenic shock). A left ventricle hypocontractility is frequently associated. The absence of a B-profile in a patient with acute circulatory failure means schematically that left cardiogenic shock cannot be considered.
Hypovolemic shock
Obstructive shock and left cardiogenic shock are eventually ruled out by applying a schematic approach. The FALLS-protocol at this stage assesses another lung artifact: the A-line. The A-profile combines A-lines with lung sliding (Figure 1). Facing an A-profile, at this step, two mechanisms of acute circulatory failure are competing: hypovolemic shock and distributive shock. In this context distributive shock is assimilated to septic shock not simply for sake of simplicity, but also because the other causes (anaphyllactic, spinal shock) are infrequent and easy to be diagnosed. The A-profile is correlated with a pulmonary artery occlusion pressure (PAOP) equal to or lower than 18 mmHg with a 93% specificity and 97% positive predictive value [2]. A shocked patient who displays the A-profile, at this step, is called a FALLS-responder. This patient can, and needs to receive fluid. The FALLS-protocol is a therapeutic test. It administers fluid with strict monitoring of the clinical parameters of circulation and lung ultrasound. This step is also exploited for searching causal disorders (site of bleeding, site of sepsis, etc.). The FALLS-protocol defines hypovolemic shock, whatever the cause, as the improvement of the circulatory function after fluid therapy (with unchanged A-profile). Hemorrhagic shock with ongoing bleeding is a particular case, since continuous “blind” fluid therapy would result in extreme hemodilution, yet hemorrhagic shock generates more therapeutic than diagnostic issues.
Distributive shock
If the clinical data do not improve, fluid therapy will eventually create a fluid overload in the tissues, particularly in the lung, which should not contain any water. Eventually, lung rockets appear. The change from A-lines to B-lines under fluid therapy occurs for a value of PAOP of 18 mmHg [2]. Interstitial edema is an early and infraclinical step of pulmonary edema. The FALLS-protocol has the peculiarity of detecting this pulmonary edema a minima, an early marker of fluid overload. At this step, called FALLS-endpoint, fluid therapy is discontinued. It indicates that the mechanism of shock is vasoplegic, since all other causes have been ruled out. At this point, the clinician should correct one of the parameters. The parameter to change is not the interstitial syndrome, which is infraclinical and does not require more oxygen [6], but rather the position of the heart on the Frank-Starling curve, since it begins to work on its flat portion. Withdrawing some fluid should position the patient on the ideal point of the curve. This is achieved using either invasive (hemodiafiltration) or noninvasive options (putting down previously raised patient’s legs, a maneuver called FALLS.PLR-protocol), the most logical one being the prescription of several blood cultures. This blood-letting will benefit to the patient’s prognosis, because the only remaining cause of shock (rarities excluded) is precisely septic shock. The fluid therapy is now judged optimal, and the classical therapy of septic shock (vasoactive agents etc.) is initiated.
The transformation from an A-profile to a B-profile under fluid therapy without clinical improvement defines, according to the protocol, the septic shock (Figure 3).
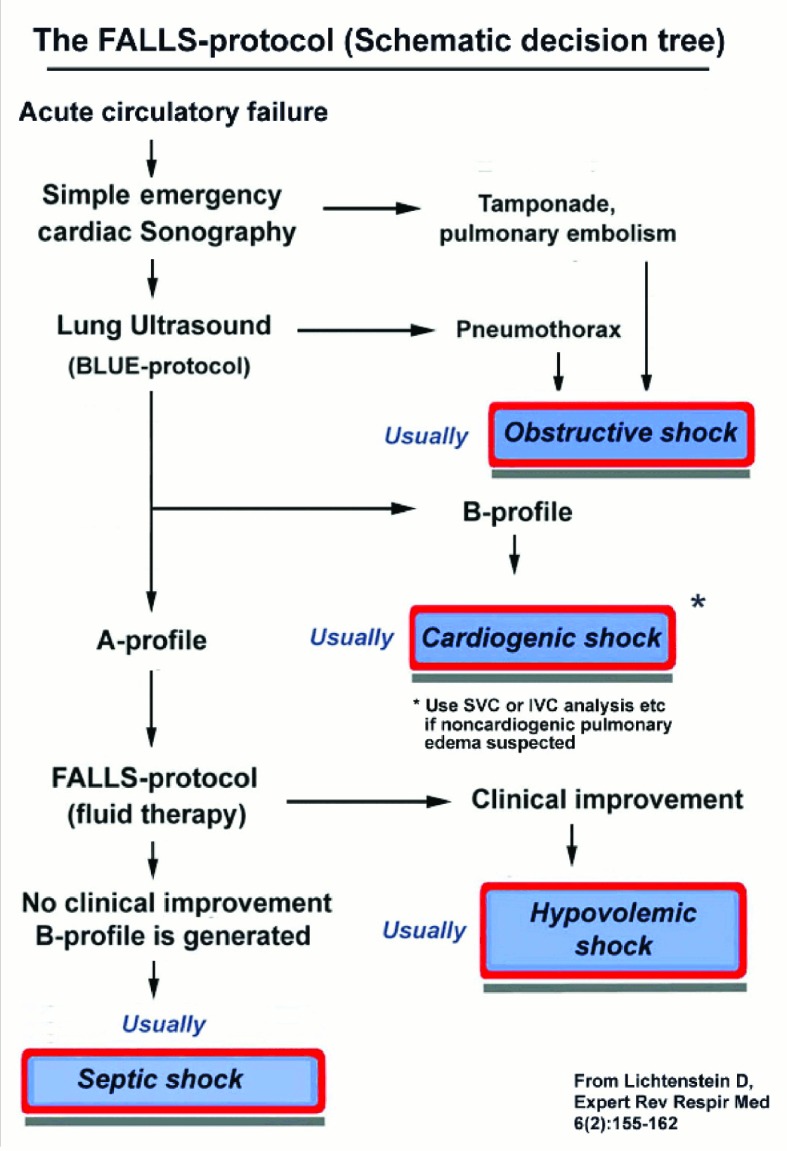
Figure 3.
Decision tree of FALLS-protocol.
A decision tree facilitating the understanding of the FALLS-protocol. According to Weil classification, cardiac and lung ultrasound sequentially rule out obstructive, then cardiogenic (from left heart) then hypovolemic then distributive shock, i.e. septic shock in current practice. Adapted from Expert Review of Respiratory Medicine, April 2012, Vol. 6, No. 2, Pages 155-162 with permission of Expert Reviews Ltd.
FALLS-protocol = Fluid Administration Limited by Lung Sonography; BLUE-protocol =Bedside Lung Ultrasound in Emergency
Septic shock is the last step of a complete FALLS-protocol. The FALLS-protocol follows the standard of care in septic shock [7], that is an early and massive fluid therapy. In addition, the FALLS-protocol takes advantage of the following recommendations: the therapy can be initiated before the usual delay required for the diagnosis of septic shock, i.e. at the admission; the administration cannot exceed the highest admissible dose. This endpoint is chosen in function of an objective change, taking into consideration the pathophysiological concept of clinical volemia. The change from A-lines to B-lines provides an ON-OFF parameter which is independent from the limitations and constraints of the usual methods.
The mortality rate in septic shock is high. One ambition of the FALLS-protocol is to decrease it. In addition, the FALLS-protocol should be mainly understood as a modern tool for diagnosing hypovolemia. This will be appreciated even more when considering patients with more complicated conditions, such as patients affected by complex disorders, peri-operative patients, obese patients where everything is more difficult to assess (but providentially, not lung ultrasound).
Limitations and questions
The main limitation of the FALLS-protocol is the B-profile seen on admission, that prevents from the PAOP assessement. The FALLS-protocol in this context cannot be applied since it is not possible to determine any endpoint. The B-profile is usually due to hemodynamic pulmonary edema. A simple cardiac sonography will usually show left heart anomalies. More rarely, the B-profile is related to a permeability-induced pulmonary edema. Exceptionally, lung fibrosis or any chronic interstitial disease can be seen in shocked patients. According to the clinical suspicion of non-hemodynamic pulmonary edema, it is possible to administer fluid therapy following other criteria, such as heart chambers volume, inferior caval vein, as well as superior caval vein (using external approach, read below). In some occasions, more classical tools will be used, including Doppler (not used in the FALLS-protocol), able to point out valvular disorders, diastolic dysfunction and other disorders. It is worth of notice that this issue (i.e. B-profile at the onset, due to a pulmonary disease) is limited by the fact that these diseases generate a respiratory failure rather than a circulatory one.
Cardiogenic shock with a low wedge pressure (e.g. right ventricle infarction) appears a limitation, but it remains a rare event. A condition of low wedge pressure should be associated with an A-profile, which is consistent with the administration of fluids proposed in the FALLS-protocol, that is indeed the appropriate therapy chosen in this setting. Moreover, note that among the tests involved in shock assessment a 12-derivations ECG is performed, which usually indicate the right ventricle infarction.
The FALLS-protocol is currently assessed for validation in a peri-operative setting, even though a completely appropriate golden standard has not been identified, yet. For this reason, the FALLS-protocol remains open to any criticism.
Answers to many questions are available [8]. In this paper we briefly focus on two of them. The first one concerns the machine that should be chosen when applying the FALLS-protocol. It needs a fast, quite simultaneous assessment of heart, lungs, and veins. In order to fulfill this requirement, we use a simple Japanese gray-scale unit from 1992, associated with a universal probe appropriate for a total body approach. Our ultrasound machine, differently from most up-to-date ones, has a suitable design for lung assessment.
Generally speaking, the more simple the machine, the more suitable for lung ultrasound. In addition, this unit has a suitable width which allows it to be used in tiny places (Intensive care unit, Operating room, Emergency room).
Our microconvex probe has a resolution, a penetration and an ergonomy allowing analysis of heart, veins, lung, as well as superior caval vein in most cases.
Another frequent question coming out is whether it is possible to manage a patient without knowing his/her cardiac output. The FALLS-protocol discriminates among patients who may benefit from fluid therapy, and when exactly should the fluid therapy be interrupted, which is the reasons why cardiac index is usually analyzed. Note that traditional tools remain still valuable, and can be associated with no drawback to FALLS-protocol [9].
Conclusion
The advantages of the FALLS-protocol are its rapid learning curve, the simple equipment required, the possibility to be performed at the bedside, its simplicity (FALLS-protocol makes no measurement) and a possible direct vision of volemia.
These features are even more useful in particular conditions, whenever transthoracic echocardiography is difficult to perform, or transesophageal echocardiography presents with problems, such as time-dependent patients, possible contraindications, or it is not performable due to inappropriately equipped settings.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic assessment of shock. Heart, Lung and Vessels. 2013; 5(3): 142-147.
References
- Jardin F, Farcot J C, Boisante L. et al. Influence of positive end-expiratory pressure on left ventricle performance. New Engl J Med. 1981;304:387–392. doi: 10.1056/NEJM198102123040703. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D, Mezière G, Lagoueyte J F. et al. A-lines and B-lines: Lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136:1014–1020. doi: 10.1378/chest.09-0001. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D. Fluid Administration Limited by Lung Sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Med. 2012;6:155–162. doi: 10.1586/ers.12.13. [DOI] [PubMed] [Google Scholar]
- Weil M H, Shubin H. Proposed reclassification of shock states with special reference to distributive defects. Adv Exp Med Biol. 1971;23:13–13. doi: 10.1007/978-1-4615-9014-9_3. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D, Mezière G. Relevance of lung ultrasound in the diagnosis of acute respiratory failure. The BLUE-protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargani L, Lionetti V, Di Cristofano C. et al. Early detection of acute lung injury uncoupled to hypoxemia in pigs using ultrasound lung comets. Crit Care Med. 2007;35:2769–2774. doi: 10.1097/01.CCM.0000287525.03140.3F. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S. et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D. 2010. Limited Investigation considering hemodynamic therapy. In: Whole Body Ultrasonography in the Critically Ill. 4th Ed; pp. 223–242. [Springer] [Google Scholar]
- Lichtenstein D, Karakitsos D. Integrating lung ultrasound in the hemodynamic evaluation of acute circulatory failure (the FALLS-protocol). J Crit Care. 2012;27:533–533. doi: 10.1016/j.jcrc.2012.03.004. [DOI] [PubMed] [Google Scholar]