Abstract
Background
Optic pathway gliomas (OPGs) are common pediatric brain tumors that pose significant clinical challenges with regard to predicting which tumors are likely to become symptomatic and require treatment. These tumors can arise sporadically or in the context of the inherited cancer predisposition syndrome, Neurofibromatosis type 1 (NF1). Few studies have suggested biological or imaging markers which predict the clinical course of this disease.
Objective
In this cross-sectional study, we hypothesize that the clinical behavior of OPGs in children can be differentiated by diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI).
Methods
27 patients with OPG were studied using DWI and DCE MRI protocols. Diffusivity and permeability were calculated and correlated to OPG clinical behavior.
Results
Mean diffusivity values of 1.39 μm2/ms and mean permeability values of 2.10 ml min−1 per 100 cc of tissue were measured. “Clinically-aggressive” OPGs had significantly higher mean permeability values (P=.05) than “clinically stable” tumors. In addition, there was a strong correlation between clinical aggressiveness and the absence of NF1 (P < 0.01).
Conclusion
These results suggest that DCE might be a useful biomarker for “clinically aggressive” OPG, which should be confirmed in larger prospective longitudinal studies.
Introduction
Low-grade gliomas are one of the most common brain tumors seen in the pediatric population. The majority of these tumors are classified by the World Health Organization as grade I pilocytic astrocytomas, and are most commonly found in the cerebellum and along the optic pathway [1]. Compared to low-grade gliomas involving the cerebellum where surgical resection is often curative, surgery plays a limited role when these tumors develop within the optic pathway. Most of these young children are evaluated by serial ophthalmologic examinations, which can be challenging in very young children, and those with declining visual acuity are treated with chemotherapy. For these reasons, it would be desirable to develop surrogate indicators that predict optic glioma growth to select children most likely to benefit from close observation.
Optic pathway gliomas (OPGs) may arise sporadically or in the context of the tumor predisposition syndrome, Neurofibromatosis 1 (NF1) or von Recklinghausen’s disease. NF1 affects approximately 1 in 2500 individuals worldwide [2, 3], and is the most common genetic cause for these tumors. 15–20% of individuals with NF1 will develop an optic glioma, usually within the first decade of life (mean age at presentation of 4.5 years) [4, 5]. NF1-associated OPGs are histologically identical to pilocytic astrocytomas that occur in individuals without NF1 [6]. However, some reports have suggested that NF1-associated optic gliomas behave in a clinically less aggressive manner than those that occur in the general population [7, 8, 9, 10].
Although OPGs are low-grade tumors, their clinical course is highly variable. Some patients with OPG will exhibit progressive tumor growth and visual loss [11, 12], while other similarly appearing OPGs will spontaneously regress [13, 14, 15, 16]. Unfortunately, our current insights derive from studies on small numbers of subjects with various types of treatments, and no macroscopic or microscopic features have been identified that reliably predict a given patient’s clinical course [7]. Thus, identifying patients with OPGs that are likely to progress remains a major clinical problem. In this study, we hypothesized that advanced MR imaging methods, including diffusion-weighted (DWI) and dynamic-contrast enhanced (DCE) imaging, might offer insight into this particular clinical management issue.
Materials and Methods
Patients
A total of 27 patients were identified from our clinical practice at St. Louis Children’s Hospital (SLCH). Fourteen of these patients had a clinical diagnosis of NF1 and were cared for by one of the investigators (DHG), who directs the Neurofibromatosis Clinic at SLCH. Based upon established guidelines for the management of patients with NF1 [17], patients in the NF program are examined clinically at least annually, and more frequently if clinically indicated. This study was performed in accordance with an approved Washington University IRB Human Studies protocol. All patients enrolled in the study had been identified as having an OPG.
In this cross-sectional study, tumors were classified as either clinically aggressive or clinically stable. This definition was based upon two specific clinical features: decline in visual acuity and a need for therapeutic intervention. “Clinically aggressive” tumors were defined as patients who had experienced a decline in their visual acuity significant enough (typically ≥2 line reduction in visual acuity) that their treating neuro-oncology team (neurologist, oncologist and ophthamologist) felt that they required treatment (surgery, chemotherapy, and/or radiation). If the patient’s treating physicians determined that the patient required therapeutic intervention for any reason other than visual decline, these patients were also classified as “clinically aggressive”. Patients who had asymptomatic or clinically non-progressive symptomatic OPGs and did not require therapeutic intervention were defined as “clinically stable”. This classification was based upon the patient’s status at the time of his/her last recorded medical visit during the time course of the study (ten months).
MRI Protocol
All patients were subject to at least one MR imaging evaluation over the course of the study. All examinations were performed at SLCH with sedation on either a 1.5T system (Sonata; Siemens, Erlangen, Germany) or a 3T system (Trio; Siemens, Erlangen, Germany) using a dedicated head RF coil. The clinical protocol included multiplanar, multiweighted imaging of the orbits and optic pathway. The diffusion and DCE sequences are described below.
Diffusion Weighted and DCE Imaging
The diffusion sequence consisted of single-shot, multisection, spin-echo EPI with FOV of 230 × 230 mm2, in-plane resolution of 1.9 × 1.9 mm2 interpolated to 256 × 256 for display and analysis, TR of 5000 milliseconds, and TE of 85 milliseconds. Three orthogonally-oriented diffusion weighted images (diffusion sensitivity, b = 1000 s/mm2), and a reference T2-weighted intensity image (b = 0 s/mm2) were obtained at each transverse section. The DWI acquisition and reconstruction and subsequent computation of ADC maps required ~ 2 minutes.
Dynamic contrast enhanced MRI was performed using a T1-weighted 3D FLASH sequence (TR = 30 ms, TE = 6 ms). A matrix of 128 × 128 × 16 was used to achieve 1 × 1 × 3 mm3 resolution. Each 3D image was acquired in less than 1 minute. A pre-contrast, variable flip angle experiment (flip angles of 10, 15, and 25 degrees) was performed to estimate T1 on a voxel-by-voxel basis. A bolus of gadolinium-based contrast agent, 0.1 mmole per kilogram, was injected, and dynamic scanning was performed for at least 6 minutes using the T1-weighted 3D sequence described above.
Identification of Tumor Volumes and Regions of Interest
A region of interest (ROI), in this case the presumed OPG tissue, was identified for each patient using T1-weighted, contrast-enhanced images that were simultaneously loaded with the DWI and DCE sequences. Images were co-registered using Intelli-link software and tumor boundaries identified manually on the anatomic T1+Gd images. On all serial slices containing tumor tissue, a free-hand ROI was drawn to circumscribe the entire tumor area (all areas determined to be tumor by the collaborating neuron-radiologist excluding cyst and CSF spaces). The tumor volume in each slice was computed by multiplying the area of the ROI by the slice thickness and the total tumor volume was then computed by serially adding the volumes measured for each slice.
Processing of Diffusion and DCE data
For processing, diffusion (ADC) maps were loaded into the Synapse software system (Fuji Medical Systems; Roselle, United States). We used the Intelli-link feature of the Fuji Synapse, which automatically cross-references a selected voxel in the reference T1-weighted images with the target voxel in the ADC map. This allowed us to precisely outline, using a free-hand ROI definition technique, the margins of the tumor on the anatomical image and apply that region definition to the diffusion map. We used T2-weighted images for comparison to avoid frankly cystic areas and those containing cerebrospinal fluid. If the ADC images were distorted at the skull base, the target ROI was modified to conform to the tumor margins and exclude CSF. For each slice, the ROI used for volumetric measurement was overlaid on the ADC map and mean ADC values were computed for the overall tumor volume.
DCE data were analyzed via a standard Patlak analysis [18, 19] using customized, Matlab-based (The MathWorks, Natick, MA) software. The Voxa analysis package in Matlab allows for voxel-by-voxel region definition. Raw DCE data were loaded into Voxa, and the regions of interest used for ADC analysis were used to guide region definition for DCE analysis. The DCE regions, however, excluded non-enhancing voxels; thus the DCE regions tend to be smaller than the ADC regions.
Voxels in both the tissue and blood ROIs with intensities less than the standard deviation of a selected noise region were excluded from the calculations. Values of the tissue permeability-surface area product, KPS (ml min−1 per 100 cc of tissue), were calculated on a voxel-by-voxel basis using standard equations. In performing this analysis, non-enhancing tumor voxels having a negative or zero slope and blood vessels whose initial slope was greater than 0.45 were excluded. Average values of KPS for each tumor ROI are reported.
Statistical Analysis
Values for tumor volume, mean diffusivity, and mean permeability (KPS) were calculated for each enrolled patient in a blinded fashion. Data were then unblinded and imaging data values were compared with the inheritance pattern (sporadic versus NF1-associated) and with clinical aggressiveness (requiring treatment: yes or no). Mean and range values were compared for each of these groups and student’s t-tests and chi-square analysis were performed to compare imaging data values for individual groups.
Results
A total of 27 patients with OPG having correlative diffusion and dynamic contrast-enhanced imaging were enrolled in the study over ten months between November 2004 and August 2005 (Table 1). Fourteen patients met diagnostic criteria for NF1; thirteen patients had a sporadic OPG without clinical features of NF1. All patients had tumors located within the prechiasmal optic nerves or the optic chiasm. Three patients had chiasmal tumors that extended into the hypothalamus (Table 2). Eleven patients were classified as “clinically stable”. Sixteen patients were classified as “clinically aggressive”, and required treatment with chemotherapy (n=10) radiation (n=3) or surgical resection (n=3) during the study or prior to enrollment. Two initially asymptomatic patients developed clinical progression and required treatment during the study. These tumors were defined as “clinically aggressive”. Nine patients had more than one imaging examination during the research study, with a mean interval of 3.1 months between MRI scans. For the three patients who underwent surgical resection, pathology confirmed pilocytic astrocytoma. Biopsy was not performed for tissue diagnosis for any of the other patients in this study, as this is not a part of the routine treatment for patients with OPG. In no cases were there indications that the tumors were higher grade gliomas.
Table 1.
A strong correlation exists between the presence of NF1 and OPG clinical aggressiveness (chi square statistic = 5.78; P < 0.05). Clinically aggressive tumors are defined as requiring medical or surgical therapeutic intervention. Stable tumors did not require clinical intervention.
| NF1-associated | Sporadic | Total | ||
|---|---|---|---|---|
| Tumor Clinical Phenotype | “clinically stable” | 9 | 2 | 11 |
| “clinically aggressive” | 5 | 11 | 16 | |
| Total | 14 | 13 | 27 | |
Table 2.
The anatomic locations of OPG tumors in all patients enrolled in the study are shown. All tumors were located in the prechiasmatic or chiasmatic regions. Three patients had tumors that also extended into the hypothalamus. Tumor location was not found to be a statistically significant factor with respect to outcome.
| Patient Number | Tumor Location |
|---|---|
| 1 | Chiasm |
| 2 | Bilateral Prechiasmal Optic Nerve and Chiasm |
| 3 | Chiasm |
| 4 | Chiasm |
| 5 | Chiasm |
| 6 | Chiasm |
| 7 | Chiasm |
| 8 | Chiasm |
| 9 | Left optic nerve, intraconal |
| 10 | Chiasm |
| 11 | Left Prechiasmal Optic Nerve and Chiasm |
| 12 | Chiasm |
| 13 | Chiasm |
| 14 | Chiasm |
| 15 | Chiasm |
| 16 | Chiasm and Hypothalamus |
| 17 | Chiasm |
| 18 | Chiasm and Hypothalamus |
| 19 | Chiasm |
| 20 | Chiasm |
| 21 | Right Prechiasmal Optic Nerve |
| 22 | Right Prechiasmal Optic Nerve |
| 23 | Chiasm |
| 24 | Chiasm and Hypothalmus |
| 25 | Left Prechiasmal Optic Nerve |
| 26 | Chiasm |
| 27 | Chiasm |
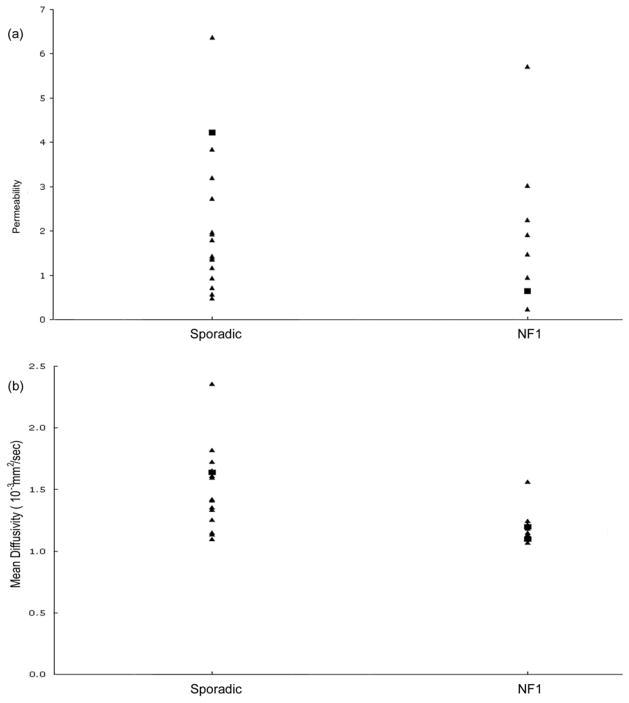
Mean diffusivity, permeability, and tumor volume were calculated for the tumor ROI for each patient at each imaging time point. For the group as a whole, OPGs had mean diffusivities of 1.39 μm2/ms (range = 1.0 – 2.35) and mean permeabilities (KPS) of 2.10 ml min−1 per 100 cc of tissue (range = 0.23 – 6.37). Both sporadic and NF1-associated OPGs had a broad range of diffusivity and permeability measurements (Figure 1). The two tumors that progressed clinically over the course of the imaging study did not differ significantly in either their mean diffusivity or permeability from the tumors that did not progress.
Figure 1.
Sporadic and NF1-associated OPGs have a large dynamic range of permeability (A) and diffusion (B) values, and there is no significant difference in either between these two groups. The few patients whose OPGs progressed over the course of the study, indicated by the closed boxes, cannot be separated from the remainder of patients whose OPGs were clinically stable over the course of the study (closed triangles).
NF1 status was strongly correlated with clinical aggressiveness. Patients with sporadic OPGs exhibited more clinically-aggressive disease: 75% of patients with “clinically stable” disease met diagnostic criteria for NF1, while the remaining 25% of patients with “clinically stable” disease had sporadic tumors. Correspondingly, 71% of patients with “clinically aggressive” disease had sporadic tumors; 29% of patients with “clinically aggressive” disease had NF1. These data are summarized in Table 1 (Chi Square statistic = 5.78; P < 0.05).
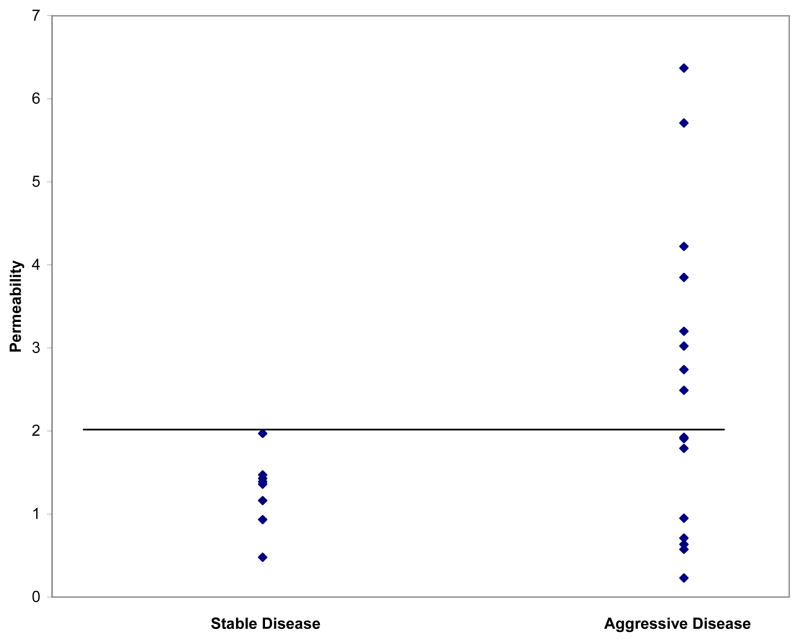
Mean permeability values correlated with “clinical aggressive” disease. These “clinically aggressive” OPGs had significantly higher mean permeability values (2.24) than the “clinically stable” tumors (1.38) (P=.05). While “clinically aggressive” tumors had a dynamic range of permeability values (0.23 – 6.37), nearly half (47%) of the permeability values for the “clinically aggressive” tumors were > 2.0. No “clinically stable” tumors had permeability values > 2.0. These results are displayed graphically in Figure 2. Further supporting the observed correlation between tumor aggressiveness and vascular permeability, sporadic tumors classified as “clinically aggressive” had significantly higher permeability values (2.77) than “clinically stable” OPGs (P < .05).
Figure 2.
Permeability values for OPGs were divided based upon tumors’ apparent clinical aggressiveness at enrollment. While “clinically aggressive” tumors had a dynamic range of permeability values (.23 – 6.37), 47% of permeability values for “clinically aggressive” tumors were > 2.0, while no “clinically stable” tumors had permeability values > 2.0. This cutoff value is indicated graphically as well.
In contrast, mean diffusivity values did not correlate with either clinical aggressiveness or with the presence of NF1, and there was no significant correlation between tumor size and either mean diffusivity or permeability. Correspondingly, there was no correlation between permeability and mean diffusivity for each individual tumor ROI.
Discussion
Optic pathway gliomas, whether associated with NF1 or not (sporadic), are difficult tumors to manage. These gliomas tend to grow slowly, their clinical course is variable, and there are no clinical, imaging, or pathologic markers that accurately predict tumor progression. Once vision is compromised, therapeutic intervention often does little to reverse existing visual loss. Consequently, it is critical to identify patients most likely to progress early during the course of their disease, preferably prior to clinical deterioration, so that vision-saving treatment can be initiated. In this study, we hypothesized that advanced imaging techniques, including diffusion and dynamic contrast-enhanced MR imaging, might be useful tools for predicting optic glioma clinical behavior.
MR imaging has provided important insights germane to the management of a variety of cancers, including brain tumors. One of the clear advantages of MRI is its ability to enable detailed anatomic localization and delineation of tumor size. However, our study as well as previous studies found no correlation between tumor size and clinical behavior.
Diffusion-weighted imaging (DWI) is sensitive to the microstructure of biological tissues on a distance scale of 1– 10 microns and, as such, reflects changes in tumor cell density. DWI has been used to measure the mean diffusivity of water in vivo (i.e., apparent diffusion coefficient or ADC). Elegant studies by Ross and colleagues [20, 21, 22] have demonstrated that changes in the ADC of water correlate with tumor response to cytotoxic therapy in both rat models of malignant gliomas and in human malignant brain tumors. Previous studies in patients with pilocytic astrocytomas have shown mean ADC values of 1.3–2.09 μm2/ms [23, 24, 25], which is comparable to our mean value of 1.41. Similarly, a previous study found mean ADC values of 1.18 μm2/ms in a one-year-old child with NF1 and an extensive optic nerve/chiasmatic optic glioma [26]. However, we found that ADC did not distinguish between “clinically stable” and “clinically aggressive” OPG. This most likely reflects the low cellularity of these tumors and their relatively modest proliferative indices.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is an imaging method with the ability to measure vascular permeability. Increased vascular permeability has been observed in gliomas, particularly malignant gliomas [27], and DCE imaging is currently being used in clinical trials of anti-angiogenic therapies for malignant brain tumors. The usefulness of DCE methods for assessing the clinical behavior of lower grade tumors, like OPG, has yet to be clearly established. In our study, we found a statistically significant correlation between increased DCE values and OPG clinical behavior. It is worth noting that pilocytic astrocytomas are highly vascular tumors, similar to high-grade glioma. Moreover, in a mouse model of Nf1 optic glioma, vascular endothelial proliferation correlates with tumor growth in vivo, providing supportive evidence for our findings in pediatric OPG [28].
Previous studies have suggested that patients with NF1-associated OPG exhibit a more indolent course than sporadically-occurring tumors [6]. Our study population included a good mix of patients with NF1-associated versus sporadic tumors, and a range of patients with “clinically stable” and “clinically aggressive” disease phenotypes. Similar to others, our data support the claim that NF1 patients with OPG have a comparatively less clinically aggressive disease course [7, 8, 9, 10].
While our conclusions are limited by a relatively small sample size, this study was intentionally designed as a cross-sectional study, involving a cohort of patients with OPG cared for at a single institution over a relatively short time interval. Over half of these patients had already progressed clinically, and many had undergone treatment with chemotherapy, radiation, or surgery. In this regard, only two patients had evidence of clinical progression during the course of the study. It is possible that treatment, including corticosteroid therapy, impacted on the imaging results, particularly for diffusion and perfusion imaging [20, 29, 30]. However, none of the patients in our study were receiving corticosteroid therapy at or near the time of MR imaging.
Due to the suprasellar location of many of the lesions, susceptibility effects from the sphenoid sinus can potentially distort the EPI-based DWI images. This could have complicated quantitative analysis of ADC, owing to the presence of magnetic field gradients; however after careful review, this distortion was a significant factor in only one patient in the study, and in this particular case the data presumed to be confounded by artifact were excluded from the analysis.
Despite these caveats, our study provides important new information regarding the potential utility of dynamic contrast enhancement imaging in the management of OPG. Future studies will be required on larger cohorts of children with OPG to validate these findings. In particular, prospective studies using DCE and DWI will be critical to confirm the prognostic specificity of our findings and to distinguish tumor physiology from changes associated with consequences of therapeutic intervention. If non-invasive biomarkers, such as ADC and permeability, prove useful in identifying a subset of patients whose tumors are likely to be more aggressive and cause visual deterioration, early identification and intervention could be key in minimizing permanent visual compromise in this challenging pediatric patient population.
Conclusions
Presently, the proposed correlation between increased diffusivity and treatment response/clinical behavior seen in patients with malignant gliomas cannot be directly applied to patients with low-grade optic pathway gliomas. However, our study suggests that patients whose OPGs have high permeabilities (KPS > 2.0) may be at increased risk for clinical progression. Based on these preliminary findings, such patients may warrant close follow-up, as their risk for symptomatic progression may be increased.
Acknowledgments
Grant support: Partial support for this project was provided by Schnucks Markets, Inc. to DHG.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. IARC; Lyon, France: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman JM. Epidemiology of neurofibromatosis type 1. American Journal of Medical Genetics. 1989;89:1–6. [PubMed] [Google Scholar]
- 3.Friedman JM, Gutmann DH, MacCollin MM, et al. Neurofibromatosis. 3. Johns Hopkins Press; Baltimore: 1999. [Google Scholar]
- 4.Listernick R, Louis DN, Packer RJ, et al. Optic pathway gliomas in children with NF1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Annals of Neurology. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 5.Listernick R, Ferner RE, Liu GT, et al. Optic pathway glioma in neurofibromatosis-1: controversies and recommendations. Annals of Neurology. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King A, Listernick R, Charrow J, et al. Optic Pathway Gliomas in Neurofibromatosis Type 1: The Effect of Presenting Symptoms on Outcome. American Journal of Medical Genetics. 2003;122A:95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- 7.Czyzyk E, Jozwiak S, Roszkowski M, et al. Optic Pathway Gliomas in Children With and Without Neurofibromatosis 1. J Child Neurol. 2003;18:471–478. doi: 10.1177/08830738030180070401. [DOI] [PubMed] [Google Scholar]
- 8.Shamji MF, Benoit BG. Syndromic and sporadic pediatric optic pathway gliomas: review of clinical and histopathological differences and treatment implications. Neurosurg Focus. 2007;23:E3. doi: 10.3171/foc.2007.23.5.4. [DOI] [PubMed] [Google Scholar]
- 9.Singhal S, Birch HM, Kerr B, et al. Neurofibromatosis type 1 and sporadic optic gliomas. Arch Dis Child. 2002;87:65–70. doi: 10.1136/adc.87.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tow SL, Chandela S, Miller NR. Long-term outcome in children with gliomas of the anterior visual pathway. Ped Neurol. 2003;28:262–70. doi: 10.1016/s0887-8994(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 11.Adams C, Fletcher WA, Myles ST. Chiasmal glioma in neurofibromatosis type 1 with severe visual loss regained with radiation. Pediatr Neurol. 1997;17:80–82. doi: 10.1016/s0887-8994(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 12.Champion MP, Robinson RO. Screening for optic gliomas in neurofibromatosis type 1 The role of neuroimaging. J Pediatr. 1995;127:507–8. doi: 10.1016/s0022-3476(95)70099-4. [DOI] [PubMed] [Google Scholar]
- 13.Brzowski AE, Bazan C, Mumma JV, et al. Spontaneous regression of optic glioma in a patient with neurofibromatosis. Neurology. 1992;42:679–681. doi: 10.1212/wnl.42.3.679. [DOI] [PubMed] [Google Scholar]
- 14.Liu GT, Lessel S. Spontaneous visual improvement in chiasmal gliomas. Am J Ophthalmol. 1992;114:193–201. doi: 10.1016/s0002-9394(14)73984-4. [DOI] [PubMed] [Google Scholar]
- 15.Parazzini C, Triulzi F, Bianchini E, et al. Spontaneous involution of optic pathway lesions in neurofibromatosis type 1: Serial contrast MR evaluation. Am J Neuroradiol. 1995;16:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 16.Shuper A, Horev G, Kornreich L, et al. Visual pathway glioma: An erratic tumour with therapeutic dilemmas. Arch Dis Child. 1997;76:259–263. doi: 10.1136/adc.76.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. Journal of the American Medical Association. 1997;278:51–57. [PubMed] [Google Scholar]
- 18.Ewing JR, Knight RA, Nagaraja TN, et al. Patlak plots of Gd-DTPA MRI data yield blood-brain transfer constants concordant with those of 14C-sucrose in areas of blood-brain opening. Magn Reson Med. 2003;50:283–292. doi: 10.1002/mrm.10524. [DOI] [PubMed] [Google Scholar]
- 19.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood to brain transfer constants from multiple uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 20.Chenevert TL, McKeever PE, Ross BD. Monitoring Early response of Experimental Brain Tumors to Therapy Using Diffusion Magnetic Resonance Imaging. Clinical Cancer Research. 1997;3:1457–1466. [PubMed] [Google Scholar]
- 21.Hall DE, Moffat BA, Stojanovska J, et al. Efficacy of DTI-015 using Diffusion MRI as an Early Surrogate Marker. Clinical Cancer Research. 2004;10:7852–7859. doi: 10.1158/1078-0432.CCR-04-1218. [DOI] [PubMed] [Google Scholar]
- 22.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional Diffusion Map: A noninvasive MRI biomarker for stratification of clinical brain tumor response. PNAS. 2005;102:5524–29. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauvain KM, McKinstry RC, Mukherjee P, et al. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. Am J Roentgenol. 2001;177:449–54. doi: 10.2214/ajr.177.2.1770449. [DOI] [PubMed] [Google Scholar]
- 24.Rumboldt Z, Camacho DL, Lake D, et al. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR. 2006;27:1362–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–91. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- 26.Sener RN. Diffusion MRI in neurofibromatosis type 1: ADC evaluations of the optic pathways, and a comparison with normal individuals. Comput Med Imag Graph. 2001;26:59–64. doi: 10.1016/s0895-6111(01)00014-3. [DOI] [PubMed] [Google Scholar]
- 27.Pauliah M, Saxena V, Haris M, et al. Improved T(1)-weighted dynamic contrast-enhanced MRI to probe microvascularity and heterogeneity of human glioma. Magn Reson Imaging. 2007;25:1292–9. doi: 10.1016/j.mri.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Bajenaru ML, Garbow JR, Perry A, et al. Natural history of neurofibromatosis 1-associated optic nerve glioma in mice. Ann Neurol. 2005;57:119–27. doi: 10.1002/ana.20337. [DOI] [PubMed] [Google Scholar]
- 29.Law M, Oh S, Babb JS, et al. Low Grade Gliomas: Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging – Prediction of Patient Clinical Response. Radiology. 2006;238:658–667. doi: 10.1148/radiol.2382042180. [DOI] [PubMed] [Google Scholar]
- 30.Robinson SP, Howe FA, Griffiths JR, et al. Susceptibility contrast Magnetic Resonance Imaging Determination of Fractional Tumor Blood Volume: A Noninvasive Imaging Biomarker of Response to the Vascular Disrupting Agent ZD6126. Int J Radiation Oncology Biol Phys. 2007;69:872–879. doi: 10.1016/j.ijrobp.2007.06.061. [DOI] [PubMed] [Google Scholar]