SUMMARY
Disease relapse remains a major obstacle to the success of allogeneic hematopoietic stem cell transplantation (HSCT), yet little is known about the relevant prognostic factors after relapse. We studied 1080 patients transplanted between 2004 and 2008, among whom 351 relapsed. The 3-year post-relapse overall survival (prOS) was 19%. Risk factors for mortality after relapse included shorter time to relapse, higher disease risk index at HSCT, myeloablative conditioning, high pre-transplantation co-morbidity index, and graft-versus-host disease (GVHD) occurring prior to relapse. Important prognostic factors did not vary by disease type. Based on this, we could stratify patients into 3 groups, with 3-year prOS of 36%, 14% and 3% (p<0.0001). This score was validated in a historical cohort of 276 patients. Post-relapse donor lymphocyte infusion or repeat HSCT was associated with improved prOS, as was the development of GVHD after relapse. These differences remained significant in models that accounted for other prognostic factors and in landmark analyses of patients who survived at least 2 months from relapse. The results of this study may aid with prognostication and management of patients who relapse after HSCT, as well as motivate the design of clinical trials aimed at relapse prevention or treatment in the higher-risk patients.
INTRODUCTION
Advances in hematopoietic allogeneic stem cell transplantation (HSCT) have improved the safety of the procedure and significantly broadened its applicability. Despite this, disease relapse still represents a major barrier to success for patients transplanted for hematologic malignancies. In fact, relapse is the principal cause of treatment failure for patients undergoing reduced intensity conditioning (RIC) or non-myeloablative (NMA) HSCT. Much work has focused on identifying the factors at the time of HSCT which increase the risk for relapse, and on devising strategies for its prevention and management (1–8), but little is known about the determinants of outcome after relapse. Recent studies, notably from the European Group for Blood and Marrow Transplantation (EBMT), describe the outcomes in subgroups of relapsing patients, especially patients with acute leukemia receiving RIC HSCT (9–11), but no study has yet identified the factors that influence survival after relapse in broader cohorts of patients across multiple disease and transplantation types. This information is necessary both to assess the prognosis of patients who relapse after HSCT and to optimally select patients for clinical trials of post-relapse treatment strategies.
We therefore undertook an observational study of 1080 consecutive adult patients with hematologic malignancies who underwent HSCT at Dana-Farber Cancer Institute/Brigham and Women’s Hospital between 2004 and 2008, with the following goals: (1) elucidating the important prognostic factors after relapse; (2) determining whether those factors are disease-specific or whether the disease risk itself is an independent prognostic factor; and (3) describing the outcome of various post-relapse intervention strategies. Three-hundred and fifty-one patients (33%) relapsed and form the basis of this report. We determined prognostic factors for post-relapse overall survival (prOS), devised a simple risk score to stratify patients into different risk groups for prOS, validated this score in a historical control population, and examined the impact of post-relapse strategies on outcome.
MATERIALS AND METHODS
Patients
We analyzed consecutive adult patients who underwent their first HSCT with myeloablative or reduced intensity conditioning at Dana Farber Cancer Institute/Brigham and Women’s Hospital (DFCI/BWH) within the 4-year period 2004–2008. Patients receiving transplantation for benign hematologic conditions were excluded. The individual medical records of all relapsed patients (defined as progression or relapse of disease any time after HSCT) were examined. Molecular or cytogenetic relapses were not considered as relapse events. We collected data on co-morbidities necessary to calculate the HCT-CI8, when available. Co-morbidity information was extracted retrospectively for patients who underwent HSCT between 2005 and 2007, and prospectively collected for patients who underwent HSCT after 2007. Disease risk index (DRI) was assigned as previously described (12), using the latest available tumor cytogenetics information for patients with acute myeloid leukemia or myelodysplastic syndromes. The DRI accounts for disease risk, including cytogenetics risk for AML and MDS, as well as disease status at the time of transplantation, in general separating patients in complete or partial remission from those with active disease. To validate the post-relapse risk score, we used a historical control cohort of 869 patients who received their first HSCT between 1998 and 2003, among whom 32% relapsed. IRB approval was obtained from the Office for Human Research Studies of the Dana-Farber/Harvard Cancer Center to conduct this study.
Transplantation
Patients were transplanted on a variety of treatment plans and investigational protocols. Myeloablative conditioning (MAC) regimens consisted mostly of cyclophosphamide (3600 mg/m2 or 120 mg/kg) plus total body irradiation (1400 cGy in 7 fractions), or busulfan (12.8 mg/kg intravenously) plus cyclophosphamide (3600 mg/m2). RIC regimens consisted principally of fludarabine (120 mg/m2) plus intravenous low-dose busulfan (3.2–6.4 mg/kg). Patients received bone marrow or peripheral blood stem cells from HLA-matched or mismatched, related or unrelated donors, or double umbilical cord blood (DUCB) units. Graft-versus-host disease (GVHD) prophylaxis consisted mostly of tacrolimus combined with methotrexate, with or without sirolimus. Supportive care for all patients followed institutional standards. The practice at our center is to attempt immunosuppression (IS) withdrawal in all patients at relapse, except for patients whose condition makes it unlikely that they would survive for more than a few weeks, or for patients for whom the severity and activity of GVHD contraindicates IS withdrawal. Donor lymphocyte infusion (DLI) is generally attempted when patients are IS-free without significant GVHD, with >~20% donor chimerism, and when DLI can be obtained from the donor. In cases where no DLI can be obtained or donor chimerism is too low, patients are considered for repeat HSCT. This practice did not change over the course of the study.
Statistical Analysis
Patient baseline characteristics were reported descriptively. Post-relapse overall survival (prOS) was defined as the time from documentation of relapse or progression to death from any cause, and calculated using the Kaplan-Meier method. Patients who were alive or lost to follow-up were censored at the time last seen alive. The log-rank test was used for comparisons of Kaplan-Meier curves. Potential prognostic factors for OS were examined in the proportional hazards model; in the multivariable models, variables were added by stepwise selection. The variables considered are detailed in Table 4. The proportional hazards assumption for each variable of interest was tested and interaction terms were examined. The linearity assumption for continuous variables was examined using restricted cubic spline estimates of the relationship between the continuous variable and log relative hazard and the cutoff points of these variables were based on the change of the log relative hazards. All p-values are two-sided with a significance level of 0.05. The c-statistic (13) was used to compare model fit using the Hmisc package in R. In order to build a risk score, points were assigned roughly following the hazard ratio for prOS in the multivariable model. The only exception was for high/very high DRI which was assigned an integral number of points to keep the score simple. All calculations were done using SAS 9.3 (SAS Institute Inc, Cary, NC), and R version 2.13.2 (the CRAN project).
RESULTS
Patients
Among the 1080 studied patients, 351 (33%) relapsed at a median time of 4.5 (range, 0–59) months after HSCT. Their characteristics are listed in Table 1. The median age was 52 (range, 19–71) years. Most had intermediate or high risk disease by DRI. Two-thirds had received a RIC HSCT. Seventy-two percent were on immunosuppression at the time of relapse (51% for GVHD prophylaxis and 21% for GVHD treatment). In all, 35% had had GVHD prior to the time of relapse.
Table 1.
Baseline patient characteristics
| Variable | N (%)a |
|---|---|
| Number of patients | 351 |
| Age (years) (median, range) | 52 (19–71) |
| Gender | |
| Male | 192 (55) |
| Female | 159 )45) |
| Disease | |
| ALL | 31 (9) |
| AMLb | 145 (41) |
| Favorable cytogenetics | 3 (1) |
| Intermediate cytogenetics | 97 (28) |
| Adverse cytogenetics | 33 (9) |
| Cytogenetics not available | 12 (3) |
| CLL | 20 (6) |
| CML | 8 (2) |
| Hodgkin lymphoma | 28 (8) |
| MDSc | 43 (12) |
| Intermediate cytogenetics | 22 (6) |
| Adverse cytogenetics | 17 (5) |
| Cytogenetics not available | 4 (1) |
| Multiple myeloma | 24 (7) |
| Myeloproliferative Neoplasms | 11 (3) |
| Non-Hodgkin lymphoma | 41 (12) |
| Stage at HSCT | |
| CRd | 134 (38) |
| PR | 75 (21) |
| Induction Failure | 49 (14) |
| Active Relapsed | 55 (16) |
| Untreated | 38 (11) |
| Disease Risk Indexe | |
| Low | 24 (7) |
| Intermediate | 188 (54) |
| High | 126 (36) |
| Very high | 13 (4) |
| HCT-CIf | |
| 0 | 78 (35) |
| 1–2 | 73 (33) |
| 3+ | 70 (32) |
| HSCT performed on protocol | |
| Yes | 122 (35) |
| No | 229 (65) |
| Donor match | |
| MRD | 152 (43) |
| Non-MRD | 199 (57) |
| MUD | 145 (41) |
| MM | 54 (15) |
| Mismatched URD | 51 (15) |
| Mismatched relative | 3 (1) |
| Graft source | |
| PBg | 306 (87) |
| BM | 16 (5) |
| UCB | 29 (8) |
| Conditioning | |
| Myeloablative | 117 (33) |
| Reduced intensity | 234 (67) |
| GVHD prophylaxis | |
| CnI + Mtx | 84 (24) |
| CnI + Siro +/− Mtx | 236 (67) |
| TCD/Other | 31 (9) |
| CMV serostatush | |
| Recipient or donor + | 223 (64) |
| Gender matchingi | |
| Female to male | 81 (23) |
| Male to female | 77 (22) |
| Female to female | 81 (23) |
| Male to male | 11 (32) |
| Year of HSCT (median, range) | 2006 (2004–2008) |
| Months from HSCT to relapse | |
| Median (range) | 4 (0–59) |
| 0–3 months | 118 (34) |
| 3–6 months | 93 (27) |
| 6–24 months | 118 (34) |
| >24 months | 22 (6) |
| Pre-relapse immune status | |
| On immunosuppression at relapse | 252 (72) |
| For GVHD prophylaxis | 179 (51) |
| For GVHD treatment | 73 (21) |
| Any prior GVHD | 123 (35) |
| Prior acute GVHD | 76 (22) |
| Prior chronic GVHD | 60 (17) |
| Post-relapse treatment | |
| Chemotherapy/Radiotherapy | 193 (55) |
| Any immune manipulation | 259 (74) |
| Immunosuppression withdrawal | 216 (62) |
| Donor lymphocyte infusion | 88 (25) |
| Repeat HSCT | 32 (9) |
| Months of follow-up for survivors following relapse (median, range) | 39 (5–90) |
Percentages may not add to 100 because of rounding
Classified according to Armand et al.(17)
Classified according to Armand et al.(18)
CR includes CML in chronic phase; Active relapse includes CML in advanced or blast phase.
Classified according to Armand et al.
Classified according to Sorror et al.; data available on 221 patients; percentages are given relative to patients with available data only.
Including patients who received combined BM and PB.
Data missing on 11 patients.
Data missing on 4 patients.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; NHL, Non-Hodgkin lymphoma;; CR, complete remission; PR, partial remission; HCT-CI; HCT Comorbidity index; MRD, matched related donor; MUD, matched unrelated donor; MM, mismatched donor; PB, peripheral blood; BM, bone marrow; UCB, umbilical cord blood; GVHD, graft versus host disease; CnI, calcineurin inhibitor; MTX, methotrexate; Siro, sirolimus; TCD, T-cell depletion; CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplantation.
Prognostic Factors for Post-Relapse Survival
Table 2 shows the results of univariable and multivariable analyses for prOS among the 351 relapsed patients. In univariable analyses, variables associated with inferior prOS were higher DRI, shorter time to relapse (TTR), myeloablative conditioning, and HCT-CI of 3 or above. The 3-year prOS for patients who received myeloablative conditioning was 10%, compared to 23% for those who received a RIC HSCT (p=0.002). In addition, being on immunosuppression at the time of relapse was associated with worse prOS. As TTR was the most important prognostic factor, we examined its association with other important baseline variables. A shorter TTR was associated with higher DRI, older age, and RIC. We compared the prognostic value of the DRI with that of two other possible classification schemes: myeloid versus lymphoid, and the low-risk/high-risk system used in a recent Blood and Marrow Transplant Clinical Trials Network (BMT CTN) trial (14). The c-statistic was highest for the Disease Risk Index, which we therefore retained as the risk stratification scheme for further analyses.
Table 2.
Univariable and multivariable analyses for overall survival
| Variable | Univariable | Multivariablea | ||
|---|---|---|---|---|
| HR | p | HR | p | |
| Disease Risk Index | ||||
| Low | Ref | Ref | ||
| Intermediate | 1.8 | 0.044 | 2.1 | 0.062 |
| High/Very High | 3.0 | 0.0002 | 2.6 | 0.017 |
| Time to Relapse | ||||
| 0–3 months | 2.6 | <0.0001 | 3.7 | <0.0001 |
| 3–6 months | 1.6 | 0.004 | 1.8 | 0.003 |
| 6–24 months | Ref | Ref | ||
| ≥24 months | 0.7 | 0.2 | 0.4 | 0.043 |
| Conditioning | ||||
| Myeloablative | Ref | Ref | ||
| Reduced intensity | 0.7 | 0.002 | 0.6 | 0.0001 |
| HCT-CIa | ||||
| 0–2 points | Ref | Ref | ||
| 3+ points | 1.8 | 0.0001 | 1.4 | 0.024 |
| Prior GVHD | ||||
| Nob | Ref | Ref | ||
| Yes | 1.1 | 0.4 | 1.9 | 0.0005 |
| On immunosuppression at relapse | ||||
| No | Ref | |||
| Yes | 1.8 | <0.0001 | ||
| Age | ||||
| <50 | Ref | |||
| ≥50 | 1.2 | 0.2 | ||
| Male | 0.9 | 0.5 | ||
| Gender mismatching | 1.0 | 0.9 | ||
| CMV seropositive donor or recipient | 1.0 | 0.8 | ||
| Donor type | ||||
| MRD | Ref | |||
| MUD | 1.1 | 0.5 | ||
| Mismatched | 1.1 | 0.8 | ||
| Graft source | ||||
| PB | Ref | |||
| BM | 1.2 | 0.5 | ||
| UCB | 0.8 | 0.3 | ||
| HSCT on protocol | ||||
| No | Ref | |||
| Yes | 0.8 | 0.2 | ||
| GVHD prophylaxis | ||||
| CnI + Mtx | Ref | |||
| CnI + Siro +/− Mtx | 0.9 | 0.2 | ||
| TCD/Other | 0.8 | 0.3 | ||
| Year of HSCT | ||||
| 2004–2005 | Ref | |||
| 2006–2008 | 1.0 | 0.8 | ||
Model built for only the 221 patients with available data. The same variables were selected if HCT-CI was not included and all 351 patients were included (not shown).
Including patients with a history of GVHD without any active disease and off systemic immunosuppression at the time of relapse.
Abbreviations are as in Table 1.
In multivariable analyses, the same factors remained significant except for being on immunosuppression at the time of relapse (hazard ratio for mortality [HR]=0.8, p=0.4); instead, the occurrence of GVHD (acute or chronic) prior to relapse was associated with significantly inferior prOS in the multivariable models. This discrepancy can be explained by the strong association between being on immunosuppression, history of GVHD, and time of relapse, the latter of which remained very strongly associated with prOS in the multivariable models. Patients who relapsed earlier were more likely to be on immunosuppression at the time of relapse (95% of patients who relapsed within 3 months were still on immunosuppression, compared with 87% of those who relapsed within 3–6 months, 47% of those who relapsed within 6–24 months and 18% of those who relapsed after 2 years, p<0.0001). Conversely, patients who relapsed earlier were less likely to have had prior or active GVHD at the time of relapse (15% among those with time to relapse <3 months, 27% for 3–6 months, 53% for 6–24 months and 77% for >24 months, p<0.0001), which likely explains why GVHD was not significant in the univariable models, even when only grade 3 or 4 acute GVHD was considered. GVHD was an adverse factor for prOS in the multivariable models regardless of the type of GVHD (acute versus chronic), or whether the GVHD was active or not at the time of relapse. There were no relevant significant interactions between prognostic variables in the multivariable models.
We obtained similar results in multivariable models built separately for MAC and RIC patients, although the impact of the DRI was less pronounced among patients who received myeloablative conditioning. Because RIC was associated with shorter time to relapse in this cohort, which could inflate the apparent benefit of RIC in the prOS multivariable model, we also checked models that did not include TTR; RIC remained significantly associated with superior prOS even in those models (HR for mortality associated with RIC = 0.7, p=0.022).
Prognostic Score for Post-Relapse OS
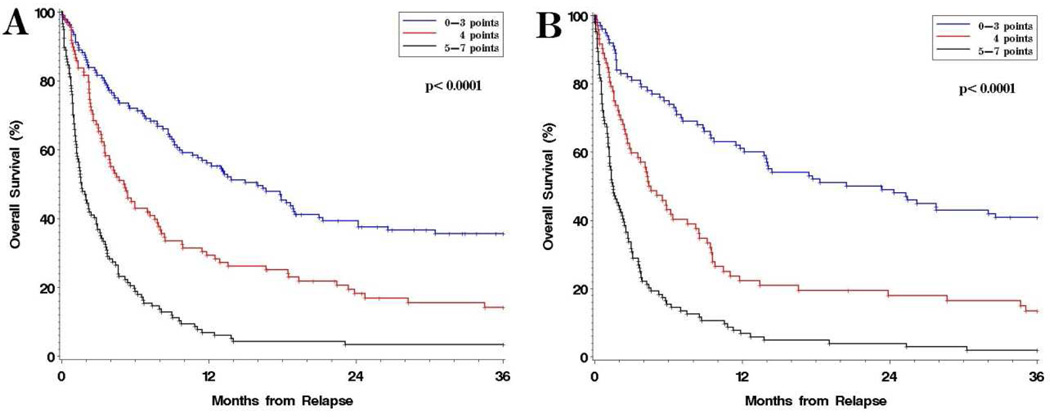
We constructed a simple score using the significant factors established above (Table 3). Because the HR associated with a high HCT-CI was only 1.4, and because the addition of the HCT-CI to the model did not noticeably improve model fit (c-statistic of model with HCT-CI 0.680 versus 0.675 for a model without HCT-CI), it was not included in the score. The score can be calculated by summing the points for a given patient among TTR (1 point for 6–24 months, 2 for 3–6 months, 3 for <3 months), DRI (1 point for intermediate, 2 for high/very high index), conditioning intensity (1 point for myeloablative), and prior GVHD (1 point). This score stratified the cohort into 3 groups with very different prOS (Figure 1A and Table 3). Patients with 0–3 points had a 3-year prOS of 36%; patients with 4 points a 3-year prOS of 14%; and patients with 4–7 points a 3-year prOS of 3%. Among the low-risk group, 46 patients (13% of the total population) had fewer than 3 risk factors, and a 3-year prOS of 51%. Because this is a surprisingly high survival rate, we examined the characteristics of this group: 65% were 50 years or older; only 4% had relapsed within 6 months of HSCT, while 67% had relapsed within 6–24 months of transplant and 28% had relapsed more than 24 months after HSCT; 35% had low DRI, 65% had intermediate DRI, and none had high or very high DRI; 89% had received a RIC transplant; 48% had ALL, AML or MDS; and 50% were transplanted in complete remission.
Table 3.
Outcomes by risk group.
- DRI (0 points for low, 1 point for intermediate, 2 points for high or very high)
- TTR (0 points for >24 months, 1 point for 6–24 months, 2 points for 3–6 months, 3 points for <3 months)
- Myeloablative conditioning (1 point)
- Prior GVHD (1 point)
| Number of Risk Factors | # of patients (%) Among 351 patients |
3-year OS (95CI) p<0.0001 |
Hazard Ratio for mortality |
|---|---|---|---|
| 0–3 | 136 (39%) | 36% (27–44) | Ref |
| 4 | 98 (28%) | 14% (8–22) | 2.0 (p<0.0001) |
| 5–7 | 117 (33%) | 3% (1–8) | 4.3 (p<0.0001) |
Figure 1. Post-relapse overall survival (prOS) stratified by risk score.
A. prOS in the main cohort of 351 patients.; B. prOS in a historical cohort of 276 patients.
In order to validate this score, we considered a cohort of 869 patients transplanted between 1998 and 2003, among whom 276 had relapsed. The 3-year prOS in this control cohort was 19%, as it was in the training cohort (p=0.9). The median time to relapse was 5.6 months, and the median age was 46. Fewer patients in the historical control had high/very high risk disease by DRI (33% versus 40%), fewer underwent RIC HSCT (37% versus 67%), and fewer relapsed without prior GVHD (42% versus 65%), compared with the main study cohort. Nonetheless, the prOS among the 3 risk groups in the historical cohort were very similar to that of the training cohort (Figure 1B).
Disease-specific risk factors
The foregoing models were all built in a cohort of patients that is heterogeneous with respect to disease, which was accounted for through the use of a general risk index (the DRI). However, it is possible that post-relapse risk factors depend on the specific disease type. We therefore also built multivariable models on specific subgroups, including patients with myeloid diseases (AML, MDS, myeloproliferative diseases or CML), and patients with lymphoid diseases (ALL, Hodgkin and non-Hodgkin lymphoma, and multiple myeloma). We also built a model only for patients with AML and one for all non-AML patients, since AML was the dominant group in our cohort. For those analyses we combined the training and testing sets, with a total of 627 relapsed patients; we therefore omitted HCT-CI from the models, since the testing set patients did not have this data available. In all 4 models, the same factors remained significant, i.e., TTR, conditioning intensity, and prior GVHD. The only difference was that advanced age (≥50) was also an adverse risk factor in the group of patients with myeloid disease, and in the entire non-AML cohort. The difference in model fit for non-AML patients with or without age was small. The difference in the Akaike information criterion (a measure of multivariable model fit) from inclusion of age in the model was 4, compared to 17 for conditioning intensity, 18 for DRI, 23 for prior GVHD, and 86 for TTR. We therefore did not incorporate age in the score, and more importantly confirmed that a non disease-specific scoring system that incorporates a general disease risk term is appropriate for estimating the prognosis of relapsed patients.
Post-Relapse Treatment
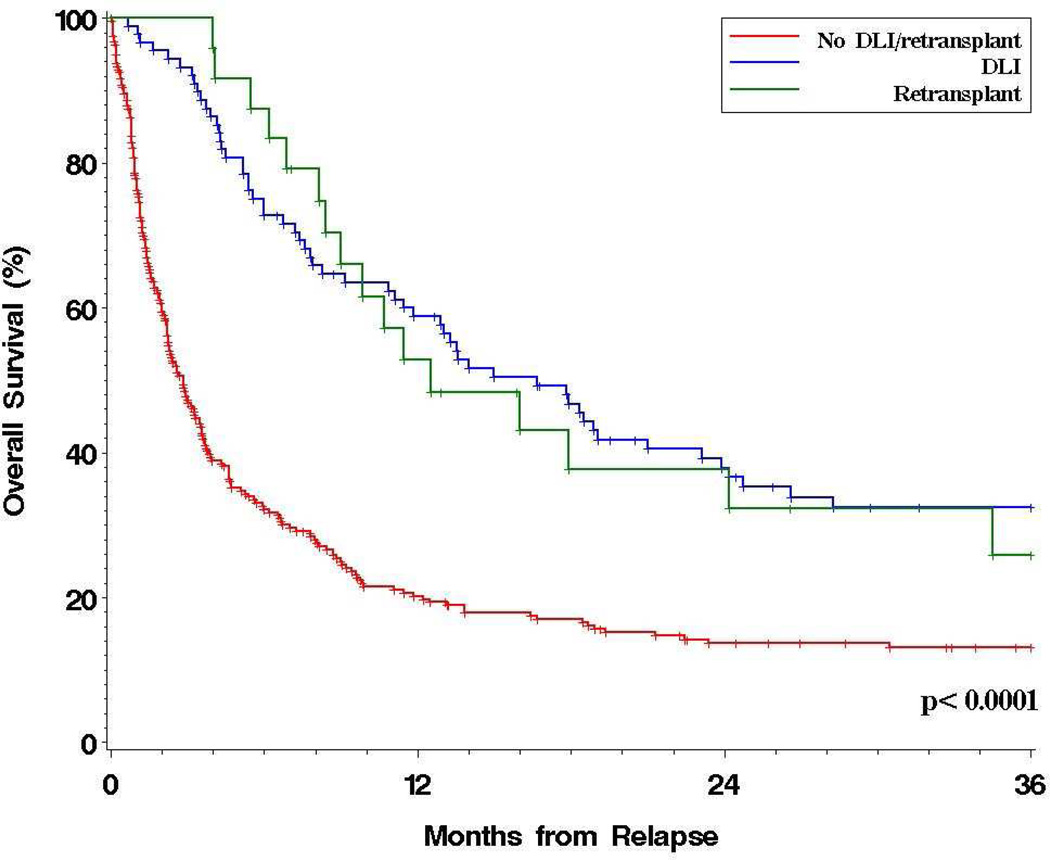
We examined the outcomes of the 351 relapsed patients based on the treatment received. Fifty-five percent received cytotoxic therapy after relapse (chemotherapy or radiotherapy); 74% received immune manipulation in the form of immunosuppression withdrawal (62%), DLI (25%), or repeat HSCT (9%). Withdrawal of immunosuppression at the time of relapse did not appear to confer a benefit in univariable or multivariable models. In contrast, receipt of DLI was associated with significantly better prOS (HR=0.4, p<0.0001), as was receipt of a second HSCT (HR=0.4, p=0.0003). The 3-year prOS appeared superior in patients who received DLI or repeat HSCT compared with those who did not (31% versus 13%, p<0.0001); the outcomes after administration of DLI were similar to those after repeat HSCT (Figure 2). Patients who received cytotoxic therapy after relapse also appeared to have a better prOS (p=0.0005); however, the benefit was only apparent early (1-year prOS was 39% versus 24%, while 3-year prOS was 19% versus 18%), and was not apparent in multivariable models, possibly because receipt of cytotoxic therapy was associated with a longer TTR. In exploratory analyses, the apparent benefit of DLI or repeat HSCT extended across disease groups except for CLL and ALL, but fewer than 10 patients with each of those diseases received DLI/HSCT.
Figure 2. Post-relapse overall survival stratified by post-relapse treatment.
Naturally, there is a selection bias associated with the use of post-relapse therapies. Indeed, patients with shorter TTR or with higher HCT-CI were less likely to receive DLI/HSCT (p=0.002 and p=0.0008, respectively). Despite this, the benefit of DLI/HSCT persisted in multivariable models in which all prognostic factors were added (HR for mortality associated with DLI/HSCT=0.4, p<0.0001; in this model all other factors remained significant). Interestingly, the benefit of DLI/HSCT was evident whether or not patients had a history of GVHD by the time of relapse. In contrast, receipt of cytotoxic therapy was not associated with a significant benefit when DLI/HSCT and the other risk factors in the relapse score were included in the model (HR=0.8, p=0.10). Because patients who die early after relapse are less likely to receive DLI/HSCT, we conducted a landmark analysis considering only patients who were alive 2 months after relapse. In this group, both the prognostic score and DLI/HSCT remained associated with improved survival in a multivariable model (HR of DLI/HSCT 0.6, p=0.001).
We also examined the association between GVHD that occurred after relapse and prOS. Among the 351 relapsed patients, 85 (24%) developed acute or chronic GVHD after relapse. Twenty-one of these patients (25%) had received DLI/HSCT. We built multivariable models for prOS that included TTR, DRI, conditioning intensity, pre-relapse GVHD, and receipt of DLI/HSCT, with the addition of post-relapse GVHD considered as a time-dependent variable. All factors were significant: the HR for mortality of DLI/HSCT (compared to neither) was 0.4 (p<0.0001), and the HR of post-relapse GVHD (as a time-dependent variable) was 0.5 (p<0.0001). In those models, there was no apparent benefit for patients who received DLI/HSCT compared with those who developed GVHD but never received DLI/HSCT (HR=0.7, p=0.08). These findings were unchanged in landmark analyses of patients who survived at least 2 months past relapse.
DISCUSSION
It is generally accepted that the prognosis for patients who relapse after HSCT is very poor. Nonetheless, by analyzing a large cohort of patients with different diseases and transplant types, we were able to distinguish important prognostic factors in this population. The size of our observational cohort allowed us to untangle some of the confounding issues in this setting, such as time-to-relapse confounding the association between immunosuppression at the time of relapse and prOS, as well as to adjust for the well-recognized difference in post-relapse outcome between different diseases. In this respect it is notable that the major prognostic factors for post-relapse survival do not appear to depend on the disease type, and that a generalized disease risk index seems adequate to capture disease-specific features. Because of the retrospective nature of this study, we could not analyze whether the presence minimal residual disease (MRD), which is becoming increasingly recognized as an important prognostic factor across many hematologic malignancies, significantly affects pre-relapse or post-relapse outcomes.
TTR was a very strong prognostic factor in our cohort, which is a recurrent finding in most stem cell transplantation outcome studies. TTR and disease risk likely both capture the aggressiveness of the tumor and its relative susceptibility to the graft-versus-tumor (GVT) effect. Similarly, since MAC is usually associated with lower disease relapse compared to RIC transplantation (15, 16), patients who relapse after MAC may have more aggressive or immuno-refractory disease, which could explain their worse prognosis in our study. In our analyses, the worse prognosis of patients relapsing after MAC HSCT was not solely due to the longer time-to-relapse in those patients. The HCT-CI was also weakly prognostic in our study, which may reflect the ability of patients to tolerate further therapy. It is possible that HCT-CI calculated at the time of relapse would be a better prognostic marker than HCT-CI at the time of HSCT, but this data was not available in our cohort.
The negative effect of prior GVHD on prOS is noteworthy. It appeared to be independent of the type of GVHD or its status (remitted or active) at the time of relapse; however, it is possible that with a larger cohort differences between those subsets or differences between patients with different diseases (with different GVT susceptibility) might emerge. The association between prior GVHD and prOS could be explained by the fact that patients who relapse after having experienced GVHD may have disease that is less sensitive to the GVT effect than patients who relapse without ever having developed GVHD, in whom the GVT effect may not yet have been tested. It is important to note that the adverse prognosis of prior GVHD on prOS does not contradict the observation that GVHD may be protective after HSCT. Rather, it implies that patients who still relapse after developing GVHD fare worse than those who relapse without having developed GVHD. This is also consistent with the apparent beneficial effect of post-relapse GVHD, regardless of whether or not this is brought about by DLI/HSCT. Patients who are treated after relapse with immunosuppression taper, DLI or HSCT are likely those who have had no or minimal active GVHD at the time of relapse, and in whom a GVL effect may yet be obtained with immune manipulation. It is also notable that immune manipulation (DLI/HSCT) was associated with a clear prOS benefit even in patients with prior GVHD; therefore, while a history of prior GVHD may portend a worse outcome, it does not necessarily contraindicate the use of immunotherapy. Here again the selection bias inherent in the use of DLI/HSCT must be acknowledged, making it unlikely that those therapies were used in patients with severe active GVHD at the time of relapse. Even though no single prognostic variable eliminated the benefit of DLI/HSCT, their combination did, in that patients with a high risk score (6–7 points) had a 3-year prOS of 0% with or without DLI/HSCT. Naturally, the quantitative difference in prOS provided by different post-relapse therapies may well differ between diseases, and our study was not designed or powered to detect those differences.
While we did not find a prognostic relevance to being on IS at the time of relapse in multivariable model, this issue is quite complex. This variable is inextricably related to the time of relapse which appears in our study to be the dominant driver of outcome. Aside from that, patients who relapse on IS without prior GVHD could be expected to do better as they may not yet have been exposed to a full blown GVL effect; whereas patients who relapse on IS with prior GVHD may be expected to do worse given that they have relapsed after evidence of immunologic graft activity. Similarly, although we did not find a benefit to immune withdrawal per se, patients the appearance of GVHD after immune withdrawal without additional immunotherapy was associated with a survival advantage in time-dependent multivariable models, suggesting that immune withdrawal alone, if it is associated with new-onset GVHD, may have a beneficial impact similar to that of DLI/HSCT.
Even though the relapse prognostic score proposed here showed good stratification ability in an independent cohort of patients transplanted earlier than those in the present cohort, our study is still based on a single institution’s experience, and it would be useful to validate this in other centers. In the meantime, we hope that the results of this study help clinicians to assess the prognosis of patients who relapse after HSCT; to target higher-risk patients for investigational interventions for relapse treatment or prevention; and perhaps –again with the caveat of the unavoidable selection bias— to inform the use of immunotherapy in this setting.
ACKNOWLEDGMENTS
P.A. is supported by a career development award from the Conquer Cancer/ASCO Foundation. This work was also supported by P01 CA142106 and the Jock and Bunny Adams Research Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
G.T. designed the study, collected data, and wrote the manuscript.
H.T.K. designed the study, analyzed the data, and wrote the manuscript.
C.C. collected data, and edited the manuscript.
V.T.H. collected data, and edited the manuscript.
J.K. collected data, and edited the manuscript.
E.P.A. collected data, and edited the manuscript.
J.H.A. collected data, and edited the manuscript.
R.J.S. designed the study, collected data, and edited the manuscript.
P.A. designed the study, collected data, analyzed the data, and wrote the manuscript.
All authors approved the submitted version of the manuscript.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Alyea EP, DeAngelo DJ, Moldrem J, et al. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2010;16:1037–1069. doi: 10.1016/j.bbmt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop MR, Alyea EP, 3rd, Cairo MS, et al. National Cancer Institute's First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant. 2011;17:443–454. doi: 10.1016/j.bbmt.2010.12.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairo MS, Jordan CT, Maley CC, et al. NCI first International Workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the biological considerations of hematological relapse following allogeneic stem cell transplantation unrelated to graft-versus-tumor effects: state of the science. Biol Blood Marrow Transplant. 2010;16:709–728. doi: 10.1016/j.bbmt.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroger N, Bacher U, Bader P, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on Disease-Specific Methods and Strategies for Monitoring Relapse following Allogeneic Stem Cell Transplantation. Part I: Methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2010;16:1187–1211. doi: 10.1016/j.bbmt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller JS, Warren EH, van den Brink MR, et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol Blood Marrow Transplant. 2010;16:565–586. doi: 10.1016/j.bbmt.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavletic SZ, Kumar S, Mohty M, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:871–890. doi: 10.1016/j.bbmt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter DL, Alyea EP, Antin JH, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:1467–1503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oran B, de Lima M. Prevention and treatment of acute myeloid leukemia relapse after allogeneic stem cell transplantation. Curr Opin Hematol. 2011;18:388–394. doi: 10.1097/MOH.0b013e32834b6158. [DOI] [PubMed] [Google Scholar]
- 9.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 10.Spyridonidis A, Labopin M, Schmid C, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 11.Pollyea DA, Artz AS, Stock W, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40:1027–1032. doi: 10.1038/sj.bmt.1705852. [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–913. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 14.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 16.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armand P, Kim HT, Zhang MJ, et al. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2012;18:280–288. doi: 10.1016/j.bbmt.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, Deeg HJ, Kim HT, et al. Multicenter validation study of a transplantation-specific cytogenetics grouping scheme for patients with myelodysplastic syndromes. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]