Abstract
Eosinophilia–myalgia syndrome (EMS) is characterized by subacute onset of myalgias and peripheral eosinophilia, followed by chronic neuropathy and skin induration. An epidemic of EMS in 1989 was linked to L-tryptophan consumption originating from a single source. Following the Food and Drug Administration (FDA) ban on the sale of L-tryptophan, the incidence of EMS declined rapidly. Moreover, no new cases have been published since the FDA ban was lifted in 2005. We report the clinical, histopathological and immunogenetic features of a new case of L-tryptophan-associated EMS along with evidence of activated transforming growth factor-ß and interleukin-4 signaling in the lesional skin.
Introduction
Eosinophilia–myalgia syndrome (EMS), a chronic multisystem disorder first recognized in 1989, is characterized by subacute onset of myalgias and peripheral eosinophilia associated with chronic muscle, nerve, fascia, and skin involvement.1,2 This apparently new disease occurred in an epidemic outbreak that over a 6-month period affected 1,500 individuals and was associated with over 30 deaths.3 The Centers for Disease Control (CDC) proposed a surveillance case definition of EMS that included peripheral blood eosinophilia and severe generalized myalgias.4 Toxico-epidemiologic studies linked EMS to L-tryptophan (L-TRP) containing dietary supplements manufactured using genetically-engineered bacteria.5 Initial analysis of implicated L-TRP revealed an impurity that was identified as 1'1'-ethylidenebis[tryptophan] (EBT).6 Removal of L-TRP from the market was followed by swift resolution of the EMS epidemic.
The pathobiological basis of EMS remains unknown. Epidemiologic studies tracing implicated L-TRP to a single manufacturer, and quantitative analyses of EBT suffered from methodological limitations. EBT was just one of more than 60 minor impurities detected in implicated L-TRP, six of which were associated with EMS.7 One of these, 3-(phenylamino)alanine (PAA), shares chemical properties with 3-(N-phenylamino)-1,2-propanediol (PAP) that was implicated in the 1981 Spanish toxic oil syndrome (TOS) epidemic linked to consumption of aniline-denatured rapeseed oil.8 Although rodents exposed to implicated L-TRP developed EMS-like histopathologic changes, similar changes were also observed in rodents that received L-TRP without EBT.9 Moreover, some cases of EMS had no known L-TRP exposure, while in others the disease antedated the epidemic.10 Even among epidemic L-TRP users, the estimated attack rate of EMS was less than 2%.11
The FDA lifted the ban on L-TRP in 2005, allowing L-TRP-containing products to again be available as dietary supplements. Since that time it has been recognized that some individuals possess putative immunogenetic risk and protective factors for EMS.12 We report the first new case, to our knowledge, of L-TRP-associated EMS occurring since the reintroduction of L-TRP.
Case report
A 44 year-old woman started using L-TRP (1500 mg daily) for insomnia in January 2009. She had undergone duodenal switch weight loss surgery in 2007 for obesity, and during the ensuing 12 months lost 140 lbs of weight. She consumed L-TRP, sold under the trade name “Uber rest” distributed by Heartland Products, which she purchased from a Chicago apothecary. In addition to L-TRP, she also used L-carnitine, flax oil and alpha-lipoic acid, but took no medications. Within three weeks of starting L-TRP, she developed swelling in the upper and lower extremities followed by severe myalgia and weakness. During the ensuing four weeks, she noted progressive skin induration in her upper and lower extremities but sparing the fingers and toes. Physical examination in August 2009 was remarkable for woody induration of the forearms and legs (Fig. 1A). Proximal extremity palpation provoked myalgias. Manual muscle testing revealed Medical Research Council grade 4/5 strength in proximal upper and lower extremity muscles with normal distal strength. Cranial nerve, sensory, and reflex examinations were normal.
Figure 1. Clinical presentation of EMS.
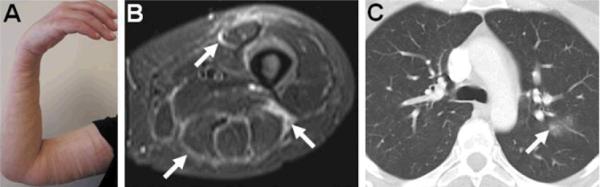
A. Skin induration and edema in the left arm is shown and was also present in both upper and lower extremities with palpably thickened fascia. B. Axial magnetic resonance imaging STIR sequence images of the thigh revealed high signal intensity in the fascia surrounding the anterior and posterior compartment muscles (arrows). C. High resolution computed chest tomography showing ground glass changes and air space opacity in the left lung (arrow).
Laboratory investigation demonstrated an elevated white blood cell (WBC) count with 24% eosinophils (absolute count 1600 cells/mm3). Serum aldolase was mildly elevated, but creatine kinase (CK) was normal. Results of the following test results were negative or normal: anti-nuclear, anti-double-stranded DNA, anti-neutrophil cytoplasmic, anti-Ro, anti-La, anti-Scl-70 and anti-CCP antibodies, rheumatoid factor, erythrocyte sedimentation rate, serum immunofixation, thyroid stimulating hormone, and albumin. Nerve conduction studies (NCS) revealed normal motor and sensory responses in the upper and lower extremities. Electromyography (EMG) of proximal muscles showed evidence in the form of small amplitude, short duration, polyphasic motor unit potentials with early recruitment to support the presence of a myopathic process. Magnetic resonance imaging (MRI) of the thighs showed muscle and fascial edema (Fig. 1B). High resolution computed tomography (HRCT) of the chest revealed ground glass opacification in the left lung (Fig. 1C). Biopsies of the skin, vastus lateralis fascia, and muscle taken prior to initiation of immunotherapy showed histopathological changes characteristic of EMS (Fig. 2). Immunostaining with antibodies to the eosinophil degranulation products major basic protein (MBP) and eosinophil-derived neurotoxin (EDN) were strongly positive (Figs. 2 H,I). HLA-DR typing revealed that the patient had the HLA-DRB1*04 allele, a risk factor among L-TRP users for the development of EMS (OR 3.9, 95% CI 1.1–16.4), and the absence of any of the reported protective HLA alleles.12
Figure 2. Tissue inflammation, fibrosis, and eosinophil degranulation.
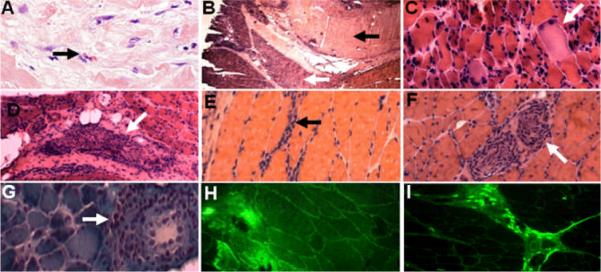
Skin (A) and muscle (B – I) biopsy specimens stained with hematoxylin and eosin (A – F), Gomori trichrome (G), and antibodies to eosinophil derived neurotoxin (EDN) (H) and major basic protein (MBP) (I). A. Skin fibrosis with dermal collagen accumulation and eosinophil infiltration (arrow). B. Fascia and muscle specimen demonstrates dense fibrosis of epidermal fascia (dark arrow) and atrophic muscle fascicles (white arrow). C. Higher power magnification reveals scattered necrotic fibers (arrow). D – G. Inflammatory infiltrate (arrows) in the perimysial connective tissue (D), endomysial connective tissue (E), around intramuscular nerve (F), and around intramuscular blood vessels (G). Eosinophils were only rarely detected. H, I. Immunofluorescence analysis. EDN (H) and MBP (I) staining is present in the perimysial and endomysial connective tissue, as well as around intramuscular blood vessel and nerve (not shown).
Although the L-TRP consumed prior to the onset of symptoms was no longer available, multiple contemporaneous samples from the same batch were subjected to high-performance liquid chromatography (HPLC) with mass spectrometry.13 No impurities were detected at greater than 10 ppm in any of the samples. In particular, the hydroxylated tryptophan derivative peak C (EBT), peak E (PAA), 2-(2-hydroxy indoline)-Trp peak FF, or 2-(3-indolyl)-L-tryptophan were not detected (data not shown).
With informed consent approved by Northwestern University's Institutional Review Board, a single 3 mm punch skin biopsy was taken from the clinically affected forearm for microarray analysis. At the time of the biopsy, the patient was taking prednisone (50 mg/d) and mycophenolate mofetil (1000 mg/bid). Skin biopsy homogenization occurred in a Qiagen TissueLyser and total RNA was isolated using RNeasy for fibrosis tissue kits with automation in the Qiagen QIAcube. Labeled RNA from the lesional skin was competitively hybridized to 44,000-element Agilent oligonucleotide microarrays representing 33,000 known and novel human genes.14 Samples were run in triplicate and compared to three independent anatomical site and age-matched healthy female control skin biopsies. Raw microarray data has been deposited into the National Center for Biotechnology Information's Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo); accession number: GSE26934. Genes differentially expressed between healthy controls and the EMS skin biopsies were identified using Significance Analysis of Microarrays (SAM). A comparison of gene expression in the EMS skin biopsy to that of the controls (Fig. 3) identified 343 genes whose expression varied significantly (SAM, false discovery rate [FDR] ≤ 0.64%). Genes showing increased expression in the EMS biopsy included multiple collagens (Collagen V, Collagen VI, Collagen VIII, Collagen XII), extracellular matrix molecules (fibronectin, tenascin C, COMP1, elastin, lumican and Cyr 61), lysyl oxidase, PAI-1, thrombospondin, cadherin 11, TIMP-1, and multiple integrins and cadherin, as well as genes implicated in TGF-ß, IL-4 and IL-13 signaling.15
Figure 3. Genes differentially expressed in EMS skin biopsy relative to healthy controls.
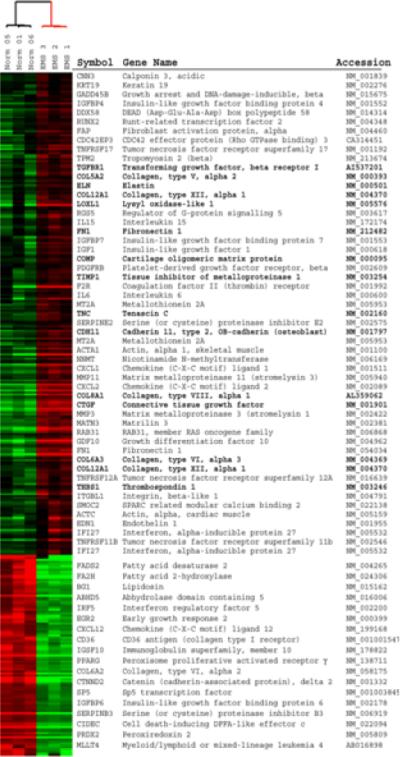
Gene expression was measured in an EMS skin biopsy in triplicate and in three independent sex-, age- and anatomical site-matched normal control skin biopsies. All samples were taken from the left forearm. 343 differentially expressed genes were selected using Significance Analysis of Microarrays (FDR = 0.64%). Select genes are indicated and those discussed in the text are in bolded. The full figure with all gene names is available as supplemental Figure S3.
To determine if the TGF-β, IL-4 and IL-13 pathways, which are each implicated in fibrosis, were significantly differentially regulated in the EMS skin biopsy, a Gene Set Enrichment Analysis was performed using experimentally defined gene sets.15 All measured genes were ranked by their correlation to an idealized distinction between the healthy phenotype and the EMS phenotype (Supplemental Figs. S1A and S2A). We then compared the ranked pathway gene lists to those of randomly selected genes (Supplemental Figs. S1B and S2B). Each pathway received an Enrichment Score (ES), which measures the statistical significance of the differential expression. To determine if there is a significant association with disease phenotype, a p-value for the ES was calculated by permuting the array labels and the ES was recomputed (Supplemental Figure S1C and S2C). To estimate the FDR we compared the ES of the experimentally determined pathway to that found with randomly selected gene subsets of the same size (Supplemental Figure S1D and S2D). This assesses how likely we are to find an ES comparable to the true ES if the pathway is actually unrelated to the disease. Both the TGF-β (ES 0.3729) and IL-4 (ES 0.2428) pathways were significantly up-regulated with p-values and FDRs <0.001. The IL-13 pathway (ES 0.2370) was also increased, with a low FDR (0.0032), but high p-value (p-value = 0.125). This suggests that while the IL-13 pathway showed evidence for up-regulation, it may not be responsible for the distinction between EMS skin and healthy control skin.
Although treatment with prednisone and mycophenolate resulted in modest improvement in proximal muscle strength and myalgia along with prompt resolution of peripheral blood eosinophilia and ground glass opacifications seen on CT scans, skin induration and neuropathy progressed. Repeat NCS revealed reduced or absent sensory and motor response amplitudes compatible with a new length-dependent axonal sensorimotor polyneuropathy affecting the upper and lower extremities. Addition of methotrexate (20 mg weekly for 5 months) followed by daily injections of anakinra for 3 months failed to yield further improvement in myalgia, neuropathy, or skin induration.
Discussion
We report a patient with EMS whose illness occurred shortly after she started taking L-TRP. To our knowledge, this represents the first case of L-TRP-associated EMS since reintroduction of LTRP as a dietary supplement. The patient was taking additional supplements concurrently with L-TRP. Although we did not subject these supplements to impurity analysis with high-performance liquid chromatography and mass spectrometry, they, unlike L-TRP, have not been associated with EMS or EMS-like disorders. Therefore, while we cannot unequivocally exclude the possibility that these additional supplements contributed to the development of EMS in this case, we think it is unlikely. The patient displayed the hallmark clinical, electrophysiologic and histopathologic features of EMS. She satisfied both the CDC case definition developed for surveillance in 19892,4,16 as well as the revised EMS criteria proposed in 2001.17 Importantly, the revised diagnostic criteria were shown to be 97% specific for EMS. In identifying new cases of EMS, it is important to keep in mind that the non-validated and imprecise 1989 CDC case definition was developed as a means of surveillance, and was not intended to be applied for clinical diagnosis or epidemiologic study classification. The revised diagnostic criteria proposed by an expert panel in 2001 acknowledge the importance of broader exclusionary conditions.
Dermal fibrosis and perimysial inflammatory cell infiltration along with muscle fiber atrophy and necrosis were prominent histopathological findings. Intact eosinophils were observed in the dermis, but only rarely in the muscle and fascia. However, the eosinophil-derived proteins MBP and EDN showed strong immunostaining in the fascia, perimysium, and endomysium as well as around intramuscular blood vessels and nerves. Paucity of intact eosinophils with prominent eosinophil degranulation was a characteristic finding in epidemic EMS, suggesting an important role for eosinophils in pathogenesis.16 Immunogenetic analysis showed the presence of a risk allele for EMS among L-TRP users, with absence of the protective immunogenetic markers.12 The composition of the L-TRP consumed by the patient could not be ascertained; however analysis of available L-TRP obtained contemporaneously with the batch that was actually consumed by the patient failed to detect previously implicated impurities.
The pathogenesis of EMS is thought to involve exposure to certain preparations of L-TRP in a genetically-susceptible host that trigger acute inflammation and eosinophil activation and degranulation with resulting chronic tissue fibrosis.18 However, because EMS has been reported in individuals who have never consumed L-TRP, it is likely that xenobiotics other than L-TRP preparations can also trigger a similar immune response. Fibrosis of the skin and subcutaneous fascia were pathological hallmarks of epidemic EMS. The deep dermis showed evidence of local fibroblast activation, increased expression of Type I and Type VI collagens, and elevated levels of TGF-ß.19 Gene expression profiling of the lesional skin in the present case showed up-regulation of multiple genes associated with extracellular matrix production and remodeling, as well as activated TGF-ß and IL-4 signaling, suggesting that TGF-ß and IL-4 may drive the fibrotic response.
Although the 1989 EMS epidemic was linked to ingestion of L-TRP originating from a single Japanese manufacturer, the precise identity of the causative agent of EMS remains unknown. Once the FDA issued an import alert in March 1990, L-TRP was effectively removed from the US market, and the EMS epidemic resolved. In 2005, the FDA lifted the L-TRP import alert. A search of the FDA passive surveillance system called the Center for Food Safety & Applied Nutrition Adverse Event Reporting System (CAERS) for single-ingredient tryptophan product adverse event reports from January 1, 2003 to August 31, 2010 yielded 2 reports of possible EMS (1 in 2005 and 1 in 2008) and 2 reports of myalgias (1 in 2005 and 1 in 2008). No adverse event reports for tryptophan sold under the “Uber Rest” or “Heartland Products” brand names were indentified, but the manufacturer of the tryptophan in all these cases is unknown. It is important to remember that interpretation of results from the CAERS database poses several limitations that are inherent with passive surveillance reporting systems. In particular, only a small fraction of adverse events are generally reported and reports may contain errors of omission or errors of commission.
The present report documents the first clear-cut case of L-TRP-associated EMS since reintroduction of L-TRP. Although analysis of contemporaneous L-TRP batches failed to detect EBT or PAA, we cannot be certain that the L-TRP actually consumed by this patient was free of impurities. The present case should raise awareness for the potential for occurrence of EMS among L-TRP users two decades after the epidemic outbreak, and prompt continued efforts to identify the causative agent for this devastating syndrome.
Supplementary Material
343 differentially expressed genes were selected using Significance Analysis of Microarrays (FDR = 0.64%). All genes are shown. This figure is best viewed in PDF format so that the zoom function can be used to read gene names.
Acknowledgments
We are grateful to Drs. Thomas Lucas (Northwestern University), Kristin Leiferman (University of Utah), Gerald Gleich (University of Utah), Mark Gourley (NIAMS) and Lori Love (National Institute of Environmental Health) for helpful suggestions and technical assistance, and to Drs. Robert Mozersky and Karen Brugge (FDA) for providing CAERS reports.
Grant support: Supported by grants from the NIH (AR42309 to JV, and NIEHS Z01 ES101074 DIR to FWM). M.L.W, T.A.W and J.M.M were supported by grants from the Scleroderma Research Foundation (SRF), Arthritis Foundation, U01AR055063 (NIAMS), R01HG004499 (NHGRI), R01CA077485 (NCI). J.M.M. received support from the NCI (R25CA134286).
References
- 1.Hertzman PA, Blevins WL, Mayer J, Greenfield B, Ting M, Gleich GJ. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N Engl J Med. 1990;322:869–73. doi: 10.1056/NEJM199003293221301. [DOI] [PubMed] [Google Scholar]
- 2.Silver RM, Heyes MP, Maize JC, Quearry B, Vionnet-Fuasset M, Sternberg EM. Scleroderma, fasciitis, and eosinophilia associated with the ingestion of tryptophan. N Engl J Med. 1990;322:874–81. doi: 10.1056/NEJM199003293221302. [DOI] [PubMed] [Google Scholar]
- 3.Swygert LA, Back EE, Auerbach SB, Sewell LE, Falk H. Eosinophilia-myalgia syndrome: mortality data from the US national surveillance system. J Rheumatol. 1993;20:1711–17. [PubMed] [Google Scholar]
- 4.Eosinophilia-myalgia syndrome and L-tryptophan-containing products--New Mexico, Minnesota, Oregon, and New York, 1989. Centers for Disease Control (CDC) MMWR Morb Mortal Wkly Rep. 1989;38:785–8. [PubMed] [Google Scholar]
- 5.Belongia EA, Hedberg CW, Gleich GJ, et al. An investigation of the cause of the eosinophilia-myalgia syndrome associated with tryptophan use. N Engl J Med. 1990;323:357–65. doi: 10.1056/NEJM199008093230601. [DOI] [PubMed] [Google Scholar]
- 6.Mayeno AN, Lin F, Foote CS, et al. Characterization of “peak E,” a novel amino acid associated with eosinophilia-myalgia syndrome. Science. 1990;250:1707–8. doi: 10.1126/science.2270484. [DOI] [PubMed] [Google Scholar]
- 7.Hill RH, Jr, Caudill SP, Philen RM, et al. Contaminants in L-tryptophan associated with eosinophilia myalgia syndrome. Arch Environ Contam Toxicol. 1993;25:134–42. doi: 10.1007/BF00230724. [DOI] [PubMed] [Google Scholar]
- 8.Mayeno AN, Belongia EA, Lin F, Lundy SK, Gleich GJ. 3-(Phenylamino)alanine, a novel aniline-derived amino acid associated with the eosinophilia-myalgia syndrome: a link to the toxic oil syndrome? Mayo Clin Proc. 1992;67:1134–9. doi: 10.1016/s0025-6196(12)61142-2. [DOI] [PubMed] [Google Scholar]
- 9.Love LA, Rader JI, Crofford LJ, et al. Pathological and immunological effects of ingesting L-tryptophan and EBT in Lewis rats. J Clin Invest. 1993;91:804–11. doi: 10.1172/JCI116300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan EA, Staehling N, Philen RM. Eosinophilia-myalgia syndrome among the non-L-tryptophan users and pre-epidemic cases. J Rheumatol. 1996;23:1784–7. [PubMed] [Google Scholar]
- 11.Henning KJ, Jean-Baptiste E, Singh T, Hill RH, Friedman SM. Eosinophilia-myalgia syndrome in patients ingesting a single source of L-tryptophan. J Rheumatol. 1993;20:273–8. [PubMed] [Google Scholar]
- 12.Okada S, Kamb ML, Pandey JP, Philen RM, Love LA, Miller FW. Immunogenetic risk and protective factors for the development of L-tryptophan-associated eosinophilia-myalgia syndrome and associated symptoms. Arthritis Rheum. 2009;61:1305–11. doi: 10.1002/art.24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson BL, Johnson KL, Tomlinson AJ, Gleich GJ, Naylor S. On-line HPLC-tandem mass spectrometry structural characterization of case-associated contaminants of L-tryptophan implicated with the onset of eosinophilia myalgia syndrome. Toxicol Lett. 1998;99:139–50. doi: 10.1016/s0378-4274(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 14.Milano A, Pendergrass SA, Sargent JL, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE. 2008;3(7):e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sargent JL, Milano A, Bhattacharyya S, et al. A TGFbeta-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol. 2010;130:694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin RW, Duffy J, Engel AG, et al. The clinical spectrum of the eosinophilia-myalgia syndrome associated with L-tryptophan ingestion. Clinical features in 20 patients and aspects of pathophysiology. Ann Intern Med. 1990;113:124–34. doi: 10.7326/0003-4819-113-2-124. [DOI] [PubMed] [Google Scholar]
- 17.Hertzman PA, Clauw DJ, Duffy J, Medsger TA, Jr, Feinstein AR. Rigorous new approach to constructing a gold standard for validating new diagnostic criteria, as exemplified by the eosinophilia-myalgia syndrome. Arch Intern Med. 2001;161:2301–6. doi: 10.1001/archinte.161.19.2301. [DOI] [PubMed] [Google Scholar]
- 18.Varga J, Uitto J, Jimenez SA. The cause and pathogenesis of the eosinophilia-myalgia syndrome. Ann Intern Med. 1992;116:140–7. doi: 10.7326/0003-4819-116-2-140. [DOI] [PubMed] [Google Scholar]
- 19.Peltonen J, Varga J, Sollberg S, Uitto J, Jimenez SA. Elevated expression of the genes for transforming growth factor-beta 1 and type VI collagen in diffuse fasciitis associated with the eosinophilia-myalgia syndrome. J Invest Dermatol. 1991;96:20–5. doi: 10.1111/1523-1747.ep12514683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
343 differentially expressed genes were selected using Significance Analysis of Microarrays (FDR = 0.64%). All genes are shown. This figure is best viewed in PDF format so that the zoom function can be used to read gene names.


