Abstract
Zinc oxide nanosheet is assessed as a selective adsorbent for the detection and adsorption of cadmium using simple eco-friendly extraction method. Pure zinc oxide nanosheet powders were characterized using field emission scanning electron microscopy, energy dispersive spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and Fourier transform infrared spectroscopy. The zinc oxide nanosheets were applied to different metal ions, including Cd(II), Cu(II), Hg(II), La(III), Mn(II), Pb(II), Pd(II), and Y(III). Zinc oxide nanosheets were found to be selective for cadmium among these metal ions when determined by inductively coupled plasma-optical emission spectrometry. Moreover, adsorption isotherm data provided that the adsorption process was mainly monolayer on zinc oxide nanosheets.
Keywords: Zinc oxide, Nanosheets, Metal uptake, Cd(II), Environmental applications
Background
With the development of industry and economy, environmental problem becomes more and more serious day by day [1-3]. Due to certain man-made activities, numerous hazardous compounds and heavy metals are introduced into the environment which is a concerning matter for monitoring agencies and regulation authorities [4-6]. Among these pollutants, toxic metals are the most sever pollutants and main environmental threat which instigate too many serious public health and cost-cutting problems [7,8]. Cadmium is known to be as highly toxic as probably carcinogenic for humans and is listed as the sixth most poisonous substance jeopardizing human health. Cadmium is introduced into water bodies from different sources, for example, smelting, metal plating, cadmium-nickel batteries, phosphate fertilizers, mining, pigments, stabilizers, alloy industries, and sewage sludge. The harmful effects of Cd(II) involve a number of acute and chronic disorders such as gastrointestinal irritation, vomiting, abdominal pain, diarrhea, renal damage, emphysema, hypertension, and testicular atrophy [9,10]. Therefore, separation and determination of Cd(III) in different matrices have continued to be of import.
In addition, the development of simple, rapid, and efficient methods has become of interest for monitoring metal ions in the environment. Several analytical methods have been applied to analyze metal ions in aqueous solutions [7,8]. However, analytical methods cannot directly measure metal ions, in particular at ultra-trace concentration, in aqueous systems due to the lack of sensitivity and selectivity of these methods. Therefore, an efficient separation procedure is usually required prior to the determination of noble metals for sensitive, accurate, and interference-free determination of noble metals.
Several analytical methods have been utilized for separation of analyte of interest, including liquid/liquid extraction, ion exchange, coprecipitation, cloud-point extraction, and solid-phase extraction (SPE) [11,12]. SPE is considered to be one of the most powerful techniques because it minimizes solvent usage and exposure, disposal costs, and extraction time for sample preparation. Several adsorbents have appeared because of the popularity of SPE for selective extraction of analytes such polymers, silica, carbon nanotube, etc. [7,8].
Nanoscience and technology have attracted significant attention due to its potential application in various fields and especially in metal ion adsorption [13,14]. ZnO, a versatile material, emerges as a challenging prospect in the field of nanotechnology. Nanosized ZnO has been widely used as a catalyst [14], gas sensor [15,16], active filler for rubber and plastic, ultraviolet (UV) absorber in cosmetics, and antivirus agent in coating [17,18] and has more potential application in building functional electronic devices with special architecture and distinctive optoelectronic properties.
In this investigation, we synthesized ZnO nanosheets by stirring method and characterized by X-ray diffraction patterns (XRD), field emission scanning electron microscopy (FESEM), Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), and energy dispersive spectroscopy (EDS). ZnO nanosheets were applied to investigate their utility and the analytical efficiency as adsorbent on the selectivity and adsorption capacity of Cd(II). The selectivity of ZnO nanosheets toward eight metal ions, including Cd(II), Cu(II), Hg(II), La(III), Mn(II), Pb(II), Pd(II), and Y(III), was investigated in order to study the effectiveness of ZnO nanosheets on the adsorption of selected metal ions. Based on the selectivity study, the ZnO nanosheets attained the highest selectivity toward Cd(II). Static uptake capacity of ZnO nanosheets for Cd(II) was found to be 97.36 mg g−1. Adsorption isotherm data of Cd(II) with ZnO nanosheets were well fit with the Langmuir adsorption isotherm, strongly confirming that the adsorption process was mainly monolayer on homogeneous adsorbent surfaces.
Methods
Chemicals and reagents
Zinc nitrate, sodium hydroxide, mercuric nitrate, lanthanum nitrate, palladium nitrate, and yttrium nitrate were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Stock standard solutions of 1,000 mgL−1 Cd(II), Cu(II), Mn(II), and Pb(II) were also obtained from Sigma-Aldrich. All reagents used were of high purity and of spectral purity grade, and doubly distilled deionized water was used throughout.
Preparation of ZnO nanosheets
ZnO nanosheets were synthesized by thermal stirring method in which 0.1 M of zinc nitrate aqueous solution was titrated with 0.1 M solution of NaOH till pH reached above 10 and stirred at 70°C for overnight. White product was washed and dried. The dried product was calcined at 450°C for 4 h.
Possible growth mechanism of ZnO nanosheets
The formations of ZnO might take place by following probable chemical reactions:
Initially, Zn(NO3)2 and NaOH undergo hydrolysis in water and produce Zn2+ and OH− which later produce Zn(OH)2. The heating causes the dehydration of Zn(OH)2 (orthorhombic structure) to ZnO (monoclinic structure). During the growth process (Figure 1), first ZnO nucleus growth takes place which then aggregates and produces ZnO nanoparticles by Ostwald ripening. Nanoparticles crystallize and aggregate with each other through Van der Waals forces and hydrogen bonding and give ZnO nanosheets.
Figure 1.
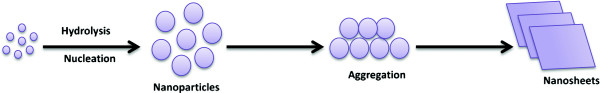
Schematic representation of ZnO nanosheets growth mechanism.
Characterization
The morphology of the synthesized product was studied at 15 kV using a JEOL Scanning Electron Microscope (JSM-7600 F, Akishima-shi, Japan). XRD was taken with a computer-controlled RINT 2000, Rigaku diffractometer (Shibuya-ku, Japan) using the Ni-filtered Cu-Kα radiation (λ = 0.15405 nm). FT-IR spectrum was recorded in the range of 400 to 4,000 cm−1 on PerkinElmer (spectrum 100, Waltham, MA, USA) FT-IR spectrometer. XPS spectrum was recorded in the range of 0 to 1,350 eV by using Thermo Scientific K-Alpha KA1066 spectrometer (Schwerte, Germany).
Samples preparation and procedure for metal uptake study
Stock solutions of Cd(II), Cu(II), Hg(II), La(III), Mn(II), Pb(II), Pd(II), and Y(III) were prepared in 18.2 MΩ·cm distilled deionized water and stored in the dark at 4°C. For studying the selectivity of ZnO nanosheets toward metal ions, standard solutions of 2 mg L−1 of each metal ion were prepared and adjusted to pH value of 5.0 with a buffered aqueous solution (0.1 mol L−1 CH3COOH/CH3COONa). Standard solutions were adjusted at pH value of 5.0 in order to avoid the formation of suspended gelatinous lanthanides hydroxides with buffer solutions at pH values beyond 5.0. Each standard solution was individually mixed with 25 mg of the ZnO nanosheets. For investigation of the Cd(II) adsorption capacity, standard solutions of 0, 5, 10, 15, 20, 25, 30, 50, 75, 125, and 150 mg L−1 were prepared as above, adjusted to pH value of 5.0 and individually mixed with 25 mg ZnO nanosheets. All mixtures were mechanically shaken for 1 h at room temperature.
Inductively coupled plasma-optical emission spectrometry (ICP-OES) measurements were acquired by use of a Perkin Elmer ICP-OES model Optima 4100 DV (Waltham, MA, USA). The ICP-OES instrument was optimized daily before measurement and operated as recommended by the manufacturers. The ICP-OES spectrometer was used with following parameters: FR power, 1,300 kW; frequency, 27.12 MHz; demountable quartz torch, Ar/Ar/Ar; plasma gas (Ar) flow, 15.0 L min−1; auxiliary gas (Ar) flow, 0.2 L min−1; nebulizer gas (Ar) flow, 0.8 L min−1; nebulizer pressure, 2.4 bars; glass spray chamber according to Scott (Ryton), sample pump flow rate, 1.5 mL min−1; integration time, 3 s; replicates, 3; wavelength range of monochromator, 165 to 460 nm. Selected metal ions were measured at wavelengths of 228.80 nm for Cd(II), 327.39 nm for Cu(II), 194.17 nm for Hg(II), 348.90 nm for La(III), 275.61 nm for Mn(II), 220.35 nm for Pb(II), 340.46 nm for Pd(II), and 361.10 nm for Y(III).
Results and discussion
Structural characterization
FESEM was used for the general structural characterization of the calcined products and demonstrated in Figure 2. It is clear from the images that the synthesized product is grown in high density. The calcined product possess sheet like structure and average thickness of the grown nanosheets is approximately 10 nm.
Figure 2.
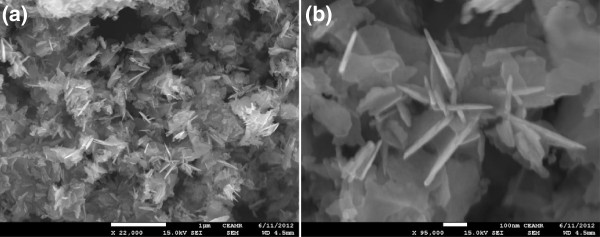
Typical (a) low-magnification and (b) high-resolution FESEM images of ZnO nanosheets.
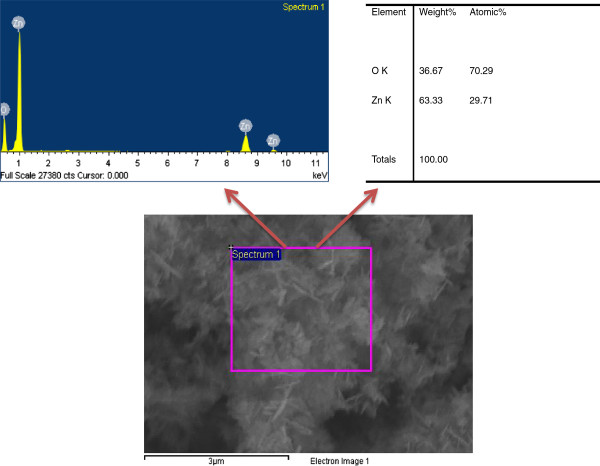
The chemical composition of the synthesized nanosheets was studied by energy dispersive spectroscopy (EDS), and the results were depicted in Figure 3. The EDS did not show any element except zinc and oxygen which suggest that the synthesized nanosheets are pure ZnO.
Figure 3.
Typical EDS spectrum of ZnO nanosheets.
To check the crystallinity of the synthesized ZnO nanosheets, X-ray diffraction technique was used, and results are shown in Figure 4a. A series of characteristic peaks were obtained which are responsible for wurtzite hexagonal ZnO. X-ray diffraction confirms that the obtained nanomaterial is pure ZnO with wurtzite hexagonal phase [19].
Figure 4.
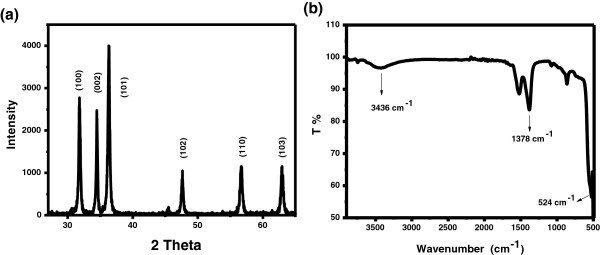
Typical (a) XRD pattern and (b) FT-IR spectrum of ZnO nanosheets.
Figure 4b shows the typical FT-IR spectra of the ZnO nanomaterial measured in the range of 420 to 4,000 cm−1. The appearance of a sharp band at 495.18 cm−1 in the FT-IR spectrum is indication of ZnO nanosheets which is due to Zn-O stretching vibration [19]. The absorption peaks at 3,477 and 1,612 cm−1 are caused by the O-H stretching of the absorbed water molecules from the environment [20].
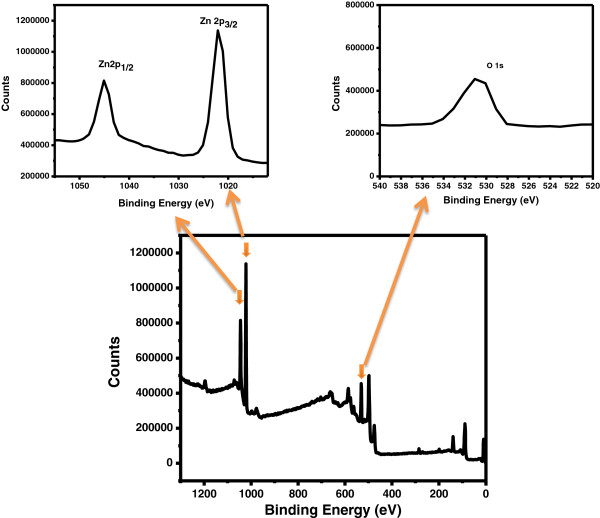
XPS was analyzed for synthesized nanosheets and described in Figure 5. XPS peaks for calcined nanosheets observed at 531.1 for O 1 s, 1,022.0 eV for Zn 2p3/2, and 1,045.0 eV for Zn 2p1/2 which are comparable to the literature values [21] which suggest pure ZnO nanosheets.
Figure 5.
Typical XPS spectrum of ZnO nanosheets.
Metal uptake
Selectivity study of ZnO nanosheets
Selectivity of the newly synthesized ZnO nanosheets toward different metal ions was investigated based on the basis of calculated distribution coefficient of ZnO nanosheets. The distribution coefficient (Kd) can be obtained from the following equation [22]:
| (1) |
where Co and Ce refer to the initial and final concentrations before and after filtration with ZnO nanosheets, respectively, V is the volume (mL), and m is the weight of ZnO nanosheets (g). Distribution coefficient values of all metal ions investigated in this study are summarized in Table 1. It can be clearly observed from Table 1 that the greatest distribution coefficient value was obtained for Cd(II) with ZnO nanosheets in comparison to other metal ions. As can be depicted from Table 1, the amount of Cd(II) was almost all extracted using ZnO nanosheets. Thus, selectivity study results indicated that the newly synthesized ZnO nanosheets were most selective toward Cd(II) among all metal ions. The incorporated donor atom of oxygen, presented in ZnO nanosheets, strongly attained the selective adsorption of ZnO nanosheets toward Cd(II). Based on the above results, the mechanism of adsorption may be electrostatic attraction or chelating mechanism between ZnO nanosheets and Cd(II).
Table 1.
Selectivity study of ZnO nanosheets adsorption toward different metal ions at pH 5.0 and 25°C (N= 5)
| Metal ion | qe(mg g−1) | Kd(mL g−1) |
|---|---|---|
| Cd(II) |
1.98 |
89,909.09 |
| Mn(II) |
1.53 |
3,237.29 |
| Cu(II) |
1.41 |
2,412.97 |
| Y(III) |
1.33 |
1,985.07 |
| Pb(II) |
1.25 |
1,666.67 |
| La(III) |
1.08 |
1,166.85 |
| Hg(II) |
0.73 |
568.63 |
| Pd(II) | 0.35 | 209.19 |
Static adsorption capacity
For determination of the static uptake capacity of Cd(II) on ZnO nanosheet adsorbent, 25 mL Cd(II) sample solutions with different concentrations (0 to 150 mg L−1) were adjusted to pH 5.0 and individually mixed with 25 mg ZnO nanosheets (Figure 6). These mixtures were mechanically shaken for 1 h at room temperature. Static adsorption capacity was obtained using Equation 2 as follows:
Figure 6.
Schematic view of Cd(II) adsorption process on ZnO nanosheets.
| (2) |
where qe represents the adsorbed metal ion by the adsorbent (mg g−1), Co and Ce are the initial and equilibrium concentrations of metal ion in solution (mg L−1), respectively, V is the volume (L), and m is the weight of adsorbent (g). Figure 7a displays the metal uptake capacity of ZnO nanosheets for Cd(II) obtained from the experiment of adsorption isotherm. Adsorption capacity of ZnO nanosheets for Cd(II) was determined to be 97.36 mg g−1. Reported adsorption capacity in this study was found to be comparable with those previously reported for Cd(II) (4.92 [23], 9.39 [24], 84.30 [25], 57.90 [26], 95.20 [27], 123.65 mg g−1[28]) in other studies. In comparison with the adsorption capacity of ZnO nanosheets toward Cd(II), uptake capacities of other nanostructures for Cd(II) were also previously reported. For example, the adsorption capacity of Cd(II) on MnO2 functionalized multi-walled carbon nanotubes was determined to be 41.60 mg g−1 by Luo et al. [29]. In addition, adsorption capacities of nano B2O3/TiO2 composite material and nanocrystallite hydroxyapatite for Cd(II) were previously evaluated and reported to be 49.00 [30] and 142.86 mg g−1[31]. As discussed above, the adsorption capacity of nanostructures for Cd(II) may vary. However, ZnO nanosheets possess the most important property in its high efficiency and selectivity for Cd(II). Thus, the high selectivity of ZnO nanosheets enables the method for accurate and precise determination of Cd(II) in complex matrices.
Figure 7.
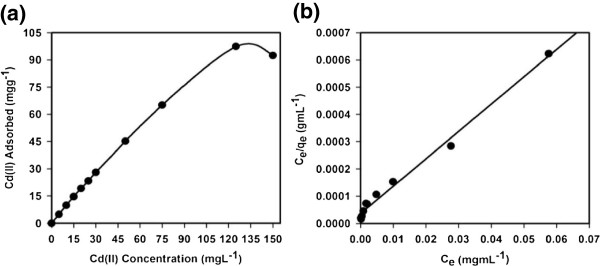
Adsorption profile of Cd(II) (a) and Langmuir adsorption isotherm model of Cd(II) adsorption (b). On 25 mg of ZnO nanosheets at pH 5.0 and 25°C. Adsorption experiments were obtained at different concentrations (0 to 150 mg L−1) under static conditions.
Adsorption isotherm models
Experimental equilibrium adsorption data were analyzed using different models in order to develop an equation that accurately represents the results. Langmuir equation is based on an assumption of a monolayer adsorption onto a completely homogeneous surface with a finite number of identical sites and a negligible interaction between the adsorbed molecules. The Langmuir adsorption isotherm model is governed by the following relation [7]:
| (3) |
where Ce corresponds to the equilibrium concentrations of Cd(II) ion in solution (mg mL−1) and qe is the adsorbed metal ion by the adsorbate (mg g−1). The symbols Qo and b refer to Langmuir constants related to adsorption capacity (mg g−1) and energy of adsorption (L mg−1), respectively. These constants can be determined from a linear plot of Ce/qe against Ce with a slope and intercept equal to 1/Qo and 1/Qob, respectively. Moreover, the essential characteristics of Langmuir adsorption isotherm can be represented in terms of a dimensionless constant separation factor or equilibrium parameter, RL, which is defined as RL = 1/(1 + bCo), where b is the Langmuir constant (indicates the nature of adsorption and the shape of the isotherm); Co the initial concentration of the analyte. The RL value indicates the type of the isotherm, and RL values between 0 and 1 represent a favorable adsorption [8].
The experimental isotherm data were best fit with the Langmuir equation (Figure 7b) based on the least square fit, confirming the validity of Langmuir adsorption isotherm model for the adsorption process. Consequently, adsorption isotherm data suggested that the adsorption process was mainly monolayer on a homogeneous adsorbent surface. Langmuir constants Qo and b are found to be 99.60 mg g−1 and 0.28 L mg−1, respectively. The correlation coefficient obtained from the Langmuir model is found to be R2 = 0.989 for adsorption of Cd(II) on ZnO nanosheets. Moreover, the Cd(II) adsorption capacity (99.60 mg g−1) calculated from Langmuir equation was consistent with that (97.36 mg g−1) of the experimental isotherm study. The RL value of Cd(II) adsorption on the ZnO nanosheets is 0.03, supporting a highly favorable adsorption process based on the Langmuir classical adsorption isotherm model.
Conclusions
ZnO nanosheets were synthesized by low-temperature eco-friendly method and evaluated their efficiency for selective adsorption and determination of Cd(II) in aqueous solution. Reasonable static adsorption capacities of 97.36 mg g−1 for ZnO nanosheet adsorbent were achieved for Cd(II) in aqueous solution. Adsorption isotherm data of Cd(II) were well fit with the Langmuir classical adsorption isotherm model. Thus, the method may play an important role for using it as an effective approach for a selective adsorption and determination of Cd(II) in complex matrices for a range of several applications.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
SBK and MMR synthesized the ZnO nanosheets, performed structural analyses of the samples, analyzed the experimental results, and contributed to the manuscript preparation. AMA and KAA coordinated the study, analyzed the data, and contributed to the manuscript preparation. HMM carried out the metal ion adsorption study and analyzed the data and contributed to the manuscript preparation. All authors read and approved the final manuscript.
Contributor Information
Sher Bahadar Khan, Email: sbkhan@kau.edu.sa.
Mohammed M Rahman, Email: mmrahman@kau.edu.sa.
Hadi M Marwani, Email: hmarwani@kau.edu.sa.
Abdullah M Asiri, Email: aasiri2@kau.edu.sa.
Khalid A Alamry, Email: kaalamri@kau.edu.sa.
Acknowledgments
This project was funded by the Center of Excellence for Advanced Materials Research (CEAMR), King Abdulaziz University, Jeddah, under grant no. (CEAMR-434-01).
References
- Khan SB, Faisal M, Rahman MM, Jamal A. Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci Tot Environ. 2011;8:2987. doi: 10.1016/j.scitotenv.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Khan SB, Akhtar K, Rahman MM, Asisir AM, Seo J, Alamry KA, Han H. A thermally and mechanically stable eco-friendly nanocomposite for chemical sensor applications. New J Chem. 2012;8:2368. doi: 10.1039/c2nj40549k. [DOI] [Google Scholar]
- Khan SB, Rahman MM, Jang ES, Akhtar K, Han H. Special susceptive aqueous ammonia chemi-sensor: extended applications of novel UV-curable polyurethane-clay nanohybrid. Talanta. 2011;8:1005. doi: 10.1016/j.talanta.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Faisal M, Khan SB, Rahman MM, Jamal A. Synthesis, characterizations, photocatalytic and sensing studies of ZnO nanocapsules. Appl Surf Sci. 2011;8:672. doi: 10.1016/j.apsusc.2011.07.067. [DOI] [Google Scholar]
- Dai G, Liu S, Liang Y, Luo T. Synthesis and enhanced photoelectrocatalytic activity of p–n junction Co3O4/TiO2 nanotube arrays. Appl Surf Sci. 2013;8:157. [Google Scholar]
- Faisal M, Khan SB, Rahman MM, Jamal A, Abdullah MM. Fabrication of ZnO nanoparticles based sensitive methanol sensor and efficient photocatalyst. App Surf Sci. 2012;8:7515. doi: 10.1016/j.apsusc.2012.04.075. [DOI] [Google Scholar]
- Khan SB, Alamry KA, Marwani HM, Asiri AM, Rahman MM. Synthesis and environmental applications of cellulose/ZrO2 nanohybrid as a selective adsorbent for nickel ion. Compos Part B-Eng. 2013;8:253. [Google Scholar]
- Asiri AM, Khan SB, Alamry KA, Marwani HM, Rahman MM. Growth of Mn3O4 on cellulose matrix: nanohybrid as a solid phase adsorbent for trivalent chromium. Appl Surf Sci. 2013;8:539. [Google Scholar]
- Leyva-Ramos R, Rangel-Mendez JR, Mendoza-Barron J, Fuentes-Rubio L, Guerrero-Coronado RM. Adsorption of cadmium(II) from aqueous solution on activated carbon. Water Sci Technol. 1997;8:205. [Google Scholar]
- Ensafi AA, Ghaderi AR. On-line solid phase selective separation and preconcentration of Cd(II) by solid-phase extraction using carbon active modified with methyl thymol blue. J Hazard Mater. 2007;8:319. doi: 10.1016/j.jhazmat.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Khan SB, Marwani HM, Asiri AM, Alamry KA, Al-Youbi AO. Selective determination of gold(III) ion using CuO microsheets as a solid phase adsorbent prior by ICP-OES measurement. Talanta. 2013;8:75. doi: 10.1016/j.talanta.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Khan SB, Marwani HM, Asiri AM, Alamry KA. Selective iron(III) ion uptake using CuO-TiO2 nanostructure by inductively coupled plasma-optical emission spectrometry. Chem Central J. 2012;8:158. doi: 10.1186/1752-153X-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Yi P, Zhu Y, Xu L, Zhang W, Yu W, Qian Y. Preparation of beta-MnO2 nanorods through a gamma-MnOOH precursor route. Mater Res Bull. 2004;8:1641. doi: 10.1016/j.materresbull.2004.05.014. [DOI] [Google Scholar]
- Kamat VP, Huehn R, Nicolaescu R. A sense and shoot approach for photocatalytic degradation of organic contaminants in water. J Phys Chem B. 2002;8:788. doi: 10.1021/jp013602t. [DOI] [Google Scholar]
- Lin HM, Tzeng SJ, Hsiau PJ, Tsai WL. Electrode effects on gas sensing properties of nanocrystalline zinc oxide. Nanostruct Mater. 1998;8:465. doi: 10.1016/S0965-9773(98)00087-7. [DOI] [Google Scholar]
- Xu JQ. Pan QY, Shun YA, Tian ZZ: Grain size control and gas sensing properties of ZnO gas sensor. Sens Actuators B Chem. 2000;8:277. doi: 10.1016/S0925-4005(00)00381-6. [DOI] [Google Scholar]
- Hu ZS. Oskam G, Searson PC: Influence of solvent on the growth of ZnO nanoparticles. J Colloid Interf Sci. 2003;8:454. doi: 10.1016/S0021-9797(03)00205-4. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Lia LH. Preparation and characterization of nanocrystalline zinc oxide by a novel solvothermal oxidation route. J Cryst Growth. 2003;8:184. doi: 10.1016/S0022-0248(02)02495-8. [DOI] [Google Scholar]
- Khan SB, Faisal M, Rahman MM, Jamal A. Low-temperature growth of ZnO nanoparticles: photocatalyst and acetone sensor. Talanta. 2011;8:943. doi: 10.1016/j.talanta.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Faisal M, Khan SB, Rahman MM, Jamal A. Role of ZnO-CeO2 nanostructures as a photo-catalyst and chemi-sensor. J Mater Sci Technol. 2011;8:594. doi: 10.1016/S1005-0302(11)60113-8. [DOI] [Google Scholar]
- Nandi SK, Chakraborty S, Bera MK, Maiti CK. Structural and optical properties of ZnO films grown on silicon and their applications in MOS devices in conjunction with ZrO2 as a gate dielectric. Bull Mater Sci. 2007;8:247. doi: 10.1007/s12034-007-0044-3. [DOI] [Google Scholar]
- Han DM, Fang GZ, Yan XP. Preparation and evaluation of a molecularly imprinted sol–gel material for on-line solid-phase extraction coupled with high performance liquid chromatography for the determination of trace pentachlorophenol in water samples. J Chromatogr A. 2005;8:131–136. doi: 10.1016/j.chroma.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Ho Y, Ofomaja AE. Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem Eng J. 2006;8:117–123. doi: 10.1016/j.bej.2006.02.012. [DOI] [Google Scholar]
- Salem Z, Allia K. Cadmium biosorption on vegetal biomass. Int J Chem React Eng. 2008;8:1–9. [Google Scholar]
- Wang X, Xia S, Chen L, Zhao J, Chovelon J, Nicole J. Biosorption of cadmium(II) and lead(II) ions from aqueous solutions onto dried activated sludge. J Environ Sci. 2006;8:840–844. doi: 10.1016/S1001-0742(06)60002-8. [DOI] [PubMed] [Google Scholar]
- Green-Ruiz C, Rodriguez-Tirado V, Gomez-Gil B. Cadmium and zinc removal from aqueous solutions by Bacillus jeotgali: pH, salinity and temperature effects. Bioresour Technol. 2008;8:3864–3870. doi: 10.1016/j.biortech.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Yu J, Tong MS, Li XB. A simple method to prepare poly(amic acid)-modified biomass for enhancement of lead and cadmium adsorption. Biochem Eng J. 2007;8:126–133. doi: 10.1016/j.bej.2006.10.012. [DOI] [Google Scholar]
- Schiewer S, Patil SB. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour Technol. 2008;8:1896–1903. doi: 10.1016/j.biortech.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Luo C, Wei R, Guo D, Zhang S, Yan S. Adsorption behavior of MnO2 functionalized multi-walled carbon nanotubes for the removal of cadmium from aqueous solutions. Chem Eng J. 2013;8:406–415. [Google Scholar]
- Kalfa OM, Yalçınkaya O, Turker AR. Synthesis of nano B2O3/TiO2 composite material as a new solid phase extractor and its application to preconcentration and separation of cadmium. J Hazard Mater. 2009;8:455–461. doi: 10.1016/j.jhazmat.2008.11.112. [DOI] [PubMed] [Google Scholar]
- Mobasherpour I, Salahi E, Pazouki M. Removal of divalent cadmium cations by means of synthetic nano-crystallite hydroxyapatite. Desalination. 2011;8:142–148. doi: 10.1016/j.desal.2010.08.016. [DOI] [Google Scholar]