Abstract
The management of infections caused by multidrug-resistant Gram-negative bacteria, particularly Pseudomonas aeruginosa, continues to be a significant challenge to clinicians. Ceftolozane/tazobactam is a novel antibacterial and β-lactamase-inhibitor combination that has shown appreciable activity against wild-type Enterobacteriaceae and potent activity against P. aeruginosa. Moreover, ceftolozane/tazobactam has not demonstrated cross-resistance to other antimicrobial classes, particularly those affected by extended-spectrum β-lactamases, AmpC β-lactamase, a loss in porin channels, or the overexpression of efflux pumps in P. aeruginosa. Ceftolozane/tazobactam has completed two Phase II clinical trials in complicated intra-abdominal and complicated urinary tract infections. A Phase III, multicenter, prospective, randomized, open-label study has been initiated to evaluate the safety and efficacy of ceftolozane/tazobactam versus piperacillin/tazobactam for the treatment of ventilator-associated pneumonia. A Medline search of articles from inception to May 2013 and references for selected citations was conducted. Data from abstracts presented at conferences were also appraised. This article reviews the antimicrobial, pharmacokinetic, and pharmacodynamic profile of ceftolozane/tazobactam, and discusses its potential role in therapy.
Keywords: CXA-201, CXA-101, FR264205, Pseudomonas aeruginosa
Introduction
In the past decade, significant attention has been given to research on multidrug-resistant Gram-positive organisms, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and associated treatment modalities.1–3 However, infections caused by resistant Gram-negative bacilli continue to cause significant morbidity and mortality without decline.4–6 A report from the Centers for Disease Control and Prevention estimated 1.7 million health care-associated infections in the US in 2002, with approximately 99,000 associated deaths.7 Emergence and spread of drug-resistant Gram-negative bacteria are particularly concerning.8–11 Health care-associated infections caused by resistant Gram-negative bacteria can lead to additional attributable hospital cost and length of stay of 29.3% and 23.8%, respectively, as reported in a single-center study.12
Pseudomonas aeruginosa is a leading nosocomial Gram-negative pathogen well known for its intrinsic as well as extraordinary ability to develop resistance to various antimicrobial agents. It can cause a wide array of infections, due to its multitude of cell-associated (eg, quorum sensing) and secreted virulence factors (eg, type III secretion system).13–17 Infections caused by P. aeruginosa remain a significant challenge to clinicians, given that therapeutic options are limited to a handful of agents in three major classes: antipseudomonal β-lactams, antipseudomonal fluoroquinolones, and aminoglycosides.18 Further complicating the matter is the rapid emergence of antibiotic-resistant strains of P. aeruginosa. Data from the 2004 National Nosocomial Infections Surveillance System Report summary showed increasing resistance rates to imipenem, quinolones, and ceftazidime.19 More specifically, the Centers for Disease Control and Prevention reported the prevalence of resistance to carbapenems, fluoroquinolones, and antipseudomonal extended-spectrum cephalosporins at 16.0%, 29.6%, and 23.3%, respectively, in a collection of strains that caused health care-associated infections.20 Of greater concern is the spread of multidrug-resistant strains of P. aeruginosa due to combinations of resistance mechanisms, including enzymatic degradation, decreased outer-membrane permeability, and efflux pumps,21,22 as the observed proportion of strains with resistance to three classes of antimicrobial agents was 10%.23 Infections caused by such strains are associated with severe outcomes, including increased mortality, increased length of hospital stay, and poorer functional capacity at discharge.24,25
Ceftolozane/tazobactam (previously referred to as CXA-201) is a novel antibacterial and β-lactamase-inhibitor combination with the potential to meet the challenges of infections caused by multidrug-resistant strains of P. aeruginosa and other resistant Gram-negative bacteria. Ceftolozane has demonstrated increased stability to AmpC β-lactamases,26–28 and is less affected by changes in porin permeability and efflux pumps due to enhanced binding of select penicillin-binding proteins (PBPs).27 The addition of tazobactam in a 2:1 ratio broadens its spectrum of activity against β-lactamase-producing Enterobacteriaceae, including those producing extended-spectrum β-lactamases (ESBLs).26,29 The primary aim of this review is to provide an overview of this novel cephalosporin and β-lactamase-inhibitor combination, and evaluate its place in therapy.
Pharmacology
Chemical structure
Ceftolozane (previously referred to as FR264205 or CXA-101) is a novel oxyimino-aminothiazolyl cephalosporin that was developed via the introduction of amino groups to the 4-position of a 3-amino-2-methylpyrazole cephalosporin (Figure 1). The addition of amino groups to the 4-position of a 3-amino-2-methylpyrazole cephalosporin improved the minimum inhibitory concentration (MIC) values against AmpC β-lactamases.30 Additionally, a conformational restriction of the 4-position substituent on the pyrazolium ring of FR264205 decreased the potential for convulsion-inducing effects.27,30 The addition of tazobactam sodium, empiric formula C10H11N4NaO5S, broadens its activity to include select ESBL-producing organisms (Figure 2).27
Figure 1.
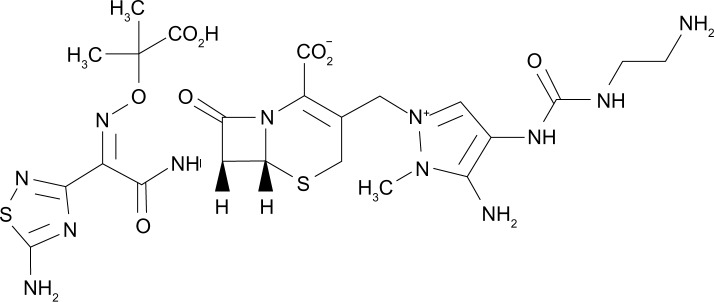
Chemical structure of ceftolozane.
Figure 2.
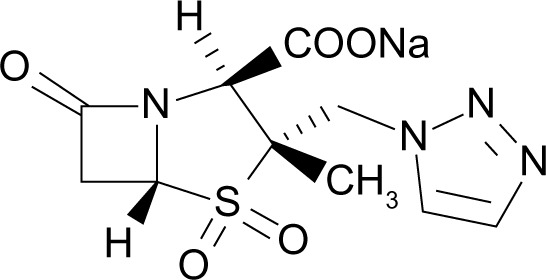
Chemical structure of tazobactam.
Mechanism of action
Ceftolozane/tazobactam is an intravenous cephalosporin combined with a β-lactamase inhibitor in a fixed 2:1 ratio.31,32 Similar to other cephalosporins, ceftolozane inhibits cell-wall synthesis via binding of PBPs. More specifically, ceftolozane exhibits greater affinity for all essential PBPs (1b, 1c, 2, and 3), in comparison to ceftazidime and imipenem.33 Tazobactam is a β-lactam sulfone that inhibits most class A β-lactamases and some class C β-lactamases.34
In vitro activity
Ceftolozane has favorable intrinsic activity against wild-type Enterobacteriaceae, and potent activity against P. aeruginosa, with MICs at four- to 16-fold dilutions below the comparative MICs for ceftazidime. However, similar to other extended-spectrum cephalosporins, ceftolozane is susceptible to enzymatic degradation by ESBLs and carbapenemases.35 The addition of the β-lactamase inhibitor tazobactam potentiates the activity of ceftolozane against select organisms producing degrading enzymes, particularly those exhibiting the ESBL phenotype.26
P. aeruginosa
Data from the Program to Assess Ceftolozane/Tazobactam Susceptibility (PACTS) surveillance study have shown greater ceftolozane/tazobactam potency (two- to eightfold) for 973 clinical isolates of P. aeruginosa in comparison with ceftazidime and cefepime. At an MIC of ≤8 mg/L, ceftolozane/tazobactam inhibited 97.7% of the isolates. Ceftazidime and cefepime inhibited 80.9% and 80.7% of P. aeruginosa isolates using the Clinical and Laboratory Standards Institute (CLSI) breakpoint criteria of 8 mg/L.29 It should also be noted that ceftolozane/tazobactam retained activity against ceftazidime nonsusceptible strains (88.2% had an MIC of ≤8 mg/L), meropenem nonsusceptible strains (89.6% had an MIC of ≤8 mg/L), and strains with concomitant ceftazidime and meropenem nonsusceptibility (78.8% at an MIC of ≤8 mg/L).29 In a study examining the effects of various known resistance mechanisms on ceftolozane/tazobactam, the agent appears to be unaffected by upregulation of efflux pumps or loss of porin channels.27P. aeruginosa strains overexpressing multidrug efflux (Mex)-CD–opioid receptor (Opr)-J and MexEF-OprN resulted in a 16-fold increase in MIC to ciprofloxacin. However, overexpression of these efflux pumps, as well as MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY, in P. aeruginosa did not appear to affect the MIC of ceftolozane in this study.27 Similarly, a 16-fold increase in MIC was observed for imipenem due to membrane impermeability (loss of OprD), while ceftolozane retained its activity against such strains.27 Stability of ceftolozane against porin OprD loss was also reported in other studies.36,37
The activity of ceftolozane/tazobactam against 100 isolates of P. aeruginosa (first and last available isolates) from 50 patients with cystic fibrosis was also evaluated.38 The MIC50 and MIC90 were 0.5 and 2 mg/L, respectively, with 95% of the isolates inhibited at MIC ≤ 8 mg/L. Ceftolozane/tazobactam maintained activity against a high distribution of isolates resistant to select antipseudomonal agents tested (ceftazidime, cefepime, imipenem, meropenem, levofloxacin, tobramycin, and piperacillin/tazobactam). In addition, ceftolozane/tazobactam retained some activity for multidrug resistant strains with MIC50 and MIC90 of 2 and 16 mg/L, respectively. It also conserved activity against 96% of the last isolates from each patient with MIC ≤ 8 mg/L. Cross-resistance from other antipseudomonal agents was not observed.
Enterobacteriaceae
The activity of ceftolozane/tazobactam against selected Gram-negative bacilli is outlined in Tables 1 and 2.29,39 Ceftolozane/tazobactam was tested against 1,244 US clinical isolates of Escherichia coli in the PACTS surveillance study, with 99.3% of E. coli strains inhibited by ceftolozane/tazobactam at the proposed MIC breakpoint of ≤8 mg/L. Comparatively, ceftriaxone and ceftazidime inhibited 87.7% and 91.5% of the isolates using the CLSI breakpoint criteria, respectively.29 Among the 840 strains of Klebsiella spp. tested, 92.1% of strains were inhibited at a ceftolozane/tazobactam MIC of ≤8 mg/L. Using the established CLSI breakpoint, 84.4%, 86.2%, and 90.0% of the strains were inhibited by ceftriaxone, ceftazidime, and cefepime, respectively.
Table 1.
Comparative activity of ceftolozane/tazobactam against selected U.S. clinical Gram-negative bacilli
| MIC50 / MIC90 (mg/L)
| |||||
|---|---|---|---|---|---|
| Organism | Ceftolozane/tazobactam | Ceftazidime | Cefepime | Meropenem | Piperacillin/tazobactam |
| Pseudomonas aeruginosa | 1/4 | 2/32 | 4/16 | 0.5/8 | 8/>64 |
| Escherichia coli | 0.25/0.5 | 0.12/2 | ≤0.5/4 | ≤0.06/≤0.06 | 2/8 |
| Klebsiella spp. | 0.25/2 | 0.12/32 | ≤0.5/8 | ≤0.06/≤0.06 | 4/>64 |
| Enterobacter spp. | 0.25/8 | 0.25/>32 | ≤0.5/2 | ≤0.06/≤0.06 | 2/64 |
| Serratias spp. | 0.5/1 | 0.12/0.5 | ≤0.5/≤0.5 | ≤0.06/≤0.06 | 2/4 |
| Proteus mirabilis | 0.5/0.5 | 0.06/0.06 | ≤0.5/≤0.5 | ≤0.06/≤0.06 | ≤0.5/1 |
| Citrobacter spp. | 0.25/8 | 0.25/>32 | ≤0.5/1 | ≤0.06/≤0.06 | 2/64 |
Notes: MIC breakpoint for ceftolozane/tazobactam has not been established. Data from Sader et al.29
Abbreviations: MIC, minimum inhibitory concentration.
Table 2.
Comparative activity of ceftolozane/tazobactam against selected European clinical Gram-negative bacilli
| MIC50 / MIC90 (mg/L) | |||||
|---|---|---|---|---|---|
| Organism | Ceftolozane/tazobactam | Ceftazidime | Cefepime | Meropenem | Piperacillin/tazobactam |
| Pseudomonas aeruginosa | 1/4 | 2/>32 | 4/>16 | 0.5/>8 | 8/>64 |
| Escherichia coli (non-ESBL) | 0.25/0.25 | 0.12/0.25 | ≤0.5/≤0.5 | ≤0.06/≤0.06 | 2/8 |
| Escherichia coli (ESBL) | 0.5/4 | 0.16/> 32 | >16/>16 | ≤0.06/≤0.06 | 8/>64 |
| Klebsiella spp. (non-ESBL) | 0.25/0.5 | 0.12/0.5 | ≤0.5/≤0.5 | ≤0.06/≤0.06 | 2/8 |
| Klebsiella spp. (ESBL) | 2/>32 | 32/>32 | ≤0.5/4 | ≤0.06/≤0.06 | 4/64 |
| Enterobacter spp. | 0.25/4 | 0.25/>32 | ≤0.5/4 | ≤0.06/≤0.06 | 4/64 |
| Proteus mirabilis | 0.5/1 | 0.06/0.25 | 0.5/≤0.5 | ≤0.06/0.12 | ≤0.5/1 |
| Citrobacter spp. | 0.25/8 | 0.25/>32 | ≤0.5/1 | ≤0.06/≤0.06 | 2/64 |
Notes: MIC breakpoint for ceftolozane/tazobactam has not been established. Data from Sader et al.39
Abbreviations: MIC, minimum inhibitory concentration; ESBL, extended-spectrum β-lactamase.
β-lactamase-producing organisms
Ceftolozane appears to be minimally affected by primitive β-lactamases, such as TEM-1, TEM-2, SHV-1, and OXA-1.27,36 However, its activity against ESBL-producing organisms is reduced. The addition of tazobactam enhanced the activity of ceftolozane against select ESBL-producing organisms in a concentration-dependent manner.26 In a checkerboard titration study of ceftolozane and tazobactam versus 57 ESBL-producing Enterobacteriaceae isolates, the addition of tazobactam at a concentration of 8 mg/L restored the MIC of ceftolozane to ≤4 and ≤8 mg/L in 76% and 93% of the isolates, respectively.26 In a larger collection of clinical isolates, data from the PACTS surveillance study suggest that the combination of ceftolozane and tazobactam retains activity against 91.1%, 60.3%, and 100.0% of E. coli, K. pneumoniae, and Proteus mirabilis with the ESBL phenotype at an MIC of 4 mg/L, and 94.6%, 68.0%, and 100% at an MIC of 8 mg/L, respectively.29
While ceftolozane is also susceptible to hydrolysis by the AmpC enzyme, the efficiency of hydrolysis may be dependent on the genus of the organism. In a checkerboard titration study of ceftolozane and tazobactam against 20 AmpC hyperproducing isolates, the MIC of ceftolozane was restored to ≤8 mg/L in 95% of the isolates at a tazobactam concentration of 8 mg/L.26 However, it should be noted that seven (35%) of the strains exhibited lower MICs to ceftolozane at baseline (MIC 1–2 mg/L) without the addition of tazobactam. This finding is consistent with a previous study that described the relative stability of ceftolozane to some AmpC enzymes, with fourfold-greater activity in comparison to ceftazidime.27,28
The activity of ceftolozane is compromised in the presence of carbapenemases, such as metallo-β-lactamases and K. pneumoniae carbapenemases. The addition of tazobactam does not appear to enhance the activity of ceftolozane against carbapenemase-producing organisms.26,27
Pharmacokinetics
Ceftolozane is parenterally administered and exhibits linear pharmacokinetics after single doses of 500/250 mg, 1,000/500 mg, and 2,000/1,000 mg, and multiple doses of 1,000/500 mg every 8 hours and 1,500/750 mg every 12 hours of ceftolozane/tazobactam.31,40 In healthy adults, the maximum plasma concentration (Cmax) and plasma half-life (t½) for ceftolozane/tazobactam 1,000/500 mg and 2,000/1000 mg infused over 60 minutes given every 8 hours were 74.4 mg/L, 3.12 hours, and 117 mg/L, 2.67 hours, respectively.40,41 Accumulation of ceftolozane after multiple dosing was negligible.31,40,42 Ceftolozane is eliminated unchanged primarily through the urine (>90%).31,40,42 Pharmacokinetic parameters such as clearance, t1/2, area under the curve (AUC), steady-state volume, and Cmax are similar when tazobactam is coadministered with ceftolozane compared to ceftolozane alone.40 Minimal increases in AUC and t1/2 were observed in patients with mild renal impairment (creatinine clearance 60–89 mL/minute). In patients with moderate renal impairment (creatinine clearance 30–59 mL/minute), the observed increases in AUC and t1/2 were 2.6- and 2.1-fold for ceftolozane, and 2.0- and 1.6-fold for tazobactam, respectively. As such, no dosage adjustment is required in patients with mild renal impairment. However, patients with moderate renal impairment may require a 50% dose reduction.43 The data describing the pharmacokinetic profile of ceftolozane/tazobactam in patients with severe renal insufficiency and hemodialysis have been submitted for presentation at a conference at the time of writing.
Ceftolozane/tazobactam exhibits rapid tissue distribution, including excellent lung penetration, and low protein binding.31,41,44 The probability of achieving 40% time of unbound drug above the MIC of the organism (T > MIC) in plasma and epithelial lining fluid was observed in >90% of the simulated ventilator-associated pneumonia (VAP) population for important Gram-negative pathogens, such as P. aeruginosa, E. coli, and K. pneumoniae.41,44
Pharmacodynamics
The bactericidal potential of ceftolozane was characterized by evaluating the in vitro killing kinetics of P. aeruginosa strain PAO1. A greater than 3-log reduction in bacterial load was observed after 8 hours at 1 × MIC (0.5 mg/L).33 In comparison to ceftazidime, ceftolozane initiated killing at two- to fourfold-lower multiples of MIC. Similarly, with the addition of tazobactam to ceftolozane, concentration-independent rapid bactericidal activity was observed against four isogenic strains of E. coli (wild type, β-lactamase-producing AmpC, CMY10, and ESBL CTX-M 15 strains), with 99.9% kill within 8 hours.45 In vivo infection models in neutropenic mice also revealed bactericidal activity against most organisms (four non-ESBL-producing E. coli and K. pneumonia strains, and four P. aeruginosa strains), with greater than 2-log reduction in bacterial load at 24 hours.46 The addition of tazobactam enhances the killing potential of ceftolozane against various β-lactamase-producing organisms. However, this observation is dependent on the dosing schedule of tazobactam. Bacterial reduction of greater than 2-log10 colony-forming units at 24 hours was observed when tazobactam was administered every 6 or every 8 hours. Administration of tazobactam every 12 or 24 hours resulted in much less bacterial killing (≤2-log reduction) at 24 hours in comparison to administration every 6 or 8 hours.47
Similar to other cephalosporins, the pharmacodynamic parameter predicting bacteriological efficacy is the T > MIC of the organism. Ceftolozane/tazobactam concentrations remain above MIC approximately 40%–50% of the time between dosage administrations, similar to other cephalosporins.32 However, the percentage of T > MIC required for ceftolozane against Enterobacteriaceae and P. aeruginosa in an infected murine thigh model is much less than observed with other cephalosporins.46 The mean (±standard deviation) percentages of T > MIC required for stasis and 1-log kill of wild-type Enterobacteriaceae, Enterobacteriaceae-producing ESBLs, and P. aeruginosa were 26.3 ± 2.1 and 31.6 ± 1.6; 31.1 ± 4.9 and 34.8 ± 4.4; and 24.0 ± 3.3 and 31.5 ± 3.9, respectively.46
The probability of pharmacokinetic and pharmacodynamic target attainment was determined using Monte Carlo modeling. The simulated target attainment revealed that 50% T > MIC of 8 mg/L was achieved in 90% of the subjects with a 1,500 mg dose infused over 60 minutes, every 8 hours. Based on observed MIC distribution of select organisms in the 2008 surveillance data, a high probability of target attainment was observed for E. coli (MIC90 = 0.25), K. pneumoniae (MIC90 = 2), and P. aeruginosa (MIC90 = 2). Thus, ceftolozane/tazobactam demonstrated a high probability of target attainment using 50% T > MIC, supporting a proposed breakpoint of 8 mg/L.48
Resistance
Ceftolozane is predominantly active against Gram-negative bacteria. Takeda et al reported a lower frequency of spontaneous resistance of P. aeruginosa to ceftolozane compared to ceftazidime at concentrations of 4 ×, 8 ×, and 16 × MIC, as well as compared to imipenem and ciprofloxacin at 4 × MIC.27 More specifically, after five serial passages, a fourfold reduction in susceptibility was observed for ceftolozane. Comparatively, 32-, 16-, and 16-fold reductions in susceptibility were observed for ceftazidime, imipenem, and ciprofloxacin, respectively.34 The most significant mechanism is the production of β-lactamases with hydrolysis of ceftolozane. While ceftolozane has demonstrated stability against AmpC β-lactamases,28 the addition of tazobactam is necessary to improve its stability and activity against organisms producing ESBLs.26 However, significant hydrolysis is observed in organisms producing carbapenemases (metallo-β-lactamase and K. pneumoniae carbapenemase), despite the combination of ceftolozane and tazobactam.26
Ceftolozane/tazobactam has demonstrated minimal cross-resistance with other antimicrobials, as well as maintained susceptibility in some organisms that exhibit resistance to other antipseudomonal agents.49 More specifically, ceftolozane/tazobactam is unaffected by upregulation of efflux pumps or loss of porin channels that may affect selected antipseudomonal β-lactams, fluoroquinolones, and aminoglycosides.27 Overexpression of MexAB-OprM, MexCD-OprJ, MexEF-OprN, or MexXY in clinical isolates of P. aeruginosa did not increase the MIC of ceftolozane.27 Similarly, ceftolozane maintained its activity against imipenem-resistant clinical isolates of P. aeruginosa demonstrating loss of OprD.27
Clinical trials
A Phase II clinical trial evaluating the safety and efficacy of ceftolozane/tazobactam in the setting of complicated intra-abdominal infections and a Phase II trial evaluating the safety and efficacy of ceftolozane alone in complicated urinary tract infections have been completed. In the Phase II, randomized, double-blind, multicenter study of ceftolozane/tazobactam (1.5 g intravenously every 8 hours) in combination with metronidazole (500 mg intravenously every 8 hours) against meropenem (1 g intravenously every 8 hours) for the management of complicated intra-abdominal infections, comparable cure rates of 91% versus 94% were reported, respectively. The cure rates for the microbiological intent-to-treat population and microbiologically evaluable population were 84% versus 96% and 89% versus 96%, for ceftolozane/tazobactam and meropenem, respectively (Cubist Pharmaceuticals, correspondence, March, 2013).
In the Phase II, randomized, double-blind studies comparing the safety and efficacy of ceftolozane and ceftazidime in complicated urinary tract infections, no difference in microbiologic cure rates at the test-of-cure visit was reported. More specifically, in the setting of complicated urinary tract, complicated lower urinary tract, and pyelonephritis, the observed microbiologic cure rates were 83% versus 76%, 82% versus 73%, and 86% versus 83% for ceftolozane and ceftazidime, respectively.31,50,51 The current formulation in clinical trials includes the addition of tazobactam to provide coverage for selected β-lactamase-producing organisms. However, the clinical implications of this combination need to be evaluated.
Currently, a Phase III, multicenter, prospective, randomized, open-label study has been initiated to evaluate the safety and efficacy of ceftolozane/tazobactam 3 g intravenously every 8 hours versus piperacillin/tazobactam 4.5 g intravenously every 6 hours for the treatment of VAP.52 Eligibility criteria include adult patients age 18 years or older, receipt of mechanical ventilation for greater than 48 hours, Acute Physiology and Chronic Health Evaluation score of 11–35, presence of new or progressive infiltrate on chest X-ray, and presence of clinical criteria consistent with VAP. Exclusion criteria include history of moderate or severe hypersensitivity to β-lactam antibiotics and known end-stage renal disease or requirement for dialysis.52 The rationale for a higher dosing regimen of ceftolozane partially stems from the results of a rabbit pneumonia experimental model that demonstrated significantly greater reduction in pulmonary bacterial load with ceftolozane human-equivalent dose of 2 g every 8 hours compared to ceftolozane human-equivalent dose of 1 g every 8 hours.53 In addition, the probability of target attainment (T > MIC 40%) of ceftolozane/tazobactam when patients with renal hyperclearance are modeled (estimated creatinine clearance 180–250 mL/minute) was 98%–100% with a 3 g every 8 hour dose compared to 71%–93% with a 1.5 g every 8 hour dose at an MIC of 8 mg/L. The primary objective measure for this Phase III VAP study is clinical response at the end-of-therapy visit in the modified intention-to-treat population.52 The estimated completion date for this study is January 2016.52 A randomized, double-blind study comparing ceftolozane/tazobactam to imipenem/cilastatin is also planned.
Adverse effects
The safety of ceftolozane/tazobactam has been evaluated in Phase I and II studies, which found the drug to be generally well tolerated. In 64 healthy volunteers who received single and multiple ascending infusions of ceftolozane/tazobactam, no serious adverse events requiring discontinuation of drug therapy or deaths were observed. The most common adverse event was peripheral infusion-site reactions, while two subjects experienced diarrhea and one experienced flushing; no dose-limiting toxicity was reported.42 In the evaluation of intrapulmonary penetration of ceftolozane/tazobactam in 51 healthy adults who received ceftolozane/tazobactam or piperacillin/tazobactam, all reported adverse events were of mild severity, and event incidence was similar between the two groups.44 Adverse events experienced in the ceftolozane/tazobactam group included diarrhea, viral upper respiratory tract infection, musculoskeletal chest pain, somnolence, hematuria, and cough; none of the reported adverse events were serious or included death.44
Safety data derived from Phase II, randomized, double-blind, controlled clinical trials comparing ceftolozane and ceftazidime in complicated urinary tract infection have shown comparable adverse-effect profiles. The most common treatment-emergent adverse events were constipation (9.4%), sleep disorder (7.1%), headache (5.9%), and nausea (5.9%).51
Discussion
The increasing prevalence of multidrug-resistant Gram-negative pathogens presents significant challenges to clinicians. The Antimicrobial Availability Task Force of the Infectious Diseases Society of America released a report titled “Bad Bugs, No Drugs: As Antibiotic R&D Stagnates, a Public Health Crisis Brews” to address the lack of antibiotic development in an era of increasing resistance.9 More specifically, infections caused by P. aeruginosa result in significant morbidity and mortality, particularly in strains demonstrating cross-resistance to the handful of agents with clinical utility against P. aeruginosa.4,54,55 Currently, several β-lactam/β-lactamase-inhibitor combinations are in clinical development (ceftazidime/avibactam, ceftaroline/avibactam, and imipenem/cilastatin).30
Ceftolozane/tazobactam is a novel antibacterial and β-lactamase-inhibitor combination consisting of ceftolozane, a novel antipseudomonal cephalosporin, and tazobactam, a well-established β-lactamase inhibitor. It has demonstrated potent bactericidal activity against select Gram-negative isolates, mainly wild-type Enterobacteriaceae and P. aeruginosa. The addition of tazobactam has enhanced the spectrum of ceftolozane to Enterobacteriaceae-producing select ESBLs. Moreover, ceftolozane/tazobactam has not demonstrated cross-resistance to other antimicrobial classes, particularly those affected by ESBLs, AmpC β-lactamase, a loss in porin channels, or the overexpression of efflux pumps in P. aeruginosa.
It is in this context that the utility of ceftolozane/tazobactam appears particularly attractive. The selection of antimicrobial agents in infectious settings where P. aeruginosa may be a likely pathogen requires careful review of the institution-specific antimicrobial susceptibility profile, an antibiogram. Specifically, in institutions lacking antipseudomonal agents with reliable susceptibility, a combination of agents (including agents with narrow therapeutic indices and high risk of toxicity) may be needed. Ceftolozane/tazobactam carries the potential to be a first-line agent in this setting, given its in vitro/in vivo data and favorable adverse-effects profile. In contrast, in institutions with favorable antipseudomonal susceptibility profiles, ceftolozane/tazobactam nonetheless requires serious consideration for addition to the formulary, for the management of challenging cases caused by strains with resistant phenotypes. However, given its early stage of development, the role of ceftolozane/tazobactam will be determined by the results of Phase III clinical data.
Acknowledgments
We would like to thank Philip Tam, PhD for building the chemical structures of ceftolozane and tazobactam. Chemical structures were developed using BKChem software (http://bkchem.zirael.org). We would also like to thank John Mohr, PharmD for provision of data.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. Donald Hsu is a speaker for Astellas, Inc, Optimer Pharmaceuticals, and Merck Inc. The views expressed in this article are solely those of the authors and do not necessarily reflect the views of the Western University of Health Sciences, St Joseph Hospital Orange, Veterans Affairs San Diego Healthcare System, nor University of California San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences.
References
- 1.Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2002;162(19):2223–2228. doi: 10.1001/archinte.162.19.2223. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 3.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 4.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 5.Giske CG, Ge J, Nordmann P. Activity of cephalosporin CXA-101 (FR264205) and comparators against extended-spectrum-{beta}-lactamase-producing Pseudomonas aeruginosa. J Antimicrob Chemother. 2009;64(2):430–431. doi: 10.1093/jac/dkp193. [DOI] [PubMed] [Google Scholar]
- 6.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 7.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007;122(2):160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 9.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42(5):657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Gram-negative bacteria infections in healthcare settings 2011Available from: http://www.cdc.gov/hai/organisms/gram-negative-bacteria.htmlAccessed April 29, 2013
- 11.Stein GE. Antimicrobial resistance in the hospital setting: impact, trends, and infection control measures. Pharmacotherapy. 2005;25(10 Pt 2):44S–54S. doi: 10.1592/phco.2005.25.10part2.44S. [DOI] [PubMed] [Google Scholar]
- 12.Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54(1):109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnello M, Wong-Beringer A. Differentiation in quinolone resistance by virulence genotype in Pseudomonas aeruginosa. PloS One. 2012;7(8):e42973. doi: 10.1371/journal.pone.0042973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosson P, Zulianello L, Join-Lambert O, et al. Pseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host system. J Bacteriol. 2002;184(11):3027–3033. doi: 10.1128/JB.184.11.3027-3033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72(12):6969–6977. doi: 10.1128/IAI.72.12.6969-6977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4(4):551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veesenmeyer JL, Hauser AR, Lisboa T, Rello J. Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med. 2009;37(5):1777–1786. doi: 10.1097/CCM.0b013e31819ff137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giamarellou H, Antoniadou A. Antipseudomonal antibiotics. Med Clin North Am. 2001;85(1):19–42. doi: 10.1016/s0025-7125(05)70303-5. [DOI] [PubMed] [Google Scholar]
- 19.National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 20.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 21.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002 Mar 1;34(5):634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 22.Rossolini GM, Mantengoli E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin Microbiol Infect. 2005;11( Suppl 4):17–32. doi: 10.1111/j.1469-0691.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 23.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31(5):528–531. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 24.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50(1):43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao B, Wang H, Sun H, Zhu Y, Chen M. Risk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa infections. J Hosp Infect. 2004;57(2):112–118. doi: 10.1016/j.jhin.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Livermore DM, Mushtaq S, Ge Y. Chequerboard titration of cephalosporin CXA-101 (FR264205) and tazobactam versus beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2010;65(9):1972–1974. doi: 10.1093/jac/dkq248. [DOI] [PubMed] [Google Scholar]
- 27.Takeda S, Nakai T, Wakai Y, Ikeda F, Hatano K. In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2007;51(3):826–830. doi: 10.1128/AAC.00860-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda S, Ishii Y, Hatano K, Tateda K, Yamaguchi K. Stability of FR264205 against AmpC beta-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30(5):443–445. doi: 10.1016/j.ijantimicag.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Sader HS, Flamm RK, Streit JM, Jones RN. Abstracts: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington: American Society for Microbiology; 2012. Activity of novel antimicrobial ceftolozane/tazobactam tested against contemporary clinical strains from USA hospitals (2011) [Google Scholar]
- 30.Toda A, Ohki H, Yamanaka T, et al. Synthesis and SAR of novel parenteral anti-pseudomonal cephalosporins: discovery of FR264205. Bioorg Med Chem Lett. 2008;18(17):4849–4852. doi: 10.1016/j.bmcl.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 31.Cubist Pharmaceuticals Ceftolozane/tazobactam – overview Available from: http://www.cubist.com/sup/img/drugs/Ceft-Taz-overview_880.pngAccessed January 10, 2013
- 32.Cubist Pharmaceuticals Gram-negative: ceftolozane/tazobactam Available from: http://www.cubist.com/products/cxa_201Accessed January 10, 2013
- 33.Moyá B, Zamorano L, Juan C, Ge Y, Oliver A. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54(9):3933–3937. doi: 10.1128/AAC.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shlaes DM. New β-lactam-β-lactamase inhibitor combinations in clinical development. Ann N Y Acad Sci. 2013;1277:105–114. doi: 10.1111/nyas.12010. [DOI] [PubMed] [Google Scholar]
- 35.Mushtaq S, Warner M, Livermore DM, et al. Abstracts: 48th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington: American Society for Microbiology; 2009. Activity of cephalosporin CXA-101 (FR264205) with β-lactamase inhibitors vs Enterobacteriaceae. [Google Scholar]
- 36.Livermore DM, Mushtaq S, Ge Y, Warner M. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int J Antimicrob Agents. 2009;34(5):402–406. doi: 10.1016/j.ijantimicag.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Moya B, Zamorano L, Juan C, Pérez JL, Ge Y, Oliver A. Activity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob Agents Chemother. 2010;54(3):1213–1217. doi: 10.1128/AAC.01104-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamorano L, Juan C, Fernández-Olmos A, Ge Y, Cantón R, Oliver A. Activity of the new cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa isolates from chronically-infected cystic fibrosis patients. Clin Microbiol Infect. 2010;16(9):1482–1487. doi: 10.1111/j.1469-0691.2009.03130.x. [DOI] [PubMed] [Google Scholar]
- 39.Sader H, Farrell D, Jones RN. Activity of the novel antimicrobial CXA-201 tested against contemporary clinical strains from European hospitals (2012); Presented at: 22nd Annual Meeting of the European Congress of Clinical Microbiology (ECCMID); March 31–April 3, 2012; London, UK. [Google Scholar]
- 40.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother. 2012;56(6):3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller B, Chandorkar G, Umeh O, et al. Abstracts: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington: American Society for Microbiology; 2012. Safety and PK of IV ceftolozane/tazobactam 3 g Q8h and cumulative fraction of response in plasma and epithelial lining fluid in a simulated VAP population. [Google Scholar]
- 42.Ge Y, Whitehouse MJ, Friedland I, Talbot GH. Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions. Antimicrob Agents Chemother. 2010;54(8):3427–3431. doi: 10.1128/AAC.01753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller B, Hershberger E, Benziger D, et al. Pharmacokinetics of CXA-101/tazobactam in subjects with mild or moderate renal impairment; Presented at: 21st Annual Meeting of the European Congress of Clinical Microbiology; May 7–10, 2011; Milan, Italy. [Google Scholar]
- 44.Chandorkar G, Huntington JA, Gotfried MH, Rodvold KA, Umeh O. Intrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjects. J Antimicrob Chemother. 2012;67(10):2463–2469. doi: 10.1093/jac/dks246. [DOI] [PubMed] [Google Scholar]
- 45.Soon RL, Forrest A, Holden PN, et al. Abstracts: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington: American Society for Microbiology; 2012. In vitro pharmacodynamics of ceftolozane/tazobactam against β-lactamase producing Escherichia coli. [Google Scholar]
- 46.Craig WA, Andes DR. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum beta-lactamases, in the thighs of neutropenic mice. Antimicrob Agents Chemother. 2013;57(4):1577–1582. doi: 10.1128/AAC.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanscoy B, Mendes RE, Nicasio AM, et al. Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model. Antimicrob Agents Chemother. 2013;57(6):2809–2814. doi: 10.1128/AAC.02513-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hershberger E, Mouksassi M, Steenbergen JN, et al. CXA-101/tazobactam probability of target attainment using population pharmacokinetic analysis; Presented at: 21st European Congress of Clinical Microbiology and Infectious Diseases and Twenty-seventh International Congress of Chemotherapy; May 7–10, 2011; Milan, Italy. [Google Scholar]
- 49.Cabot G, Mulet X, Moya B, et al. Abstracts: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington: American Society for Microbiology; 2012. Dynamics and mechanisms of resistance development to ceftazidime, meropenem and ceftolozane/tazobactam in wild-type and mutator P. aeruginosa strains. [Google Scholar]
- 50.ClinicalTrials.gov [website on the Internet] Safety and efficacy of IV CXA-101 and IV ceftazidime in patients with complicated urinary tract infections 2010Available from: http://clinicaltrials.gov/ct2/show/NCT00921024?term=CXA±and±ceftazidime&rank=1Accessed June 7, 2013
- 51.Umeh O, Cebrik D, Friedland I. Abstracts: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington: American Society for Microbiology; 2010. A double-blind, randomized, phase 2 study to compare the safety and efficacy of intravenous CXA-101 (CXA) and intravenous ceftazidime (CTZ) in complicated urinary tract infection (cUTI) [Google Scholar]
- 52.ClinicalTrials.gov [website on the Internet] Study of intravenous ceftolozane/tazobactam vs piperacillin/tazobactam in ventilator associated pneumonia 2013Available from: http://clinicaltrials.gov/ct2/show/NCT01853982?term=ceftolozane&rank=2Accessed May 17, 2013
- 53.Jacqueline C, Bretonniere C, Desessard C, et al. Abstracts: 51st Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington: American Society for Microbiology; 2011. In vivo activity of CXA-101 against Pseudomonas aeruginosa(PA) in a rabbit experimental model of pneumonia: comparison with ceftazidime (CAZ), piperacillin/tazobactam (TZP), and imipenem (IMP) [Google Scholar]
- 54.Hsu DI, Okamoto MP, Murthy R, Wong-Beringer A. Fluoroquinolone-resistant Pseudomonas aeruginosa: risk factors for acquisition and impact on outcomes. J Antimicrob Chemother. 2005;55(4):535–541. doi: 10.1093/jac/dki026. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen LH, Hsu DI, Ganapathy V, Shriner K, Wong-Beringer A. Reducing empirical use of fluoroquinolones for Pseudomonas aeruginosa infections improves outcome. J Antimicrob Chemother. 2008;61(3):714–720. doi: 10.1093/jac/dkm510. [DOI] [PubMed] [Google Scholar]


