Abstract
Dominant mutations in GJA1, the gene encoding the gap junction protein connexin43 (Cx43), cause oculodentodigital dysplasia (ODDD), a syndrome affecting multiple tissues, including the central nervous system (CNS). We investigated the effects of the G60S mutant, which causes a similar, dominant phenotype in mice (Gja1Jrt/+). Astrocytes in acute brain slices from Gja1Jrt/+ mice transfer sulforhodamine-B comparably to that in their wild-type (WT) littermates. Further, astrocytes and cardiomyocytes cultured from Gja1Jrt/+ mice showed a comparable transfer of lucifer yellow to those from WT mice. In transfected cells, the G60S mutant formed gap junction (GJ) plaques but not functional channels. In co-transfected cells, the G60S mutant co-immunoprecipitated with WT Cx43, but did not diminish GJ coupling as measured by dual patch clamp. Thus, whereas G60S has dominant effects, it did not appreciably reduce GJ coupling.
Keywords: ODDD, astrocytes, connexin
INTRODUCTION
Gap junctions (GJs) are intercellular channels that form between apposed cell membranes, permitting the diffusion of ions and small molecules typically less than 1000 Da (Bruzzone et al., 1996). In vertebrates, GJs are comprised of connexins (Cxs), a family of highly conserved integral membrane proteins that are named according to their predicted molecular mass (Willecke et al., 2002). Six Cxs oligomerize into a hemichannel (or connexon), and two apposing hemi-channels form a GJ channel; aggregates of tens to thousands of channels form a GJ plaque. Hemichannels may be homomeric, containing one type of Cx, or heteromeric, containing more than one type. GJs are termed homotypic if the apposed hemichannels are identical, and heterotypic if they differ (Kumar and Gilula, 1996). The potential diversity of GJ composition is immense, as over 20 mammalian Cxs have been described.
Dominant mutations in GJA1, the gene that encodes connexin43 (Cx43), cause oculodentodigital dysplasia (ODDD), which is characterized by developmental abnormalities of the eyes, teeth, limbs and face that can be related to the expression of Cx43 in the affected tissues (Loddenkemper et al., 2002; Paznekas et al., 2003; Richardson et al., 2004; Fenwick et al., 2008). Neurological symptoms have been reported in a subset of patients, including dysarthria, neurogenic bladder, spastic paraparesis, ataxia, mental retardation and seizures; some of these patients have abnormal signal in magnetic resonance imaging of central nervous system (CNS) white matter tracts (Gutmann et al., 1991; Loddenkemper et al., 2002). Gja1Jrt/+ mice are a model of ODDD: they have a dominantly inherited disorder with abnormal digits, dentition and facial skeleton (Flenniken et al., 2005). In these mice, a point mutation in Gja1 results in the replacement of glycine 60 with serine (G60S) in the highly conserved first extracellular loop of Cx43 (Sohl and Willecke, 2004). Because the GJ coupling between ovarian granulosa cells, which express Cx43 but no other Cx, was reported to be impaired in Gja1Jrt/+ mice, G60S was thought to have dominant effects on wild-type (WT) Cx43 (Flenniken et al., 2005).
We investigated the effects of the G60S mutation in astrocytes. In rodents, astrocytes are coupled to other astrocytes by Cx43:Cx43 and Cx30:Cx30 homotypic channels, and to oligodendrocytes by heterotypic Cx43:Cx47 and Cx30:Cx32 channels (Rash et al., 2001; Nagy et al., 2003; Nagy and Rash, 2003; Altevogt and Paul, 2004; Li et al., 2004; Orthmann-Murphy et al., 2007; Orthmann-Murphy et al., 2008). To our surprise, we found no measurable effect of the G60S mutant on astrocyte:astrocyte (A:A) coupling in acute brain slices or in culture. We corroborated these results by showing that G60S does not have a dominant effect on coupling in cells transfected to express both G60S and WT Cx43, even though they can be co-immunoprecipitated. Thus, whereas G60S has dominant effects in some cell types, these effects did not detectably affect A:A coupling.
METHODS
Constructs
The open reading frame of mouse Cx43 was generated by polymerase chain reaction (PCR) of genomic mouse DNA, and subcloned into a pIRES2–Enhanced Green Fluorescent Protein (EGFP) vector or pIRES2–DsRed vector, which was produced by replacing the coding sequence of EGFP with the monomeric DsRed. The open reading frame of this sequence was identical to a previously published sequence of mouse Cx43 (GenBank accession number NM_010288). The G60S mutation was generated by site-directed mutagenesis using QuikChange®II XL Site-Directed Mutagenesis Kits (Stratagene), by substituting G with A at position 178 of the open reading frame. To make the Flag- and Myc-epitope tagged versions of WT and G60S, we placed the open reading frames of mouse Cx43 and the G60S mutation, without the stop codon, into the pFlag-N1 or p-Myc-N1 vectors previously made from pEGFP-N1 vector (Clontech) by substituting EGFP with Flag or Myc. Sequences of all these constructs were confirmed by sequencing a large-scale plasmid preparation.
Animals
Gja1Jrt/+ mice were obtained from the Centre of Modeling Human Diseases in Ontario, Canada. The mice were on the C57BL/6J (B6) and C3H/HeJ (C3) background. All experiments were conducted according to University of Pennsylvania guidelines for laboratory animal use. Tail biopsies were taken from neonatal mice and genotyped by PCR (A. Flenniken, personal communication), followed by digestion with MspI, which does not cut into the mutant sequence, CCAG, thus giving an extra band on gel electrophoresis in Gja1Jrt/+ compared to WT (Gja1+/+) littermates. To identify astrocytes in brain slices, Gja1Jrt/+ mice were crossed to FVB/N-Tg(GFAPGfp)14Mes/J mice (FVB) (Zhuo et al., 1997), which express GFP in astrocytes; 50% of the offspring expressed GFP-positive astrocytes, and an equal proportion of these mice were Gja1Jrt/+ and Gja1+/+. For these experiments, mice were maintained by crossing Gja1Jrt/+ and Gja1+/+ littermates.
Immunoblotting and co-immunoprecipitations
Individual brains, cerebella, cervical spinal cords and hearts were obtained from euthanized P44 Gja1Jrt/+ and their Gja1+/+ littermates, homogenized in lysis buffer (150 mM NaCl, 10 mM Tris, 1 mM ethylene diamine tetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X, 1 mM Na vanadate and 1 mM NaFl; Manias et al., 2008) and separated on 4–15% Tris–HCL ReadyGel (Bio-Rad). The blots were incubated overnight at 4°C with a rabbit antiserum against Cx43 (1:8000 or 1:80,000 dilution; Sigma) or Cx30 (1:500 dilution; Zymed), washed several times, incubated in horse radish peroxidase (HRP)-conjugated donkey anti-rabbit antiserum (1:10,000 dilution; Jackson ImmunoResearch Laboratories) and developed using Amersham ECL Western Blotting Detection Reagents. For blotting with GADPH, membranes were stripped following the blotting for Cx43 and Cx30 and then re-incubated overnight at 4°C with a mouse antiserum against GADPH (1:80,000 dilution; Chemicon), washed several times and incubated in HRP-conjugated donkey anti-mouse antiserum (1:10,000 dilution; Jackson ImmunoResearch Laboratories).
For co-immunoprecipitation, N2A cells (American Type Culture Collection, Manassas, VA) were co-transfected to express WTCx43-Flag and G60S-Myc or WTCx43-Myc. After 24 h, the cells were lysed in 500 μl of ice-cold Radio immunoprecipitation assay (RIPA) buffer (10 mM sodium phosphate, pH 7.0, 150 mM NaCl, 2 mM EDTA, 50 mM NaFl, 1% Nonidet P-40, 1% sodium deoxycholate and 0.1% SDS) for 15 min on ice, scraped, and then spun at 14,000 rpm for 30 min. The supernatants were collected, incubated on ice with 5 μl rabbit antiserum against Flag (Sigma) for 1 h, followed by 100 μl protein G agarose (Sigma). After an overnight incubation at 4°C, the beads were washed in RIPA buffer, resuspended in electrophoresis Lammeli buffer with 2-mercaptoethanol (Bio-Rad), separated on 4–15% Tris–HCL ReadyGel (Bio-Rad), and transferred to a polyvinylidene fluoride membrane (Immobilon–P; Millipore). The membrane was incubated with a mouse antibody against Myc (1:5000 dilution; Sigma) overnight at 4°C, and developed as described above. Additional experiments were processed as above with co-immunoprecipitation using rabbit antibody against Flag and were blotted using a monoclonal mouse anti-Cx43 (1:1000 dilution; Chemicon). Blots for Myc using mouse antibody against Myc (1:5000 dilution; Sigma), and GADPH (1:5000 dilution; Chemicon) were carried directly on cell lysates.
Primary cell culture
Post-natal day 1 (P1) to P4 Gja1Jrt/+ and their Gja1+/+ littermates were euthanized, and their tails were kept for later genotyping. To obtain astrocytes, the cerebral cortices were dissected, individually triturated in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 5 U ml−1 penicillin/streptomycin at room temperature and incubated in 95% air/5% CO2 at 37°C. Confluent cultures (7–10 days in vitro; DIV) were trypsinized, plated into poly-L-lysine coated coverslips (22 mm), and used when confluent (14–16 DIV). Cultures were ~95% pure astrocytes as evidenced by GFAP staining (Meme et al., 2006; Retamal et al., 2007; Saura, 2007). To obtain cardiac myocytes, the atria were removed, and the ventricles from individual mice were digested overnight in Ca2+-free HEPES-buffered Hanks’ solution with 0.5% trypsin at 4°C, then triturated in Ca2+-free HPSS with 10% horse serum and 5% fetal bovine serum and 1% penicillin/streptomycin. Cells were then rinsed in Ca2+-free DMEM containing 10% horse serum, 5% fetal bovine serum 1% penicillin/streptomycin and plated on fibronectin-coated coverslips in 35-mm tissue culture dishes in Opti-MEM media with 7% horse serum, 3% fetal bovine serum and 1% penicillin/streptomycin.
Immunocytochemistry and immunohistochemistry
P40 Gja1Jrt/+ and their Gja1+/+ littermates were euthanized, their hearts were dissected, rinsed in phosphate buffered saline (PBS) and fixed for 15 min in 4% paraformaldehyde (in PBS), and embedded in Optimal Cutting Temperature compound (OCT). The brains, cerebella and spinal cords were dissected from P44 mice that had been perfused with PBS followed by 4% paraformaldehyde, fixed for another hour, infiltrated in 10% sucrose in PBS at 4°C and then embedded in OCT. Cryostat sections (10 μm thick) were thaw-mounted on Super Frost Plus glass slides (Fisher Scientific, Pittsburgh, PA) and stored at −20°C.
Cultured astrocytes, myocytes and transfected N2A cells were rinsed in PBS and fixed for 10 min in 4% paraformaldehyde; tissue sections were also permeabilized by immersion in −20°C acetone for 10 minutes. The slides/coverslips were incubated for 1 h in blocking solution (0.1% Triton X-100, 5% fish skin gelatin in PBS), and incubated overnight at 4°C with various combinations of primary antibodies – rabbit antisera against Cx30 (1:500 dilution; Zymed), Cx43 (1:2000 dilution for sections and 1:400 for cells; Sigma), Flag (1:500; Sigma), Cx30 (1:500 dilution; Zymed) and mouse monoclonal antibodies against Cx30 (1:500 dilution; Zymed), Cx43 (1:250 dilution; Chemicon), GFAP (1:500 dilution; Chemicon) or Myc (1:500 dilution; Sigma). The slides/coverslips were washed several times, incubated with the appropriate fluorescein-, rhodamine- or Cy5-conjugated donkey anti-mouse or -rabbit antisera (1:200 dilution; Jackson ImmunoResearch Laboratories). Slides were mounted with ProLongGold with DAPI (Invitrogen) or Vectorshield, and examined by epifluorescence with appropriate optical filters by epifluorescence (Leica DMR) or confocal (Leica SP2 AOBS system or Olympus FlouView FV1000) microscopy using interactive software (Improvision).
Electron microscopy
After anesthesia, P40 Gja1Jrt/+ (n = 3) and their Gja1+/+ littermates (n = 3) were transcardially perfused with 2.5% glutaraldehyde in 0.1 M PB, the brains, optic nerves and spinal cords were dissected and fixed overnight at 4°C, then osmicated, dehydrated and embedded in Epon. Transverse semi-thin sections (0.5 μm) were stained with alkaline toluidine blue and visualized through light microscopy (Leica DMR) using an interactive software (Improvision). Thin sections (90 nm thick) were mounted on 2 × 1 mm single-slot, formvar-coated grids, stained with lead citrate and uranyl acetate and examined with a JOEL 1200 electron microscope.
Electrophysiology
All recording and imaging from slice and primary cell culture was conducted using an Olympus BX51WI fixed stage microscope, fitted with a 40× water immersion objective with a long working distance, infrared differential interference contrast and videomicroscopy through an Olympus DP-71 Color camera using DP-71 software. The whole cell recordings were conducted using a Model 2400 amplifier (A-M systems); signals were digitized using National Instruments USP interface card, and analyzed using WCP software (version 3.6 up to version 4.0.7, John Dempster, Department of Physiology & Pharmacology Strathclyde Institute for Biomedical Sciences University of Strathclyde, Scotland).
Cells were recorded in the whole cell configuration 2 days after the transfection in extracellular solution composed of 135 mM NaCl, 5 mM KCl, 1.0 mM MgCl2, 4 mM dextrose and 5 mM HEPES adjusted to a pH of 7.4. Electrodes with a resistance of 3–6 MΩ were filled with the following solution – 130 mM KCL, 5 mM NaCl, 1 mM MgCl2, 0.5 mM CaCl2, 5 mM EGTA and 10 mM HEPES, adjusted to a pH of 7.3 with KOH; KCL was replaced with CsCl for cardiac myocytes. To test for dye transfer, one cell in a cluster of cells expressing DsRed was loaded with 1% lucifer yellow (LY; MW 457 Da, −2 charge) via whole cell configuration. Capacitance data were collected using a 10 mV voltage step and capacitance and analyzed as described (de Roos et al., 1996). Dye injections in confluent cultured astrocytes and spontaneously beating cardiac myocytes were conducted in a similar way. We first confirmed the absence of any specific fluorescence signal in any cell, then an individual cell in a cluster was patched with an electrode containing LY, and the cluster was observed over 1–5 min as the surrounding cells became filled with LY and showed intense fluorescence signal in the soma and nucleus. Acute brain slices were prepared from P14 to P25 Gja1Jrt/+ or Gja1+/+ mice that also expressed a GFAP–GFP transgene. Mice were euthanized, decapitated and the brain was dissected, immersed for 5 min in oxygenated (bubbled with 95% O2–5%CO2), ice-cold artificial cerebrospinal fluid (ACSF) composed of 250 mM sucrose, 2.5 mM KCl, 1.mM NaH2PO4, 1.3 mM MgSO4, 2.5 mM CaCl2, 11 mM dextrose and 26.2 mM NaHCO3, pH of 7.4 and an osmolarity of 295–305 mOsm, and sectioned horizontality into 200 μm thick sections using a Leica VT1000 S vibratome. Slices were incubated in oxygenated ACSF (125 mM NaCl, 2.5 mM KCl, 1.mM NaH2PO4, 1.3 mM MgSO4, 2.5 mM CaCl2, 11 mM dextrose and 26.2 mM NaHCO3 with a pH of 7.4 and an osmolarity of 295–305 mOsm) for 1 h, then placed in the recording chamber continuously perfused at a rate 2 ml min−1 with 100% O2 bubbled ACSF. Electrodes were filled with an intracellular solution composed of 105 mM K-gluconate, 30 mM KCL, 0.3 mM EGTA, 10 mM HEPES, 10 mM phosphocreatine, 4 mM ATP-Mg2, 0.3 mM GTP-Tris, with either 0.1% LY or 0.1% sulforhodamine-B (SR-B; MW 559; Invitrogen), adjusted to a pH 7.4 with KOH. After confirming the absence of bleed-through of GFP into the TRITC fluorescence signal, GFP-positive cells were patched and observed over 1–20 min.
For dual patch recordings, N2A calls were transfected as described above, and patching was carried out using Olympus inverted microscope and a pipette solution of 145 mM CsCl2, 5 mM EGTA, 1.4 mM CaCl2 and 5.0 mM HEPES, pH 7.2. Cells were bathed solution consist of 150 mM NaCl, 4 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM dextrose, 2 mM pyruvate and 10 mM HEPES, pH 7.4. Recordings were done using Multiclamp 700 A, and junctional conductance (Gj) was determined from isolated pairs by measuring junctional current (Ij) responses to junctional voltage (Vj) ramps from −100 to +100 mV.
Statistical analyses were performed using GraphPadPrism (GraphPad Software Inc.).
RESULTS
Cortical astrocytes in acute brain slices from Gja1Jrt/+ mice are functionally coupled
Because Cx43 contributes to A:A coupling (Giaume et al., 1991; Naus et al., 1997), we examined dye transfer in acute brain slices from P15 to P25 Gja1+/+ and Gja1Jrt/+ mice. These mice also expressed the GFAP–Gfp transgene, so that astrocytes could be recognized by their expression of GFP. In our initial experiments, we used patch electrodes to label single, GFP-positive cells with LY, a small (457 Da, −2 charge) fluorescent dye that permeates GJs comprised of Cx43 (Elfgang et al., 1995). Because the GFP obscured the fluorescence of the LY, we used SR-B, a small (559 Da, uncharged) fluorescent dye that is visualized with different fluorescence optics (TRITC). In slices from both Gja1+/+ (n = 7) and Gja1Jrt/+ (n = 10) mice, SR-B dye was observed in cortical astrocytes that were adjacent to the injected cell, demonstrating intracellular dye transfer. Figure 1 shows one example in from a Gja1Jrt/+ mouse. Unpaired two-tailed t-test with Welch’s correction showed no difference (t = 0.4798; P < 0.6417) between the number of coupled cells in Gja1Jrt/+ (mean 7.5 cells ± 1.23) and Gja1+/+ (mean 8.57 ± 1.86) mice.
Fig. 1. Astrocytes in Gja1Jrt/+ mice are dye-coupled.
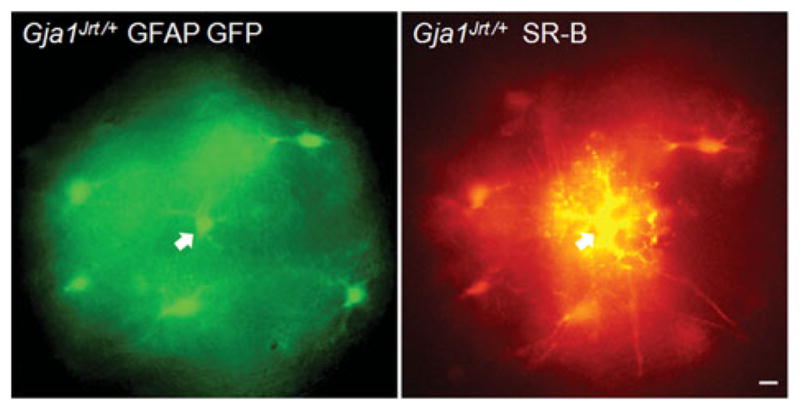
These are images from an acute slice of P23 Gja1Jrt/+ GFAP-Gfp mouse cortex. One GFP-positive cell was injected with SR-B (arrow); the adjacent GFP-positive cells were also SR-B-positive after 20 min. Scale bar: 10 μm.
Cultured astrocytes and cardiac myocytes from Gja1Jrt/+ mice are functionally coupled
The above result was surprising, because dye transfer is disrupted in cultured Gja1Jrt/+ granulosa cells, which express Cx43 (Flenniken et al., 2005). Because astrocytes also express Cx30 (Nagy et al., 1999), we considered the possibility that Cx30 homotypic channels might account for this discrepancy. To study the effect of the G60S mutant on A:A coupling in a simpler system, we examined cultured astrocytes, which express Cx43 but not Cx30 (Giaume et al., 1991; Koulakoff et al., 2008). We injected single astrocytes in confluent cultures prepared from individual Gja1Jrt/+ or Gja1+/+ pups. Because these astrocytes were not GFP-positive, we could use LY. As shown in Fig. 2A,B, LY labeled multiple cells labeled over a few minutes. By counting the number of labeled cells after 5 min, we determined that the number LY-positive cells was similar (no difference in an unpaired two-tailed t-test with Welch’s correction; t = 0.1117; P < 0.9118) between Gja1Jrt/+ (mean 7.3 ± 5.8; n = 3 animals, with 3–8 coverslips per animal) and Gja1+/+ astrocytes (mean 7.3 ± 7.3; n = 4 animals, with 1–8 coverslips per animal). We immunostained our cultures with a rabbit antiserum that specifically labels Cx30 (Yum et al., 2007), combined with a monoclonal antibody against Cx43. The astrocytes from both Gja1Jrt/+ (n = 10) and their Gja1+/+ littermates (n = 10) were Cx30-negative and Cx43-positive (red); Gja1+/+ astrocytes had more conspicuous Cx43-positive puncta on their apposed cell membranes (Fig. 2C,D).
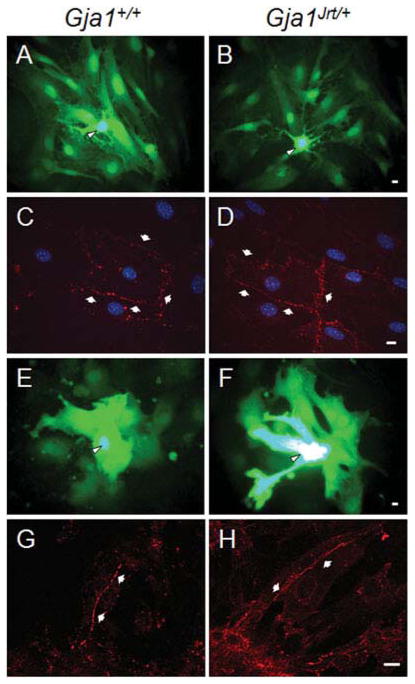
Fig. 2. Cultured Gja1Jrt/+ astrocytes and cardiac myocytes express Cx43-positive GJs and are dye coupled.
Astrocytes (A,B) and cardiac myocytes (E,F) were cultured from individual Gja1Jrt/+ mice or their Gja1+/+ littermates and either injected with LY (arrowheads indicate the injected cell) or fixed, immunostained for Cx43, and imaged by epifluorescence (C,D) or confocal (G,H) microscopy. Note that Gja1Jrt/+ and Gja1+/+ astrocytes and cardiac myocytes were extensively labeled with LY, and have Cx43-positive (red) GJ plaques on apposed cell membranes (arrows). Scale bars: 10 μm.
We also studied cultured cardiac myocytes, which express Cx43 and are affected by the loss of Cx43 (Vink et al., 2004) and by the G60S mutation (Flenniken et al., 2005; Manias et al., 2008). We cultured myocytes from single Gja1Jrt/+ and their Gja1+/+ littermates, and injected LY into single cells of spontaneously beating clusters. Both Gja1Jrt/+ (n = 5 animals, with 2–5 coverslips per animal) and Gja1+/+ (n = 5 animals, with 2–5 coverslips per animal) myocytes showed extensive LY coupling (Fig. 2E,F), in agreement with Manias et al. (2008). Because the myocytes were clustered, however, we could not perform a meaningful quantitative analysis. Immunostaining showed that most of the cultured myocytes from Gja1Jrt/+ (n = 5) mice had Cx43-positive GJ plaques on apposed cell membranes as did their Gja1+/+ littermates (n = 3; Fig. 2G,H).
G60S does not form functional channels in N2A cells
The above results provided no evidence for a dominant-negative effect of G60S on coupling. To further examine this, we expressed G60S alone, WTCx43 alone or both G60S and WTCx43 in N2A cells by transient transfection using a bicistronic vector that also expressed the monomeric DsRed. In agreement with Flenniken et al. (2005), G60S formed GJ plaques at apposed cell borders (see supplementary Fig. 1 online). As a functional test, we patched a single DsRed-positive cell in a cluster of DsRed-positive cells with an electrode containing LY. If the vector contained WTCx43, dye transfer was observed in the DsRed-positive cells of the cluster (Fig. 3A, upper panels; n = 18 injections, from three separate transfections). In contrast, if the vector contained the G60S mutant alone, no LY transfer was observed (Fig. 3A, middle panels; n = 18 injections, from three separate transfections). If the cells were transfected to express both G60S (in the DsRed vector) and WTCx43 (in the pIRES2–puro3 vector; uncolored), LY transfer was seen in the DsRed-positive cells of that cluster (Fig. 3A, lower panels; n = 8 injections, from three separate transfections). Cells transfected to express both the G60S mutant (in the DsRed vector) and WTCx43 (in the pIRES2–EGFP vector) had GJ plaques at apposed cell borders (see supplementary Fig. 2 online).
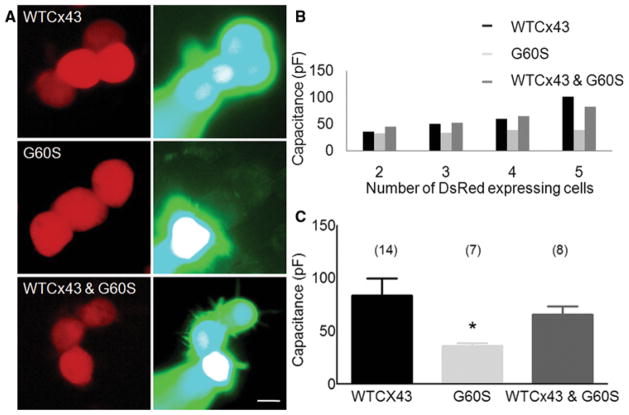
Fig. 3. G60S does not have dominant effects on coupling in cells transfected to express both G60S and WTCx43.
N2A cells were transiently transfected to express WTCx43 (in pIRES2–DsRed) alone, G60S (in pIRES2–DsRed) alone, or both G60S (in pIRES2–DsRed) and WTCx43 (in pIRES2–puro3). (A) Clusters of DsRed cells were identified with TRITC optics (left column), one cell was patched with an electrode containing 0.1% LY, and imaged after 5 min (right column). Cells expressing WTCx43 alone or WTCx43 and G60S show robust dye transfer, but cells expressing G60S alone show no dye transfer. Scale bar: 10 μm. (B) The capacitance transients (in response to a 10 mV pulse) increase progressively in DsRed-positive cell clusters expressing WTCx43 or both WTCx43 and G60S, but not in cells expressing G60S alone. (C) Compared to cells expressing WTCx43, a Kruskal–Wallis test shows a statistically significant difference for cells expressing G60S alone (asterisk; P < 0.0165), but no significant difference for cells expressing both WTCx43 and G60S.
These results from transfected cells as well as cultured astrocytes and cardiomyocytes, provided no evidence that G60S has dominant effects on coupling through WTCx43 channels, which was unexpected given the lack of coupling between granulosa cells from Gja1Jrt/+ mice (Flenniken et al., 2005). To evaluate this further, we compared the capacitance transients from these clusters of cells, as GJ coupling should increase the apparent membrane capacitance of a cell. This analysis (Fig. 3B,C) revealed a monotonic increase in membrane capacitance with increasing number of DsRed-positive cells/cluster for cells expressing WTCx43 alone (n = 14; mean capacitance 83.68 ± 59.70 pF), as previously reported (de Roos et al., 1996), as well as for cells expressing both WTCx43 and G60S (n = 8; mean capacitance 65.5 ± 23 pF). In contrast, cells from clusters expressing G60S alone (n = 7) did not show this increase in capacitance; their mean capacitance of 36.3 ± 6.5 pF. While Kruskal–Wallis analysis with Dunn’s multiple comparison test revealed statistically significant difference in the capacitance between cells expressing either the WTCx43 alone or the G60S mutant alone (8.21; P < 0.017), there was no difference between cells expressing the WTCx43 and cells expressing both the WTCx43 and G60S mutant.
As a more rigorous test, we expressed G60S alone, WTCx43 alone or both G60S and WTCx43 in N2A cells by transient transfection using a bicistronic vector that also expressed the monomeric DsRed or EGFP, and measured electrical coupling between cell pairs with dual whole-cell recordings. These results are summarized in Table 1. When both members of the cell pair expressed WTCx43 (WTCx43/WTCx43), the cell pairs were well coupled, whereas G60S/G60S cell pairs and WTCx43/G60S cell pairs were not coupled. The coupling of cell pairs in which one (WTCx43&G60S/WTCx43) or both (WTCx43&G60S/WTCx43&G60S) members expressed both WTCx43 and G60S were not statistically different than in WTCx43/WTCx43 cell pairs. Thus, these electrophysiological data provide no support for the idea that G60S has a dominant-negative effect on GJ coupling.
Table 1. Dual whole-cell patch clamp recordings.
Neuro2A cells were transiently transfected to express the indicated constructs and were paired as shown. GJ coupling was measured in nS. As expected, Cx43 homotypic pairs (WT/WT) were well coupled, as were pairs of cells in which both cells expressed Cx43WT, regardless of whether one (WT + G60S/WT) or both (WT + G60S/WT + G60S) cells also expressed G60S. Cell pairs expressing G60S alone (G60S/G60S) showed no coupling (significantly different from WT/WT at P < 0.05). Only one heterotypic Cx43G60S/Cx43WT pairing showed any coupling; the other six cell pairs showed no coupling.
| WT/WT | G60S/G60S | WT/G60S | WT + G60S/WT + G60S | WT + G60S/WT | |
|---|---|---|---|---|---|
| n | 10 | 8 | 11 | 6 | 13 |
| Mean (nS) SEM | 40.8 | 0.0 | 2.56* | 39.5 | 37.2 |
| 7.74 | 0.0 | 2.52 | 5.88 | 5.19 | |
| P (versus WT/WT) | – | <0.05 | <0.05 | NS | NS |
All due to one true outlier.
G60S interacts directly with WT Cx43
We considered the possibility that the lack of a dominant-negative effect of G60S on WTCx43 could result from a lack of direct interaction. To address this issue, we generated epitope-tagged versions of G60S (G60S-Myc) and WTCx43 (both WTCx43-Flag and WTCx43-Myc) because available antibodies do not discriminate between G60S and WTCx43. We immunoprecipitated lysates of cells co-transfected to express WTCx43-Flag and G60S-Myc (or WTCx43-Flag and WTCx43-Myc as a control) with a rabbit antibody against Flag, and immunoblotted the immunoprecipitant for Myc. As shown in Fig. 4A, G60S-Myc and WTCx43-Myc were co-immunoprecipitated, demonstrating that G60S can interact with WTCx43. This experiment was repeated three times, with similar results. We also immunoblotted the immunoprecipitate for Cx43, and found that the amount of Cx43 (the sum of Flag- and Myc-tagged Cx43) was reduced in cells expressing both WTCx43-Flag and G60S-Myc compared to cells expressing both WTCx43-Flag and WTCx43-Myc (Fig. 4B).
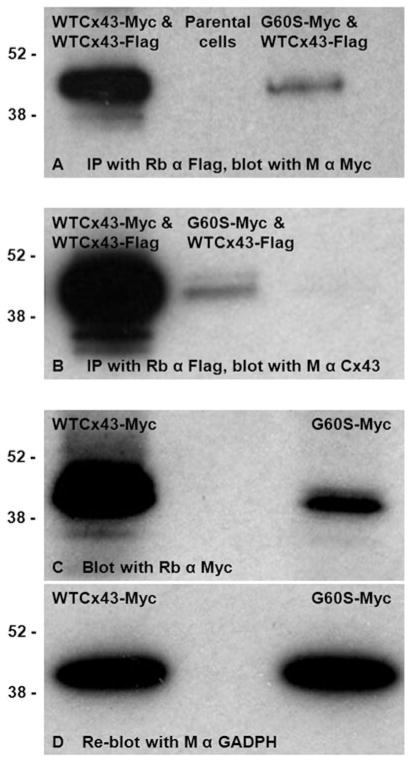
Fig. 4. G60S co-immunoprecipitates with WTCx43.
N2A cells were transiently co-transfected to express both WTCx43-Flag and WTCx43-Myc or WTCx43-Flag and G60S-Myc, and immunoprecipitated with a rabbit antiserum against Flag. The immunoprecipitants were immunoblotted with a mouse monoclonal antibody against Myc, confirming that WTCx43 and the mutant interact (A). Immunoblotting immunoprecipitates for Cx43 shows that the overall expression of Cx43 (WTCx43-Flag and either WTCx43-Myc or G60S-Myc) was lower for cells expressing G60S-Myc (B). Panels C and D are immunoblots of lysates of cells that were transiently transfected to express G60S-Myc or WTCx43-Myc, probed with a rabbit antiserum against Myc then reprobed with a monoclonal antibody against GAPDH. Note that the steady-state level of G60S-Myc is lower than that of the WTCx43-Myc (C), despite equal loading of the cell lysates as shown by blotting for GADPH (D). The positions of 38 and 52 kDa molecular markers are indicated.
The reduced amount of co-immunoprecipitated G60S-Myc could reflect a lower steady-state level of G60S-Myc or a weaker interaction of G60S-Myc with WTCx43-Flag than between WTCx43-Myc and WTCx43-Flag. To investigate this issue, we immunoblotted lysates of cells expressing G60S-Myc alone or WTCx43-Myc alone (Fig. 4C), and reblotted for GADPH (Fig. 4D), and found that the level of G60S-Myc was reduced. Immunostaining transfected cells revealed that G60S-Myc (unlike G60S without the Myc tag) is localized to the Golgi (data not shown), and in co-transfected cells, that WTCx43-Flag and G60S-Myc were co-localized in the Golgi did not form GJ plaques (in contrast to cells co-expressing WTCx43-Flag and WTCx43-Myc, which form GJ plaques; see supplementary Fig. 3 online). In summary, G60S-Myc interacts with and has a dominant effect on WTCx43-Flag, but the epitope tag confounds this analysis because it altered the localization of the G60S mutant. The interaction between G60S-Myc and WTCx43-Myc likely occurs in the Golgi, where the two proteins are largely localized, and which is the site of Cx43 oligomerization (Musil and Goodenough, 1993).
Localization of Cx43 in Gja1Jrt/+ and Gja1+/+ mice
To determine whether the G60S mutant affects the expression of WTCx43 in vivo, we immunostained sections of the cerebellum, spinal cord and heart from a litter of P40 mice. As shown in Fig. 5, apposed cell membranes of cardiac muscle cells from both Gja1+/+ and Gja1Jrt/+ had Cx43-immunoreactivity; this was stronger in Gja1+/+ mice (n = 3) than in their Gja1Jrt/+ littermates (n = 4), in accord with prior work (Flenniken et al., 2005; Manias et al., 2008). Similarly, we found that Cx43 expression was reduced in the cerebellum and spinal cord of Gja1Jrt/+ mice. The number and/or size of GJ plaques appeared reduced, without an increase in the intracellular staining (as might be expected if G60S were abnormally retained); Cx43-immunoreactivity was more pronounced in the gray matter than in the white matter (Yamamoto et al., 1990). To corroborate these findings, we compared the level of Cx43 in homogenates of three brain regions from another litter of P40 Gja1Jrt/+ and the Gja1+/+ littermates. In immunoblots (Fig. 6), the levels of Cx43 were reduced in the spinal cords and cerebella of Gja1Jrt/+ compared their Gja1+/+ littermates, but we did not see such a difference in the cerebra. The levels of Cx30 in the brains, cerebella and spinal cords of Gja1Jrt/+ mice were similar to that in Gja1+/+ littermates both by immunostaining (see supplementary Fig. 4 online) and immunoblotting (see supplementary Fig. 5 online); there was no evidence for compensatory increase in Cx30 expression.
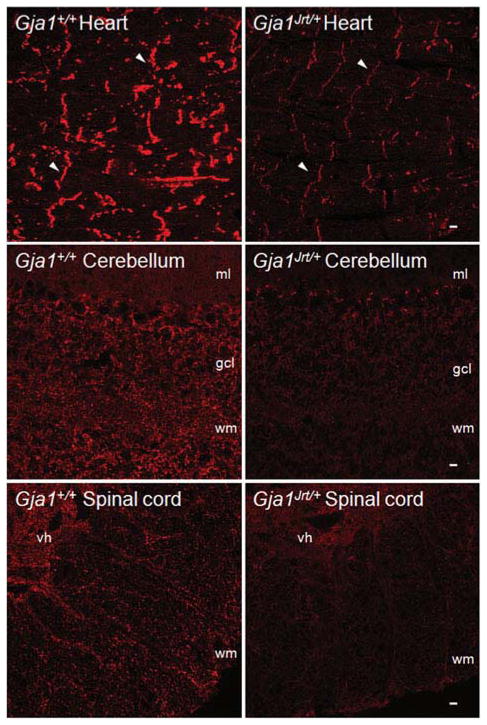
Fig. 5. Reduced Cx43-immunoreactivity in the heart, cerebellum and spinal cord of Gja1Jrt/+ mice.
These are confocal images of frozen sections from the heart, cerebellum and spinal cord of P40 Gja1Jrt/+ mice and their Gja1+/+ littermates. The sections were immunostained concurrently for Cx43 (red), and exposed for the same time to illustrate the diminished Cx43 staining in Gja1Jrt/+ tissues. In the heart, apposed membranes of cardiac myocytes from both Gja1+/+ and Gja1Jrt/+ mice are Cx43-positive. In the cerebellum, Cx43 is mostly localized in the granule cell layer (gcl) and white matter (wm) but not in the molecular layer (ml). In the spinal cord, Cx43 is more prominent in the gray matter of the ventral horn (vh) than in the white matter (wm). Scale bars: 10 μm.
Fig. 6. Reduced Cx43 in the CNS of Gja1Jrt/+ mice.
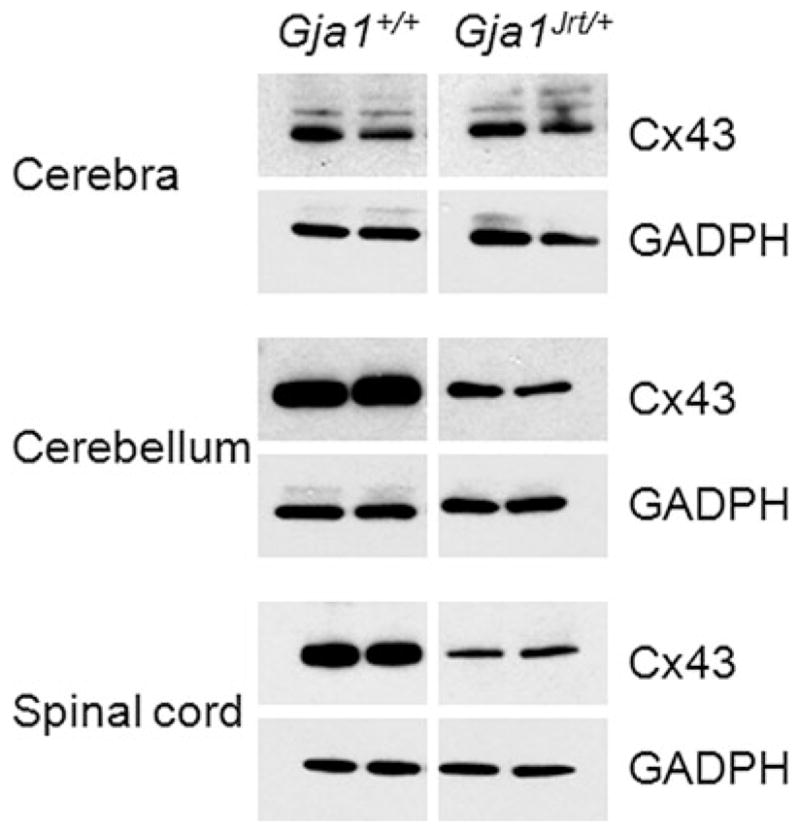
These are immunoblots of individual cerebra, cerebella and spinal cords of P40 Gja1Jrt/+ mice and their Gja1+/+ littermates. Ten μg of protein/lane was separated by electrophoresis, transferred to a membrane, probed with a rabbit antiserum against Cx43, then re-probed with mouse monoclonal antibody against GADPH, to show that the loading was comparable. The level of Cx43 is lower in Gja1Jrt/+ cerebella (B) and spinal cords (C), but not cerebra (A). The larger bands of Cx43 in the cerebra are likely phosphorylated isoforms (Manias et al., 2008). The Gja1Jrt/+ and Gja1+/+ samples were on the same membrane, but the intervening lanes were spliced out.
Cortical lamination and myelination in Gja1Jrt/+ and Gja1+/+ mice
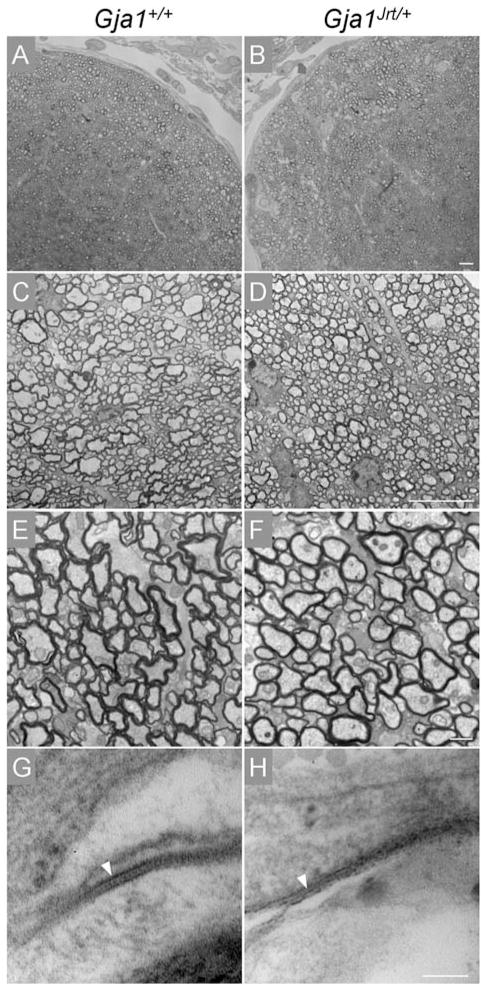
To determine whether the G60S mutant affects cortical lamination or myelination, we examined semithin sections of the cerebra, spinal cords and, optic nerves and thin sections from optic nerves from a litter of P40 mice. We did not detect any difference in myelination in Gja1Jrt/+ mice (n = 3) compared to their Gja1+/+ littermates in any of these regions; the optic nerve is shown in Fig. 7. Furthermore, using electron microscopy, we found A:A GJs between subpial astrocytes (Fig. 7) and A:O GJs in the optic nerve (data not shown). We did not detect any changes in cortical lamination in Gja1Jrt/+ mice (n = 3; see supplementary Fig. 6 online).
Fig. 7. Normal myelination in Gja1Jrt/+ CNS.
These are digital images of individual optic nerves from P40 Gja1Jrt/+ mice (n = 3) and their Gja1+/+ littermates (n = 3). Panels A and B show toluidine blue stained semi-thin sections. Panels C–H show electron micrographs from thin sections; C–F show that the myelinated axons in Gja1Jrt/+ mice appeared similar to those in their WT littermates; G and H show GJs (arrowheads) between subpial astrocytes in Gja1Jrt/+ and Gja1+/+ mice. Scale bars: 10 μm in A–D; 1 μm in E and F, and 100 nm in G and H.
DISCUSSION
We have confirmed and extended the analysis of the G60S mutant, which mediates the abnormal phenotype in Gja1Jrt/+ mice. We confirm that in transfected cells, the G60S mutant does not form functional GJs when expressed alone (Flenniken et al., 2005), and show for the first time that G60S does not detectably disrupt GJs when co-expressed with WTCx43 in co-transfected cells, even though G60S co-immunoprecipitates with WTCx43. We also demonstrate that in both acute brain slices and cultured astrocytes from Gja1Jrt/+ mice, G60S does not detectably disrupt dye coupling between astrocytes. Thus, whereas G60S has dominant effects in some cell types such as ovaries (on both dye transfer and electrical coupling (Flenniken et al., 2005)), cardiac myocytes (electrical coupling (Manias et al., 2008)), myometrial smooth muscle cells (electrical coupling (Tong et al., 2009a, b)) and mammary epithelial cells (dye transfer (Plante and Laird, 2008)), these effects were not detected in astrocytes.
Flenniken et al. (2005) expressed GFP-tagged WT and G60S in heterologous cells and found that G60S-GFP was prominently localized in the Golgi, but appeared to form at least some GJ plaques. We observed that G60S formed GJ plaques, whereas G60S-Myc and G60S-Flag did not; they were largely localized to the Golgi and appeared to sequester WTCx43 in co-transfected cells. Because oligomerization of endogenous Cx43 occurs in the trans-Golgi network (Musil and Goodenough, 1993), defective oligomerization might be the cause of the apparent retention of G60S (and WTCx43) in the Golgi. This, in turn, could increase the degradation of WTCx43 and G60S by proteosomes or lysosomes (Laing et al., 1997), and hence, lower their steady-state levels. Why the Myc- and Flag-tags resulted in little cell surface expression of Cx43 (and more apparent retention in the Golgi) is unclear. The tags were placed at the C-terminus, and should have disrupted the PDZ-binding site (Giepmans and Moolenaar, 1998), and potentially other protein–protein interactions (Chanson et al., 2007), but not oligomerization (Maass et al., 2004). A C-terminal GFP-tag affects the physiological characteristics of WTCx43, but apparently not its ability to form GJ plaques (Bukauskas et al., 2001).
Flenniken et al. (2005) also found that WTCx43-GFP, but not G60S-GFP, formed functional channels in N2A cells, but they did not analyze cells that were co-transfected to express both WTCx43 and G60S. Instead, they analyzed granulosa cells, which are thought to express exclusively Cx43, and found weak (10/27 injections) or absent (17/27) dye transfer. In contrast, we could not document diminished dye coupling in cells transfected to express both WT Cx43 and G60S, in cultured cardiac myocytes, cultured astrocytes or astrocytes in acute brain slices, and we could not document diminished electrical coupling in cells transfected to express both WTCx43 and G60S. Compensation by another Cx is unlikely, as Manias et al. (2008) did not detect increased Cx40 or Cx45 in the hearts of Gja1Jrt/+ mice, and we did not detect increased Cx30 in any brain region, or in cultured astrocytes (nor would LY be expected to diffuse across Cx30 homotypic channels (Manthey et al., 2001; Beltramello et al., 2003)). Thus, why granulosa cells are more affected in Gja1Jrt/+ mice remains to be explained.
Although GJs have long been recognized to be a prominent feature of astrocytes (Peters et al., 1991), their functions have only recently been elucidated, largely by investigating mice that have null alleles. Although Gja1-null mice were initially thought to have normal brains at birth (when they die of a cardiac defect (Dermietzel et al., 2000)), conditionally deleting Cx43 in the CNS results in abnormal cerebellar development (Wiencken-Barger et al., 2007) and abnormal neuronal migration in the cortex (Cina et al., 2009). Deleting both Cx30 and Cx43 in astrocytes completely disrupts GJ communication, with diminished K+ buffering (Wallraff et al., 2006), and decreased trans-cellular diffusion of a glucose analog between astrocytes (Rouach et al., 2008). In addition, these mice are long lived, with mild but wide spread white matter abnormalities (Lutz et al., 2009), in contrast to the more severe phenotype of mice lacking both Cx32 and Cx47, which die at ~6 weeks (Menichella et al., 2003; Odermatt et al., 2003).
In contrast, the G60S mutant did not produce a discernible effect on dye transfer of LY, although our analysis could have missed a subtle effect on electrical coupling, as was found in cardiac myocytes (Manias et al., 2008). Just as not all ODDD patients have CNS manifestations (Paznekas et al., 2009; Alao et al., 2010), perhaps the lack of detectable abnormalities in Gja1Jrt/+ astrocytes owes to the mild effect of the Gja1Jrt allele. The analysis of astrocytes coupling in mice that express mutations known to result in a CNS phenotype in humans, such as I130 T (Kalcheva et al., 2007) and G138R (Dobrowolski et al., 2008), should be informative.
ODDD mutants appear to produce their clinical phenotype in a cell autonomous manner, and a dominant-negative effect on WTCx43 is the best substantiated mechanism (McLachlan et al., 2005; Roscoe et al., 2005; Shibayama et al., 2005; Gong et al., 2006). Disrupting Cx43:Cx43 homotypic channels is the most obvious way that ODDD mutants would affect astrocytes, but mutants could also affect heterotypic Cx43:Cx47 coupling between astrocytes and oligodendrocytes. A reduction in the amount of Cx43 in certain cells might account for neurological manifestations, either by altering proteins expression (Iacobas et al., 2004) or by impairing other functions of Cx43 such as it has adhesive properties (Elias et al., 2007), or by protein–protein interactions (Chanson et al., 2007).
Supplementary Material
Acknowledgments
This work was supported by the NIH grants NS55284 (to S.S.S.) and NS050705 (to C.K.A.). We thank Drs. Ann Flenniken and Janet Rossant for the Gja1Jrt/+ mice, Dr. John Dempster for the WCP software, and Drs. Christian Giaume, Philip Haydon, Jian Li, Kenneth Morgulies, Haji Takano and Ambuin Mu for advice.
Footnotes
Statement of interests
None.
The supplementary material referred to in this article can be found online at journals.cambridge.org/ngb.
References
- Alao MJ, Bonneau D, Holder-Espinasse M, Goizet C, Manouvrier-Hanu S, Mezel A, et al. Oculo-dento-digital dysplasia: lack of genotype-phenotype correlation for GJA1 mutations and usefulness of neuroimaging. European Journal of Medical Genetics. 2010;53:19–22. doi: 10.1016/j.ejmg.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24:4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D’Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochemical and Biophysical Research Communications. 2003;305:1024–1033. doi: 10.1016/s0006-291x(03)00868-4. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. European Journal of Biochemistry/FEBS. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Bukauskiene A, Bennett MV, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophysical Journal. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson M, Kotsias BA, Peracchia C, O’Grady SM. Interactions of connexins with other membrane channels and transporters. Progress in Biophysics and Molecular Biology. 2007;94:233–244. doi: 10.1016/j.pbiomolbio.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cina C, Maass K, Theis M, Willecke K, Bechberger JF, Naus CC. Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. Journal of Neuroscience. 2009;29:2009–2021. doi: 10.1523/JNEUROSCI.5025-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, et al. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Research Brain Research Reviews. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Human Molecular Genetics. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. Journal of Cell Biology. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Richardson RJ, Butterworth J, Barron MJ, Dixon MJ. Novel mutations in GJA1 cause oculodentodigital syndrome. Journal of Dental Research. 2008;87:1021–1026. doi: 10.1177/154405910808701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, et al. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132:4375–4386. doi: 10.1242/dev.02011. [DOI] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D. Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron. 1991;6:133–143. doi: 10.1016/0896-6273(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Current Biology: CB. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- Gong XQ, Shao Q, Lounsbury CS, Bai D, Laird DW. Functional characterization of a GJA1 frameshift mutation causing oculodentodigital dysplasia and palmoplantar keratoderma. Journal of Biological Chemistry. 2006;281:31801–31811. doi: 10.1074/jbc.M605961200. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, Zackai EH, McDonald-McGinn DM, Fischbeck KH, Kamholz J. Oculodentodigital dysplasia syndrome associated with abnormal cerebral white matter. American Journal of Medical Genetics. 1991;41:18–20. doi: 10.1002/ajmg.1320410106. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Scemes E, Spray DC. Gene expression alterations in connexin null mice extend beyond the gap junction. Neurochemistry International. 2004;45:243–250. doi: 10.1016/j.neuint.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, et al. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proceedings of the National Academy of Sciences of the USA. 2007;104:20512–20516. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakoff A, Ezan P, Giaume C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia. 2008;56:1299–1311. doi: 10.1002/glia.20698. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Laing JG, Tadros PN, Westphale EM, Beyer EC. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Experimental Cell Research. 1997;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- Li X, Ionescu AV, Lynn BD, Lu S, Kamasawa N, Morita M, et al. Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain. Neuroscience. 2004;126:611–630. doi: 10.1016/j.neuroscience.2004.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddenkemper T, Grote K, Evers S, Oelerich M, Stogbauer F. Neurological manifestations of the oculodentodigital dysplasia syndrome. Journal of Neurology. 2002;249:584–595. doi: 10.1007/s004150200068. [DOI] [PubMed] [Google Scholar]
- Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. Journal of Neuroscience. 2009;29:7743–7752. doi: 10.1523/JNEUROSCI.0341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, et al. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Molecular Biology of the Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manias JL, Plante I, Gong XQ, Shao Q, Churko J, Bai D, et al. Fate of connexin43 in cardiac tissue harbouring a disease-linked connexin43 mutant. Cardiovascular Research. 2008;80:385–395. doi: 10.1093/cvr/cvn203. [DOI] [PubMed] [Google Scholar]
- Manthey D, Banach K, Desplantez T, Lee CG, Kozak CA, Traub O, et al. Intracellular domains of mouse connexin26 and -30 affect diffusional and electrical properties of gap junction channels. Journal of Membrane Biology. 2001;181:137–148. doi: 10.1007/s00232-001-0017-1. [DOI] [PubMed] [Google Scholar]
- McLachlan E, Manias JL, Gong XQ, Lounsbury CS, Shao Q, Bernier SM, et al. Functional characterization of oculodento-digital dysplasia-associated Cx43 mutants. Cell Communication and Adhesion. 2005;12:279–292. doi: 10.1080/15419060500514143. [DOI] [PubMed] [Google Scholar]
- Meme W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, et al. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB Journal. 2006;20:494–496. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. Journal of Neuroscience. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Ionescu AV, Lynn BD, Rash JE. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: implications from normal and connexin32 knockout mice. Glia. 2003;44:205–218. doi: 10.1002/glia.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Astrocyte and oligodendrocyte connexins of the glial syncytium in relation to astrocyte anatomical domains and spatial buffering. Cell Communication and Adhesion. 2003;10:401–406. doi: 10.1080/15419060390263191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus CC, Bechberger JF, Zhang Y, Venance L, Yamasaki H, Juneja SC, et al. Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin43. Journal of Neuroscience Research. 1997;49:528–540. doi: 10.1002/(SICI)1097-4547(19970901)49:5<528::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, et al. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. Journal of Neuroscience. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. Journal of Molecular Neuroscience. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. Journal of Neuroscience. 2007;27:13949–13957. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. American Journal of Human Genetics. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Human Mutation. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System, Neurons and Their Supporting Cells. 3. New York: Oxford University Press; 1991. [Google Scholar]
- Plante I, Laird DW. Decreased levels of connexin43 result in impaired development of the mammary gland in a mouse model of oculodentodigital dysplasia. Developmental Biology. 2008;318:312–322. doi: 10.1016/j.ydbio.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Communication & Adhesion. 2001;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Froger N, Palacios-Prado N, Ezan P, Saez PJ, Saez JC, et al. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. Journal of Neuroscience. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R, Donnai D, Meire F, Dixon MJ. Expression of Gja1 correlates with the phenotype observed in oculodentodigital syndrome/type III syndactyly. Journal of Medical Genetics. 2004;41:60–67. doi: 10.1136/jmg.2003.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roos AD, van Zoelen EJ, Theuvenet AP. Determination of gap junctional intercellular communication by capacitance measurements. Pflugers Archiv: European Journal of Physiology. 1996;431:556–563. doi: 10.1007/BF02191903. [DOI] [PubMed] [Google Scholar]
- Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, et al. Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. Journal of Biological Chemistry. 2005;280:11458–11466. doi: 10.1074/jbc.M409564200. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. Journal of Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, et al. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circulation Research. 2005;96:e83–e91. doi: 10.1161/01.RES.0000168369.79972.d2. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovascular Research. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Tong D, Colley D, Thoo R, Li TY, Plante I, Laird DW, et al. Oogenesis defects in a mutant mouse model of oculodentodigital dysplasia. Disease Models amd Mechanisms. 2009a;2:157–167. doi: 10.1242/dmm.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong D, Lu X, Wang HX, Plante I, Lui E, Laird DW, et al. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biology of Reproduction. 2009b;80:1099–1106. doi: 10.1095/biolreprod.108.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink MJ, Suadicani SO, Vieira DM, Urban-Maldonado M, Gao Y, Fishman GI, et al. Alterations of intercellular communication in neonatal cardiac myocytes from connexin43 null mice. Cardiovascular Research. 2004;62:397–406. doi: 10.1016/j.cardiores.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. Journal of Neuroscience. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. A role for Connexin43 during neurodevelopment. Glia. 2007;55:675–686. doi: 10.1002/glia.20484. [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biological Chemistry. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. Journal of Comparative Neurology. 1990;302:853–883. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, et al. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. American Journal of Physiology Cell Physiology. 2007;293:C1032–C1048. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Developmental Biology. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.