Abstract
Background
Ziziphus jujuba Mill. is nutritious and used as food and medicine for more than two thousand years. It has many pharmacological effects, such as elimination of fatigue, dilation of blood vessels, etc. The polysaccharide in it is one of the bioactive substances. In this paper, the ultrasonic extraction effects on the yield and activity of polysaccharide were studied.
Results
The optimum ultrasonic extraction conditions were investigated based on a Box-Behnken statistical experimental design. Response surface methodology (RSM) of three factors (ultrasonic power, extraction time and extraction temperature) and three levels was employed to optimize the yield and the antioxidant activity of the polysaccharides. The experimental data were fitted to quadratic response surface models using multiple regression analysis. The best extraction conditions were 120 W, 15 min. and 55°C for highest yield, and 80 W, 15 min. and 40°C for highest hydroxyl radical scavenging activity.
Conclusion
The study showed that high ultrasonic power was good for obtaining high yield but bad for keeping the antioxidant activity of the polysaccharides.
Keywords: Ultrasonic extraction, Response surface methodology (RSM), Polysaccharides, Ziziphus jujuba Mill., Optimization
Introduction
Ziziphus jujuba Mill. is a native plant of China and belongs to the genus Ziziphus Mill. (Rhamnaceae) [1]. Its fruits have been used in traditional Chinese medicine for more than two thousand years. The bioactivities of the polysaccharides in Ziziphus jujuba Mill. have been reported, such as immunobiological activities [1-4] and antioxidant activities [5].
Research reports revealed that the bioactivities of polysaccharides in Ziziphus jujuba Mill. were related to their structures. Chang et al.[5] isolated one neutral polysaccharide fraction (ZJPN) and three acidic polysaccharide fractions (ZJPa1, ZJPa2 and ZJPa3). Gas chromatography (GC) analysis revealed that six monosaccharides, namely, rhamnose, arabinose, xylose, mannose, glucose and galactose were present in the polysaccharide fractions. All four polysaccharide fractions were found to be more effective at scavenging superoxide anion radicals than hydroxyl radicals, while the acidic polysaccharides showed a more pronounced effect at chelating ferrous ion [5]. Zhao et al.[2] obtained a fraction, Ju-B-7, which could stimulate spleen cell proliferation and had a molecular mass of over 2000 kDa. This isolated polysaccaride was mainly composed of α-1,4-linked d-galactopyranosyluronic acid and 1,2-linked l-rhamnose at a molar ratio of 8.1:1.
Ultrasonic extraction was widely employed to extract polysaccharides from plant material due to its high extraction efficiency [6-9]. However, ultrasonicaion can change the structures of the polysaccharides to some extent [10]. In this paper, the effects of ultrasonic power, extraction time, extraction temperature on the yield and the antioxidant activity of water soluble polysaccharides of Ziziphus jujuba Mill. were investigated by response surface methodology (RSM).
RSM is an effective statistical technique, which is used to find optimum processing parameters [11-13]. It has been used to optimize the polysaccharides extraction process variables and the interactions of these variables [14-18]. In the present study, a three-variable, three-level Box–Behnken design (BBD) [19-25] was used to optimize the extraction conditions for ultrasonic extraction of water soluble polysaccharides in Ziziphus jujuba Mill.
Experimental
Chemicals and instruments
Ziziphus jujuba Mill., which grew in Xinjiang province (China) was purchased from a local shop in Zhengzhou, China. All reagents used in this study were of analytical grade. Anhydrous ethanol, 95% ethanol and acetone were obtained from Tianli Corporation (Tianjin, China). Ferrous sulfate (FeSO4), salicylic acid and petroleum ether were purchased from Kermel Corporation (Tianjin, China). Hydrogen peroxide (H2O2) was obtained from Haohua Corporation (Luoyang, China). Deionized water used in the experiments was purified by a Milli-Q system (Millipore Corporation, USA).
KQ5200DE ultrasonic cleaner, which can control ultrasonic temperature, power and time, was supplied by Kunshan Corporation (Shanghai, China). RE-52A rotary evaporator (Yarong Corporation, Shanghai, China) and 752 UV–vis spectrophotometer (Jinghua Corporation, Shanghai, China) were also employed in the experiments.
Extraction procedure
The fruit of Ziziphus jujuba Mill. was first peeled, then the kernel was removed. The obtained pulp was dried at 40°C. The dried sample was extracted in a Soxhlet apparatus, first with petroleum ether, and afterwards with 80% ethanol twice, to remove some colored materials, monosaccharides, oligosaccharides, and small molecular weight materials. The organic solvent was evaporated to yield a dried extracted powder.
5.0 g of the Soxhlet-extracted powder was placed into a beaker with 100 g water. The powder was ultrasonically extracted for different time at varied extraction temperatures and power levels. Then the extraction solution was centrifuged for 15 min. at 4000 rpm. The supernatant was collected concentrated, and treated with 95% ethanol successively; the mixture was stored in a refrigerator at 4°C for 12 h. Afterwards, the obtained mixture was filtrated and the precipitate was successively washed by 95% ethanol, anhydrous ethanol and acetone. The washed precipitant, which was the crude polysaccharides, was dried at 40°C. The crude polysaccharides yield (%) was then calculated according to the following equation:
| (1) |
Antioxidant activity
The hydroxyl radical scavenging activity of water soluble polysaccharides in Ziziphus jujuba Mill. was investigated by the following method. Approximately 2 mL of 1.8 mmol·L-1 FeSO4 and 1.5 mL of 1.8 mmol·L-1 salicylic acid were added into a tube and mixed. Then 1 mL of 3 mg·mL-1 polysaccharides solution was added along with 1 mL of 0.3% H2O2 and mixed to initiate the reaction. The tube was put into a 37°C water bath for 30 min.; afterwards, the UV–vis absorbance at 510 nm was recorded. 1 mL of water was used instead of 1 mL of 3 mg·mL-1 polysaccharides solution and other steps were same as polysaccharide sample to obtain the absorbance of the control. The hydroxyl radical scavenging activity of the polysaccharides was calculated using the following equation:
| (2) |
Design of experiments
On the basis of single factor experiment, RSM was performed on the experimental data using a commercial statistical package, Design-Expert trial version 8.0.5 (Statease Inc., Minneapolis, USA) [26-28]. As shown in Table 1, a Box–Behnken design (BBD) with three independent variables, including ultrasonic power (X1), extraction time (X2), and extraction temperature (X3), was used for the optimization. On the basis of single factor experiments of ultrasonic extraction, three levels were coded as +1, 0, and −1 for high, intermediate and low values, respectively. The response functions were yield and hydroxyl radical scavenging activity of polysaccharides. The form of quadratic response model was as follows:
Table 1.
Box–Behnken design and the response values for yield and hydroxyl radical scavenging activity of polysaccharides
|
Run |
X1: Ultrasonic Power (W) |
X2: Extraction time (min) |
X3: Extraction temperature (°C) |
Yield of polysaccharides (%) |
Hydroxyl radical scavenging activity of polysaccharides (%) |
||
|---|---|---|---|---|---|---|---|
| Actual values | Predicted values | Actual values | Predicted values | ||||
| 1 |
120 (+1) |
15 (+1) |
50 (0) |
4.44 |
4.53 |
35.61 |
35.66 |
| 2 |
80 (−1) |
5 (−1) |
50 (0) |
3.26 |
3.17 |
49.36 |
49.31 |
| 3 |
100 (0) |
10 (0) |
50 (0) |
3.98 |
4.03 |
50.14 |
50.37 |
| 4 |
100 (0) |
10 (0) |
50 (0) |
3.96 |
4.03 |
48.35 |
50.37 |
| 5 |
100 (0) |
10 (0) |
50 (0) |
4.06 |
4.03 |
51.65 |
50.37 |
| 6 |
100 (0) |
5 (−1) |
60 (+1) |
3.56 |
3.59 |
38.43 |
36.73 |
| 7 |
120 (+1) |
5 (−1) |
50 (0) |
3.80 |
3.82 |
39.86 |
41.42 |
| 8 |
80 (−1) |
10 (0) |
40 (−1) |
2.96 |
3.01 |
65.65 |
65.51 |
| 9 |
100 (0) |
10 (0) |
50 (0) |
4.12 |
4.03 |
51.26 |
50.37 |
| 10 |
100 (0) |
15 (+1) |
60 (+1) |
4.16 |
4.12 |
41.65 |
41.46 |
| 11 |
120 (+1) |
10 (0) |
40 (−1) |
3.82 |
3.76 |
47.52 |
45.77 |
| 12 |
80 (−1) |
15 (+1) |
50 (0) |
3.58 |
3.56 |
65.16 |
63.60 |
| 13 |
100 (0) |
10 (0) |
50 (0) |
4.05 |
4.03 |
50.45 |
50.37 |
| 14 |
100 (0) |
15 (+1) |
40 (−1) |
3.68 |
3.65 |
51.68 |
53.38 |
| 15 |
100 (0) |
5 (−1) |
40 (−1) |
3.04 |
3.08 |
49.36 |
49.56 |
| 16 |
80 (−1) |
10 (0) |
60 (+1) |
3.40 |
3.45 |
49.56 |
51.31 |
| 17 | 120 (+1) | 10 (0) | 60 (+1) | 4.36 | 4.31 | 35.08 | 35.22 |
| (3) |
where Y was the response variable, and βo, βi, βii, and βij, were the regression coefficients for the response surface model. Xi and Xj were the independent variables.
Statistical analyses
Design-Expert trial version 8.0.5 (Statease Inc., Minneapolis, USA) was used to statistically analyze the experimental data. The significant terms in the model were found by analysis of variance (ANOVA) for each response. The significances of all terms in the polynomial were considered statistically different when P < 0.05. The adequacy of model was checked by accounting for the coefficient of determination (R2) and adjusted-R2 (R2adj).
Results and discussion
Statistical analysis and the model fitting
Multiple regression analysis of the experimental data afforded the following quadratic response surface models for predicting polysaccharide yield (Yyield) and hydroxyl radical scavenging activity (Yactivity) based on the values of the ultrasonic extraction parameters (i.e., X1, X2 and X3):
| (4) |
| (5) |
In these equations, X1, X2 and X3 were the values of extraction parameters, ultrasonic power (W), extracting time (min.) and extraction temperature (°C), respectively. The variables, experimental data and predicted data are shown in Table 1.
The fitted quadratic surface models for yield and hydroxyl radical scavenging activity of the polysaccharides by ANOVA are shown in Tables 2 and 3, respectively. The quadratic regression model of yield of polysaccharides in Table 2 showed the coefficient of determination coefficient, R2, value was 0.9837, while the value of the adjusted coefficient of determination coefficient, R2adj, was 0.9628, indicating a high degree of correlation between the observed and predicted values. The lower the coefficient of variation (CV), the smaller the residuals were relative to the predicted value. A low CV of 2.21% suggested a good precision and higher reliability of the models to predict experimental results. The “lack-of-fit F-value” of 2.54 implied that the lack-of-fit was not significant relative to the pure error. There was a 19.44% chance that a “lack-of-fit F-value” this large could occur due to noise, which indicated that the model equation was adequate for predicting the yield of polysaccharides. Values of P-value less than 0.05 indicated that the model terms were significant (at the 95% level).
Table 2.
Analysis of variance for the fitted quadratic polynomial model of polysaccharides yield
|
Source |
Sum of squares |
Degree of freedom |
Mean square |
F-Value |
P-value |
|---|---|---|---|---|---|
| Prob > F | |||||
| Model |
2.94 |
9 |
0.33 |
47.07 |
<0.0001 |
| X1 |
1.30 |
1 |
1.30 |
186.60 |
<0.0001 |
| X2 |
0.61 |
1 |
0.61 |
87.10 |
<0.0001 |
| X3 |
0.49 |
1 |
0.49 |
70.55 |
<0.0001 |
| X1 X2 |
0.026 |
1 |
0.026 |
3.69 |
0.0964 |
| X1 X3 |
2.5×10-3 |
1 |
2.5×10-3 |
0.36 |
0.5674 |
| X2 X3 |
4.0×10-4 |
1 |
4.0×10-4 |
0.058 |
0.8172 |
| X12 |
0.060 |
1 |
0.060 |
8.66 |
0.0216 |
| X22 |
0.088 |
1 |
0.088 |
12.66 |
0.0092 |
| X32 |
0.33 |
1 |
0.33 |
47.36 |
0.0002 |
| Residual |
0.049 |
7 |
6.946×10-3 |
|
|
| Lack of Fit |
0.032 |
3 |
0.011 |
2.54 |
0.1944 |
| Pure Error |
0.017 |
4 |
4.18×10-3 |
|
|
| Cor Total |
2.99 |
16 |
|
|
|
| R2=0.9837 R2adj=0.9628 CV=2.21% | |||||
Table 3.
Analysis of variance for the fitted quadratic polynomial model of hydroxyl radical scavenging activity of polysaccharides
|
Source |
Sum of squares |
Degree of freedom |
Mean square |
F-Value |
P-value |
|---|---|---|---|---|---|
| Prob > F | |||||
| Model |
1154.38 |
9 |
128.26 |
38.82 |
<0.0001 |
| X1 |
641.89 |
1 |
641.89 |
191.75 |
<0.0001 |
| X2 |
36.51 |
1 |
36.51 |
10.91 |
0.0131 |
| X3 |
306.16 |
1 |
306.16 |
91.46 |
<0.0001 |
| X1 X2 |
100.50 |
1 |
100.50 |
30.02 |
0.0009 |
| X1 X3 |
3.33 |
1 |
3.33 |
0.99 |
0.3518 |
| X2 X3 |
0.20 |
1 |
0.20 |
0.060 |
0.8128 |
| X12 |
1.78 |
1 |
1.78 |
0.53 |
0.4897 |
| X22 |
52.24 |
1 |
52.24 |
15.61 |
0.0055 |
| X32 |
10.35 |
1 |
10.35 |
3.09 |
0.1222 |
| Residual |
23.43 |
7 |
3.35 |
|
|
| Lack of Fit |
16.86 |
3 |
5.62 |
3.42 |
0.1328 |
| Pure Error |
6.57 |
4 |
1.64 |
|
|
| Cor Total |
1177.82 |
16 |
|
|
|
| R2=0.9801 R2adj=0.9545 CV=3.79% | |||||
Table 3 showed the quadratic regression model of hydroxyl radical scavenging activity of the polysaccharides. It can be seen that R2 was 0.9801 and R2adj was 0.9545, indicating a high degree of correlation between the observed and predicted values. The coefficient of variation was low (CV=3.79%), indicating a high degree of precision and reliability of the experimental values. F-value and P-value of the lack-of-fit were 3.42 and 0.1328, respectively, which implied that it was not significant; there was a 13.28% chance that this lack-of-fit was due to noise. It can be seen from Table 3 that the three independent variables (X1, X2 and X3), one quadratic term (X22), and the interaction between X1 and X2 significantly affected the hydroxyl radical scavenging activity of the polysaccharides.
Analysis of response surface plot
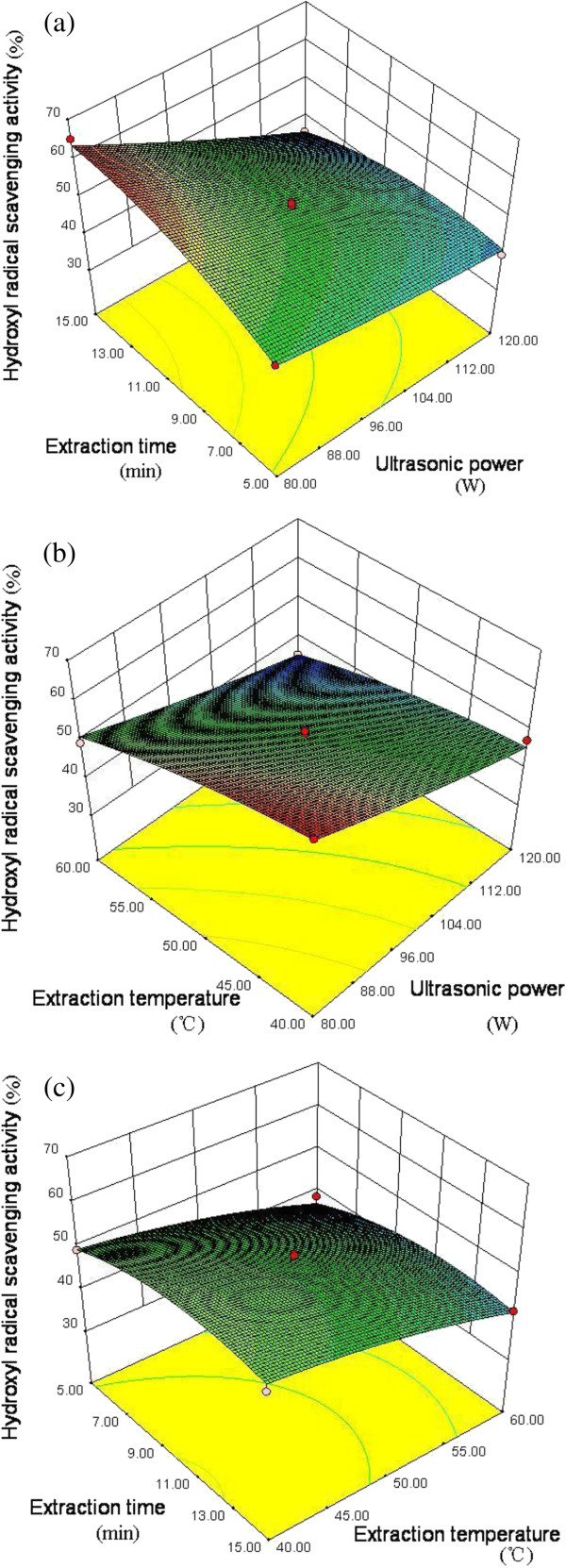
Response surface models were plotted to study the effects of parameter variables (ultrasonic power, extraction time and extraction temperature) and their interactions on yield (Figure 1) and hydroxyl radical scavenging activity (Figure 2) of the polysaccharides. When two variables within the experimental range were displayed in three-dimensional surface plots, the third variable was kept constant at the intermediate level (i.e., 0).
Figure 1.
Response surface plot results on the yield of polysaccharides. (a) Response surface plot of ultrasonic power and extraction time, and their mutual interactions on the yield of polysaccharides. (b) Response surface plot of ultrasonic power and extraction temperature, and their mutual interactions on the yield of polysaccharides. (c) Response surface plot of extraction time and extraction temperature, and their mutual interactions on the yield of polysaccharides.
Figure 2.
Response surface plot results on hydroxyl radical scavenging activity of polysaccharides. (a) Response surface plot of ultrasonic power and extraction time, and their mutual interactions on hydroxyl radical scavenging activity of polysaccharides. (b) Response surface plot of ultrasonic power and extraction temperature, and their mutual interactions on hydroxyl radical scavenging activity of polysaccharides. (c) Response surface plot of extraction time and extraction temperature, and their mutual interactions on hydroxyl radical scavenging activity of polysaccharides.
As shown in Figure 1a, when the extraction temperature (X3) was fixed at 0 level, the yield increased as the ultrasonic power (X1) and extraction time (X2) increased. Figure 1b showed the effects of ultrasonic power (X1) and extraction temperature (X3) on the yield of polysaccharides. The yield increased with the increase of ultrasonic power. The yield was positively correlated with the extraction temperature when temperature was lower than 55°C and was negatively correlated when temperature was higher than 55°C. The interactions between extraction time (X2) and extraction temperature (X3), when ultrasonic power (X1) was fixed at 0 level, were displayed in Figure 1c. The yield increased with the extraction time.
Figure 2 showed the ultrasonic parameter variables (ultrasonic power, extraction time and extraction temperature) and their interactions on hydroxyl radical scavenging activity of polysaccharides. Ultrasonic power (X1) and extraction temperature (X3) both had a negative impact on the activity. Nevertheless, longer extraction times led to an increase of the activity. Therefore, low extraction temperature and low ultrasonic power were advantageous to the hydroxyl radical scavenging activity of polysaccharides.
Optimization of extracting parameters and validation of the model
In Table 4, the optimal ultrasonic extraction condition for obtaining maximal yield of polysaccharides predicted by the quadratic model was as follows: ultrasonic power of 120 W, extraction time of 15 min. and extraction temperature of 54.69°C. The predicted yield of polysaccharides at the optimal extraction condition was 4.59%. In order to facilitate the extraction process, the optimal condition was modified as follows: ultrasonic power of 120 W, extraction time of 15 min. and extraction temperature of 55°C. The actual experimental yield under these conditions was 4.47%, which was in agreement with the predicted model value.
Table 4.
Optimum conditions, and the predicted and experimental values of response
| Ultrasonic power (W) | Extraction time (min) | Extraction temperature (°C) | Yield of polysaccharides (%) | Hydroxyl radical scavenging activity of polysaccharides (%) | |
|---|---|---|---|---|---|
| Optimum condition for yield (predicted) |
120 |
15 |
54.69 |
4.59 |
32.75 |
| Modified condition for yield (actual) |
120 |
15 |
55 |
4.47 |
30.94 |
| Optimum condition for activity (predicted) |
80 |
14.91 |
40 |
3.07 |
68.91 |
| Modified condition for activity (actual) | 80 | 15 | 40 | 2.91 | 67.30 |
The optimal predicted extraction condition for achieving the highest hydroxyl radical scavenging activity of 68.91% was ultrasonic power of 80 W, extraction time of 14.91 min. and extraction temperature of 40°C. For practical implementation, the extraction condition was modified as ultrasonic power of 80 W, extraction time of 15 min. and extraction temperature of 40°C. Using these parameters, the hydroxyl radical scavenging activity was 67.30%, which was close to the maximum predicted by the response surface model (Table 4).
Table 4 also displayed that the hydroxyl radical scavenging activity of the polysaccharides under the optimal condition for highest yield (ultrasonic power of 120 W, extraction time of 15 min. and extraction temperature of 54.69°C) was predicted as 32.75% by the quadratic response surface model (Eq. [5]), and the activity obtained at the experiment condition (ultrasonic power of 120 W, extraction time of 15 min. and extraction temperature of 55°C) was 30.94%. At the same time, the yield of polysaccharides under the optimal condition for best hydroxyl radical scavenging activity of polysaccharides (ultrasonic power of 80 W, extraction time of 14.91 min. and extraction temperature of 40°C) was predicted as 3.07% by Equation [4]. The yield in the modified condition (ultrasonic power of 80 W, extraction time of 15 min. and extraction temperature of 40°C) was 2.91%.
These data suggested that the extraction conditions for obtaining high yield of polysaccharides were not suitable for obtaining good hydroxyl radical scavenging activity, and that the optimal conditions for achieving high hydroxyl radical scavenging activity could not be applied to obtain high yield of polysaccharides. High ultrasonic power was advantageous to yield and adverse to activity, and low extraction temperature was more favorable for high radical scavenging activity. Extraction time 15 min. was good to both the yield and the activity.
Conclusion
The results indicated that the optimum extraction conditions of polysaccharides for obtaining highest yield and highest radical scavenging activity were quite different. Ultrasonic power played an important role in ultrasonic extraction.
Therefore, we should consider not only the high yield but also the sacrificed radical scavenging activity of the polysaccharides during the extraction process.
Abbreviations
RSM: Response surface methodology; GC: Gas chromatography; BBD: Box–Behnken design; ANOVA: Analysis of variance; Yyield: Polysaccharide yield; Yactivity: Hydroxyl radical scavenging activity; CV: Coefficient of variation.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CQ participated in the design of the study and performed the statistical analysis. CQ and YZ participated in the sequence alignment and drafted the manuscript. SY and LL carried out the experiments. YH participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Chenling Qu, Email: quchenling@163.com.
Songcheng Yu, Email: yusongcheng@163.com.
Li Luo, Email: yinxiangzhe222@163.com.
Yan Zhao, Email: zhaoyanss10@126.com.
Yawei Huang, Email: ywei0371@163.com.
Acknowledgements
This research was supported by grants from the Doctor Research Fund of Henan University of Technology (No: 2009BS027).
References
- Zhao Z, Liu M, Tu P. Characterization of water soluble polysaccharides from organs of Chinese Jujube (Ziziphus jujuba Mill. cv. Dongzao) Eur Food Res Technol. 2008;226:985–989. doi: 10.1007/s00217-007-0620-1. [DOI] [Google Scholar]
- Zhao Z, Dai H, Wu X, Chang H, Gao X, Liu M, Tu P. Characterization of a pectic polysaccharide from the fruit of Ziziphus jujuba. Chem Nat Compd. 2007;43(4):311–312. [Google Scholar]
- Zhao Z, Li J, Wu X, Dai H, Gao X, Liu M, Tu P. Structures and immunological activities of two pectic polysaccharides from the fruits of Ziziphus jujuba Mill. cv. jinsixiaozao Hort. Food Res Int. 2006;39(8):917–923. doi: 10.1016/j.foodres.2006.05.006. [DOI] [Google Scholar]
- Li J, Shan L, Liu Y, Fan L, Ai L. Screening of a functional polysaccharide from Zizyphus Jujuba cv. Jinsixiaozao and its property. Int J Biol Macromol. 2011;49(3):255–259. doi: 10.1016/j.ijbiomac.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Chang SC, Hsu BY, Chen BH. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int J Biol Macromol. 2010;47(4):445–453. doi: 10.1016/j.ijbiomac.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Zhong K, Wang Q, He Y, He X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in S180 tumor mice models. Int J Biol Macromol. 2010;47(3):356–360. doi: 10.1016/j.ijbiomac.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Lai F, Wen Q, Li L, Wu H, Li X. Antioxidant activities of water-soluble polysaccharide extracted from mung bean (Vigna radiata L.) hull with ultrasonic assisted treatment. Carbohyd Polym. 2010;81(2):323–329. doi: 10.1016/j.carbpol.2010.02.011. [DOI] [Google Scholar]
- Pan Y, Dong S, Hao Y, Zhou Y, Ren X, Wang J, Wang W, Chu T. Ultrasonic-assisted extraction process of crude polysaccharides from Yunzhi mushroom and its effect on hydroxyproline and glycosaminoglycan levels. Carbohyd Polym. 2010;81(1):93–96. doi: 10.1016/j.carbpol.2010.01.060. [DOI] [Google Scholar]
- Chen X, Tang Q, Chen Y, Wang W, Li S. Simultaneous extraction of polysaccharides from Poria cocos by ultrasonic technique and its inhibitory activities against oxidative injury in rats with cervical cancer. Carbohyd Polym. 2010;79(2):409–413. doi: 10.1016/j.carbpol.2009.08.025. [DOI] [Google Scholar]
- Zhou C, Yu X, Zhang Y, He R, Ma H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Ueda. Carbohyd Polym. 2012;87(3):2046–2051. doi: 10.1016/j.carbpol.2011.10.026. [DOI] [Google Scholar]
- Galai S, Touhami Y, Marzouki MN. Response surface methodology applied to laccases activities exhibited by stenotrophomonas maltophilia AAP56 in different growth conditions. Bioresources. 2012;7(1):706–726. [Google Scholar]
- Liu J, Hu HR, Xu JF, Wen YB. Optimizing enzymatic pretreatment of recycled fiber to improve its draining ability using response surface methodology. Bioresources. 2012;7(2):2121–2140. [Google Scholar]
- Xu P, Bao JS, Gao JJ, Zhou T, Wang YF. Optimization of extraction of phenolic antioxidants from tea (Camellia Sinensis L.) fruit peel biomass using response surface methodology. Bioresources. 2012;7(2):2431–2443. [Google Scholar]
- Chen Y, Gu X, Huang S, Li J, Wang X, Tang J. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int J Biol Macromol. 2010;46(4):429–435. doi: 10.1016/j.ijbiomac.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Zhong K, Wang Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohyd Polym. 2010;80(1):10–25. [Google Scholar]
- Huang S, Ning Z. Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity. Int J Biol Macromol. 2010;47(3):336–341. doi: 10.1016/j.ijbiomac.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Tian Y, Zeng H, Xu Z, Zheng B, Lin Y, Gan C, Lo YM. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus) Carbohyd Polym. 2012;88(2):522–529. doi: 10.1016/j.carbpol.2011.12.042. [DOI] [Google Scholar]
- Zhang B, Yan P, Chen H, He J. Optimization of production conditions for mushroom polysaccharides with high yield and antitumor activity. Carbohyd Polym. 2012;87(4):2569–2575. doi: 10.1016/j.carbpol.2011.11.042. [DOI] [Google Scholar]
- Chen W, Wang W, Zhang H, Huang Q. Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from Boletus edulis mycelia using response surface methodology. Carbohyd Polym. 2011;87(1):614–619. doi: 10.1016/j.carbpol.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Kennedy JF, Wang X, Yuan X, Zhao B, Peng Y, Huang Y. Optimization of ultrasonic circulating extraction of polysaccharides from Asparagus officinalis using response surface methodology. Int J Biol Macromol. 2011;49(2):181–187. doi: 10.1016/j.ijbiomac.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Yang W, Fang Y, Liang J, Hu Q. Optimization of ultrasonic extraction of Flammulina velutipes polysaccharides and evaluation of its acetylcholinesterase inhibitory activity. Food Res Int. 2011;44(5):1269–1275. doi: 10.1016/j.foodres.2010.11.027. [DOI] [Google Scholar]
- Zou Y, Chen X, Yang W, Liu S. Response surface methodology for optimization of the ultrasonic extraction of polysaccharides from Codonopsis pilosula Nannf.var.modesta L.T. Shen. Carbohyd Polym. 2011;84(1):503–508. doi: 10.1016/j.carbpol.2010.12.013. [DOI] [Google Scholar]
- Yan Y, Yu C, Chen J, Li X, Wang W, Li S. Ultrasonic-assisted extraction optimized by response surface methodology, chemical composition and antioxidant activity of polysaccharides from Tremella mesenterica. Carbohyd Polym. 2011;83(1):217–224. doi: 10.1016/j.carbpol.2010.07.045. [DOI] [Google Scholar]
- Gan C, Latiff AA. Extraction of antioxidant pectic-polysaccharide from mangosteen (Garcinia mangostana) rind: Optimization using response surface methodology. Carbohyd Polym. 2011;83(2):600–607. doi: 10.1016/j.carbpol.2010.08.025. [DOI] [Google Scholar]
- Ma L, Gan D, Wang M, Zhang Z, Jiang C, Zeng X. Optimization of extraction, preliminary characterization and hepatoprotective effects of polysaccharides from Stachys floridana Schuttl. ex Benth. Carbohyd Polym. 2012;87(2):1390–1398. doi: 10.1016/j.carbpol.2011.09.032. [DOI] [Google Scholar]
- Pan WJ, Liao AM, Zhang JG, Dong Z, Wei ZJ. Supercritical carbon dioxide extraction of the oak silkworm (Antheraea pernyi) pupal oil: process optimization and composition determination. Int J Mol Sci. 2012;13(2):2354–2367. doi: 10.3390/ijms13022354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZJ, Liao AM, Zhang HX, Liu J, Jiang ST. Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Biores Technol. 2009;100(18):4214–4219. doi: 10.1016/j.biortech.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Wei ZJ, Zhou LC, Chen H, Chen GH. Optimization of the fermentation conditions for 1-deoxynojirimycin production by streptomyces lawendulae applying the response surface methodology. Int J Food Eng. 2011;7(3):16. 12 pp. [Google Scholar]