Abstract
Semi-interpenetrating networks (sIPNs) designed to mimic extracellular matrix via covalent crosslinking of poly(ethylene glycol) diacrylate in the presence of gelatin have been shown to aid in wound healing, particularly when loaded with soluble factors. Ideal systems for tissue repair permit an effective release of therapeutic agents and flow of nutrients to proliferating cells. Appropriate network characterization can, consequently, be used to convey an understanding of the mass transfer kinetics necessary for materials to aid in the wound healing process. Solute transport from and through sIPNs has not yet been thoroughly evaluated. In the current study, the diffusivity of growth factors and nutrients through the polymeric system was determined. Transport of keratinocyte growth factor was modeled by treating the sIPN as a plane sheet into which the protein was loaded. The diffusion coefficient was determined to be 4.86 × 10−9 ± 1.86 × 10−12 cm2/s. Glucose transport was modeled as flow through a semi-permeable membrane. Using lag-time analysis, the diffusion coefficient was calculated to be 2.25 × 10−6 ± 1.98 × 10−7 cm2/s. The results were evaluated in conjunction with previous studies on controlled drug release from sIPNs. As expected from Einstein-Stokes equation, diffusivity decreased as molecular size increased. The results offer insight into the structure-function design paradigm and show that release from the polymeric system is diffusion controlled, rather than dissolution controlled.
Keywords: Semi-interpenetrating network, Transport, Diffusion, Controlled release, Physical characterization
Introduction
Biomaterials are currently being explored to promote the wound healing process. Natural and synthetic matrices can be used to provide sites of attachment for cell migration and as vehicles for the controlled delivery of therapeutic molecules, particularly tissue promoting growth factors. For example, photocrosslinked poly(vinyl alcohol) hydrogels with hydrophilic fillers have been used for the sustained release of platelet derived growth factor (PDGF-β, β) [1], fibrin matrices have allowed for the controlled delivery of keratinocyte growth factor (KGF) [2], and hyaluronate-heparin conjugate gels have been used to supply basic fibroblast growth factor (FGF-2) [3]. Other polymer based wound dressings have further been utilized as conduits for antimicrobials and analgesics. Ag+ and doxycycline have been incorporated into sulfonated elastomers [4] and minocycline has been included in chitosin-polyurethane films [5] to reduce bacterial growth, while bupivacaine-loaded poly(acrylamide(A)-co-monomethyl itaconate) hydrogels have been generated to provide prolonged pain relief [6].
In addition to controlling the delivery of therapeutic agents, optimal systems for wound healing allow for the effective flow of nutrients to proliferating cells. In general, release of solutes from and flow of solutes through polymer-based materials is controlled by a combination of network swelling, molecular diffusion, and polymer degradation/dissolution [7]. For example, hydrocortisone alcohol release from partially esterified methyl vinyl ether-maleic anhydride copolymers is regulated by dissolution, while release of larger drug molecules, such as chloramphenicol and idoxuridine, is controlled by a combination of both dissolution and diffusion [8]. In contrast, papaverine release from a poly(l-lactic acid) matrix is solely diffusion-controlled [9]. Identification and modeling of the methods by which nutrients and therapeutics are delivered to a wound by a particular biomaterial can provide valuable insight into the design paradigm.
Our lab has developed a novel, binary hydrogel system that aids tissue repair and wound healing. Poly(ethylene glycol) diacrylate (PEGdA) is photocrosslinked in the presence of gelatin to yield biodegradable, mechanically stable semi-interpenetrating networks (sIPNs) that mimic extracellular matrix [10–12]. Fibronectin-derived peptides can be tethered to the gelatin through poly(ethylene glycol) (PEG) linkers prior to photopolymerization. RGD and PHSRN have been shown to have a significant impact upon adhesion and response of a number of cell types involved in the wound healing process [13–15]. An sIPN grafted with RGD through a PEG tether and loaded with KGF resulted in regenerated tissue with cellularity and extracellular matrix organization comparable to undamaged tissue when applied to a full thickness wound model [14]. Additionally, sIPNs loaded with silver sulfadiazine showed a significant reduction in bacterial load [16]. Current work is aimed at obtaining a better understanding of the transport of therapeutic molecules from the sIPN to the wound bed, as well as the transport of nutrients through the sIPN to regenerating cells. Characterization will provide valuable information pertaining to the structure-function relationship of polymeric delivery matrices, as well as offer insight into the mass transfer kinetics necessary to promote wound healing.
In the current study, mass transfer of solutes relevant to wound healing through sIPNs prepared from unmodified gelatin and PEGdA was characterized. The effective diffusion coefficient of KGF was determined by release from the gel, while that of glucose was evaluated by flow through the gel. The results are viewed in light of previous work showing controlled release of silver sulfadiazine and bupivacaine HCl from the sIPNs. Therefore, the solutes evaluated encompass a number of functions and sizes.
Experimental
Materials
Anhydrous N,N-dimethylformamide (DMF) was obtained from a dry solvent system. PEG was received from Fluka with a molecular weight range of 1,900–2,200 Da. Type A gelatin from porcine skin with a bloom strength of ∼ 300 g was ordered from Aldrich Chemical Company. Gelatin molecular weight was estimated as 76 kDa by gel permeation chromatography. KGF was obtained from R&D Systems. All other commercial reagents were purchased from Aldrich Chemical Company and used without further purification.
Preparation of semi-interpenetrating networks (sIPNs)
sIPNs were prepared as described elsewhere [14]. Briefly, 0.3 g of gelatin was dissolved in 3 mL of Millipore filtered water (10% w/v) by heating at 60 °C. The gelatin solution was added to 0.38 g of PEGdA (Mn ≈ 575 Da). A stock initiator solution was prepared by dissolving 0.2 g of 2,2-dimethoxyphenylacetophenone (DMPA) in 0.5 g of PEGdA. About 60 μl of initiator solution were added to the gelatin-PEGdA solution to give a gelatin:PEGdA weight ratio of 2:3. Depending upon the diffusion experiment, the solution was transferred to either a disc-shaped Teflon® mold or a well of a Millicell® Multiwell Cell Culture Insert Plate (Millipore, Billerica, MA) from which the original membrane was removed. Exposure to UV light with a CF1000 LED (λmax = 365 nm, Clearstone Technologies, Inc., Minneapolis, MN) for 3 min yielded the sIPNs. For experiments involving release from the sIPN, the solute was added to the mold immediately prior to photopolymerization.
Keratinocyte growth factor (KGF) release
sIPNs containing KGF were formed by adding the sIPN solution to Teflon® molds with d = 8 mm and t = 0.75 mm. About 8–10 μl of KGF solution (100 ng/ml in DI water) was added to each mold prior to photopolymerization. The sIPNs were submerged in 35 ml of 1× PBS within a 50 ml centrifuge tube and incubated at 37 °C. About 0.220 ml aliquots were removed at regular intervals for 48 h. The volume change was accounted for in all concentration calculations. KGF concentrations at each time point were determined using a Human KGF/FGF-7 Quantikine ELISA kit (R&D Systems, Minneapolis, MN). Diffusion coefficients were found from plots of the fraction of KGF released by the gel versus time. The percentage of KGF released was calculated by assuming that the final measured concentration was equivalent to the initial loaded concentration. Calculations showed that the latter assumption was valid. Each experiment was performed in triplicate.
Glucose flow through a sIPN
SIPNs prepared in Millicell® Multiwell Cell Culture Insert Plates were equilibrated in 1 × PBS overnight. The sIPNs were submerged in 32 ml of glucose solution (2 g/ L in PBS), and 0.4 ml of PBS were added to each well. The assembly was placed in an incubator at 37 °C. About 0.25 ml samples were removed at regular intervals over the course of 4 h. To ensure that the glucose concentration in chamber 2 remained low, an identical volume of PBS immediately replaced the volume removed. The samples were diluted with 0.2 ml of PBS and concentration was determined using the established dinitrosalicylic acid assay method. Briefly, a dinitrosalicylic acid reagent solution was prepared by combining 10 g of dinitrosalicyclic acid with 2 g phenol, 0.5 g sodium sulfite, and 10 g of sodium hydroxide and adding water to 1 L. About 0.45 ml of the latter solution were added to the 0.45 ml aliquots, and the resultant mixture was heated at 95 °C for 10 min. About 0.15 ml of 40% sodium potassium tartrate solution was added, and the absorbance was measured at 575 nm [17]. Sample concentrations were calculated from a calibration curve constructed with standards of known concentration. Plots of total glucose flux versus time yield the lag time, which was used to calculate the diffusion coefficient. The height of the sIPN was found using digital calipers with an accuracy of ±0.2 mm (Thermo Fisher Scientific, Inc., Waltham, MA) upon completion of all concentration measurements. The experiment was repeated in triplicate.
Results and discussion
Exogenous KGF has been shown to accelerate wound healing by promoting re-epithelialization [2, 18]. In the current study, KGF release from thin, disc-shaped sIPNs was monitored. By minimizing transport in the radial direction, diffusion could be treated as unidirectional desorption from a membrane. As described by Crank, the following was derived from Fick' s second law of diffusion by assuming that the solute was initially distributed uniformly through the sIPN at concentration c0, that the sIPN was symmetrical, and that the bulk fluid was immediately removed the solute from the surface (cs = 0) [19].
| (1) |
The rate of disappearance at the surface (z = L) was subsequently found by letting m(t) equal the amount of solute remaining within the sIPN at time t and m0 equal the initial amount of solute loaded into the sIPN.
| (2) |
As shown in Fig.1, KGF release was successfully modeled using the latter equation, and the diffusion coefficient was determined to be 4.86 × 10−9 ± 1.86 × 10−3 cm2/s (Table 1).
Fig. 1.
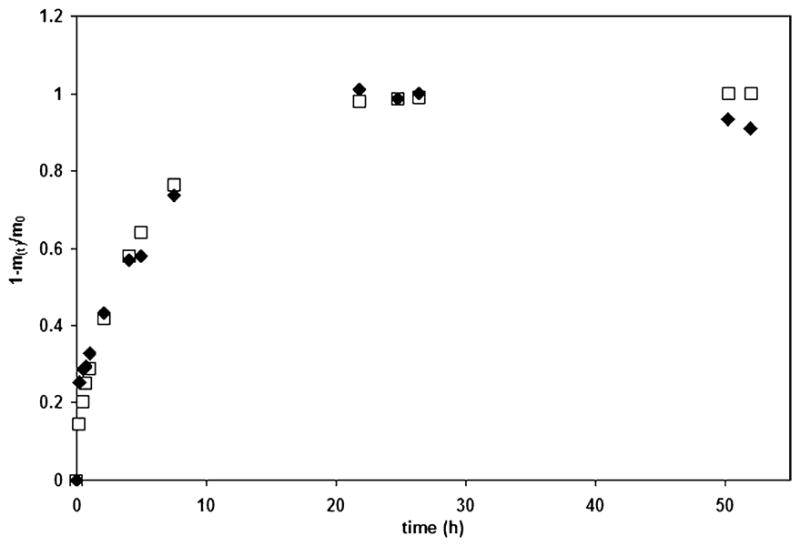
A representative plot of KGF release from a small sIPN. The KGF concentrations found experimentally (diamonds) were directly compared with those predicted using a diffusion coefficient D, estimated from the experimental data and Eq. (2) (squares)
Table 1. Diffusion coefficents as a function of molecular size.
| Molecule | MW (g/mol) | Approximate radius (Å) | D × 10−6(cm2/s) | Standard deviation × 10−6 (cm2/s)a |
|---|---|---|---|---|
| Glucose | 180.16 | 3.30 | 2.25 | 1.98 × 10−1 |
| Bupivacaine HCl | 324.89 | 5.15 | 1.54 × 10−1 | 1.45 × 10−2 |
| Silver Sulfadiazine | 357.14 | 6.15 | 3.31 × 10−2 | 5.81 × 10−3 |
| KGF | 19,000 | 19.74 | 4.86 × 10−3 | 1.86 × 10−3 |
Standard deviation was determined for a sample size of n = 3
Glucose diffusion could not successfully be evaluated by release from the sIPNs due to the Maillard reaction (data not shown) [20], which occurred upon addition of glucose to the warm gelatin-PEGdA solution prior to photopolymerization. Hence, transport was instead characterized as flow through a membrane, as depicted in Fig. 2. The lag time, or time required for glucose flow to reach a steady-state, can be derived from Fick's second law and be used to calculate the diffusion coefficient [21].
Fig. 2.
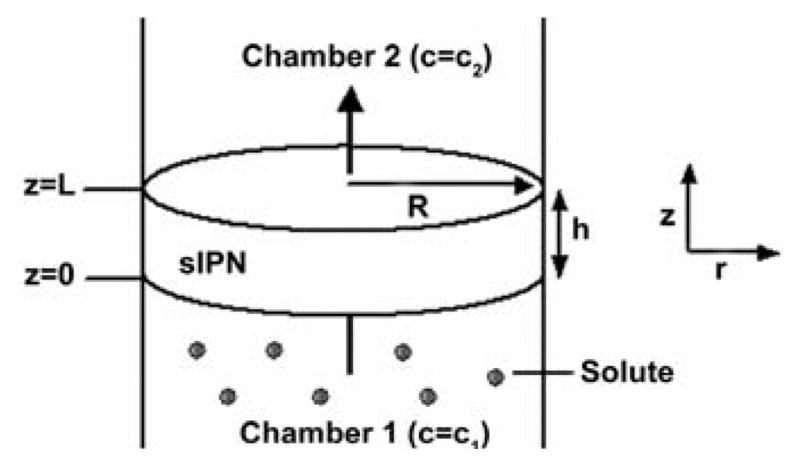
Glucose flow through a sIPN. Flux was unidirectional in the z direction from chamber 1 to chamber 2
| (3) |
As illustrated in Fig. 3, glucose transport was effectively characterized using the latter technique. A lag time of 4.76 × 103 ± 0.45 × 103 s and, consequently, a diffusion coefficient of 2.25 × 10−6 ± 1.98 × 10−7 cm2/s were determined.
Fig. 3.
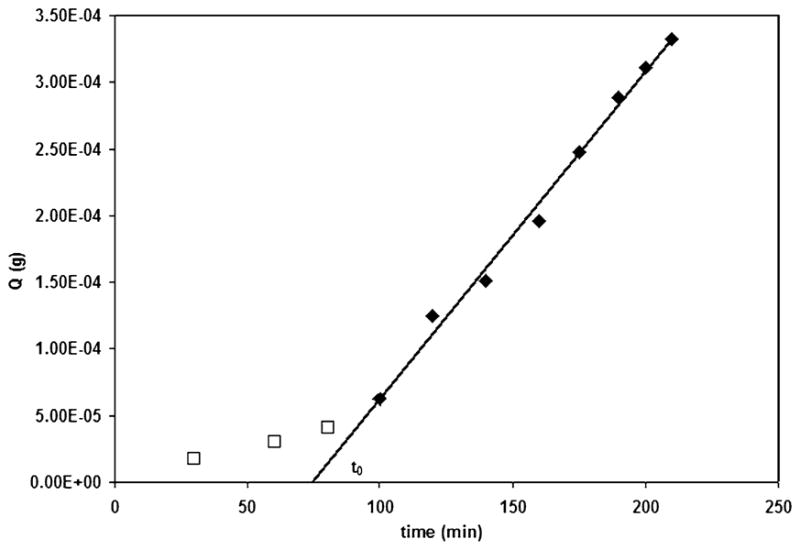
A representative plot of glucose flux through a sIPN membrane. The lag time t0, was used to determine the diffusion coefficient D, of glucose
Previous studies have shown that sIPNs can be used for the concurrent release of multiple drugs to limit bacterial growth, while simultaneously reducing pain. The diffusion coefficients for silver sulfadiazine (AgSD), a common, topical antimicrobial agent, and bupivacaine HCl (BupHCl), a local anesthetic, were evaluated as desorption from a membrane, as described for KGF. The diffusivities were determined to be 3.31 × 10−8 ± 5.81 × 10−3 cm2/s and 1.54 × 10−7 ± 1.45 × 10−8 cm2/s, respectively (Table 1) [16].
The diffusion coefficients of the solutes evaluated in the current study could be roughly correlated with size (Table 1), as expected from the Stokes-Einstein relation
| (4) |
where k is Boltzmann's constant, T is temperature in Kelvin, η is the viscosity of the fluid, and a is the radius of the molecule. Small molecules, including BupHCl, AgSD, and glucose, can be considered as spheres, and the molecular radius can be calculated as
| (5) |
where M is the molecular weight, N0 is Avagadro's number, and υρ is the specific gravity. Although KGF is a large molecule, proteins of similar size, such as myoglobin, have successfully been treated as spheres in diffusion calculations; therefore, Eq. (5) was also used in the evaluation of KGF. In accord with other proteins, the specific volume of KGF was estimated to be 0.74 cm3/g [22]. The diffusion coefficients drastically decreased as molecular radius increased, as anticipated based upon diffusion controlled release from the polymer network, rather than dissolution/degradation controlled release.
Conclusions
SIPNs prepared from unmodified gelatin and PEGdA to mimic extracellular matrix were characterized in regards to the transport of therapeutic molecules and nutrients critical to the wound healing process. Diffusion coefficients were determined for keratinocyte growth factor and glucose and were evaluated in conjunction with diffusivities previously calculated for AgSD and BupHCl. The calculated diffusion coefficients in sIPNs are not equivalent to those in pure water, indicating that transport is impacted by the network structure. As molecular size increased, transport was further hindered, as shown by a reduction in the calculated diffusivities. The results offer insight into the structure-function relationship of sIPNs and can be used to predict the ease by which molecules can be transferred to the wound. Additionally, the sIPNs were shown to be effective matrices for the delivery of drugs and growth factors, as well as for the transport of nutrients to proliferating cells, during wound healing. As indicated by the fit of the models to the experimental data, in conjunction with the dependence of diffusivity upon molecular size, solute transport through the network appears to be diffusion controlled, rather than dissolution/degradation controlled.
Acknowledgments
The work presented herein was supported by NIH grant R01 EB6613. The authors thank the following members of the Kao laboratory: Amy Chung, Sean Zuckerman, David Schmidt, and Heather Waldeck.
Contributor Information
Rebecca Ann Bader, Email: babader@syr.edu, Department of Biomedical and Chemical Engineering, Syracuse University, Syracuse, NY 13244, USA.
Kyle T. Herzog, School of Pharmacy, University of Wisconsin-Madison, Madison, WI 53705, USA
W. John Kao, School of Pharmacy, University of Wisconsin-Madison, Madison, WI 53705, USA; Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI 54706, USA.
References
- 1.Bourke SL, Al-Khalili M, Briggs T, Michniak BB, Kohn J. A photo-crosslinked poly(vinyl alcohol) hydrogel growth factor release vehicle for wound healing applications. AAPS Pharm Sci. 2003;5:E33. doi: 10.1208/ps050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geer DJ, Swartz DD, Andreadis ST. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am J Pathol. 2005;167:1575–1586. doi: 10.1016/S0002-9440(10)61242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Ng C, Thompson AY, Poser JW, Spiro R. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2) J Biomed Mater Res A. 2002;62:128–135. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 4.Vachon DJ, Yager DR. Novel sulfonated hydrogel composite with the ability to inhibit proteases and bacterial growth. J Biomed Mater Res A. 2005;76:35–43. doi: 10.1002/jbm.a.30440. [DOI] [PubMed] [Google Scholar]
- 5.Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm. 2007;330:138–145. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Blanco MD, Bernardo MV, Teijón R, Sastre L, Teijón JM. Transdermal application of bupivacaine-loaded poly(acrylamide(A)-co-monomethyl itaconate) hydrogels. Int J Pharm. 2003;255:99–107. doi: 10.1016/s0378-5173(03)00036-x. [DOI] [PubMed] [Google Scholar]
- 7.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2007 doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 8.Heller J, Baker RW, Gale RM, Rodin JO. Controlled drug release by polymer dissolution. I. Partial esters of maleic anhydride copolymers—properties and theory. J Appl Polym Sci. 1978;22:1991–2009. [Google Scholar]
- 9.Miyajima M, Koshika A, Okada J, Kusai A, Ikeda M. Factors influencing the diffusion-controlled release of papaverine from poly(L-lactic acid) matrix. J Control Release. 1998;56:85–94. doi: 10.1016/s0168-3659(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 10.Burmania JA, Martinez-Diaz GF, Kao WJ. Synthesis and physicochemical analysis of interpenetrating networks containing modified gelatin and poly(ethylene glycol) diacrylate. J Biomed Mater Res A. 2003;67:224–234. doi: 10.1002/jbm.a.10106. [DOI] [PubMed] [Google Scholar]
- 11.Witte RP, Blake AJ, Palmer C, Kao WJ. Analysis of poly(ethylene glycol)-diacrylate macromer polymerization within a multicomponent semi-interpenetrating polymer network system. J Biomed Mater Res A. 2004;71:508–518. doi: 10.1002/jbm.a.30179. [DOI] [PubMed] [Google Scholar]
- 12.Toth M, Williams K, Hayes S, Kao WJ. Tensile creep properties of interpenetrating networks containing gelatin and poly(ethylene glycol) diacrylate. J Biomater Sci Polym Ed. 2005;16:925–932. doi: 10.1163/1568562054255745. [DOI] [PubMed] [Google Scholar]
- 13.Gao Q, Chung AS, Kao WJ. Monocytic U937 adhesion, tumor necrosis factor-alpha and interleukin-1 beta expression in response to gelatin-based networks grafted with arginine-glycine-aspartic acid and proline-histidine-serine-arginine-asparagine oligopeptides. Tissue Eng. 2007;13:179–185. doi: 10.1089/ten.2006.0007. [DOI] [PubMed] [Google Scholar]
- 14.Waldeck H, Chung AS, Kao WJ. Interpenetrating polymer networks containing gelatin modified with PEGylated RGD and soluble KGF: synthesis, characterization, and application in in vivo critical dermal wound. J Biomed Mater Res A. 2007;82:861–871. doi: 10.1002/jbm.a.31054. [DOI] [PubMed] [Google Scholar]
- 15.Chung A, Gao Q, Kao WJ. Macrophage matrix metalloproteinases-2/-9 gene and protein expression following adhesion to ECM-derived multifunctional matrices via integrin complexation. Biomaterials. 2007;28:285–298. doi: 10.1016/j.biomaterials.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Kleinbeck KR, Bader RA, Stajkowski AL, Kao WJ. Concurrent in vitro release of silver sulfadiazine and bupivacaine from semi-interpenetrating networks for wound management. J Burn Care Res. 2008 doi: 10.1097/BCR.0b013e3181921ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 18.Putnins EE, Firth JD, Lohachitranont A, Uitto V, Larjava H. Keratinocyte growth factor (KGF) promotes keratinocyte cell attachment and migration on collagen and fibronectin. Cell Adhes Comm. 1999;7:211–221. doi: 10.3109/15419069909010803. [DOI] [PubMed] [Google Scholar]
- 19.Crank J. The mathematics of diffusion. Oxford University Press; Oxford: 1975. [Google Scholar]
- 20.Nursten HE. The Maillard reaction. Royal Society of Chemistry; Cambridge: 2005. [Google Scholar]
- 21.Hannoun BJM, Stephanopoulos G. Diffusion coefficients of glucose and ethanol in cell-free and cell-occupied calcium alginate membranes. Biotechnol Bioeng. 1986;28:829–835. doi: 10.1002/bit.260280609. [DOI] [PubMed] [Google Scholar]
- 22.Benedek GB, Villars FMH. Statistical physics. Vol. 2. Springer; New York: 2000. Physics with illustrative examples from medicine and biology. [Google Scholar]


