Abstract
Distinguishing self from other is necessary for self-awareness and social interactions. This distinction is thought to depend on multisensory integration dominated by visual feedback. However, self-awareness also relies on the processing of interoceptive signals. We contrasted the exteroceptive and interoceptive models of the self to investigate the hitherto unexplored interaction between the perception of the self from the outside and from within. Multisensory stimulation between self and other was used to induce controlled changes in the representation of one’s identity. Interoceptive sensitivity predicted the malleability of self-representations in response to multisensory integration across behavioral, physiological and introspective responses, suggesting that interoception plays a key modulating role in the self-recognition system. In particular, only participants with low interoceptive sensitivity experienced changes in self-other boundaries in response to multisensory stimulation. These results support the view that interoceptive predictive coding models are used to monitor and assign the sources of sensory input either to the self or to others, as well as support the hypothesis of the insular cortex as a convergence zone in the processing and global representation of the material self given its involvement in both interoceptive feelings, multisensory integration and self-processing.
Keywords: interoception, multisensory, face, self-awareness, self-recognition
1. Introduction
Recent models of the self show how the brain’s processing of multisensory information underpins self-awareness (Lenggenhager, Tadi, Metzinger, & Blanke, 2007). For example, synchronous visuo-tactile stimulation between the participant’s body and a foreign body results in an illusory sense of ownership of the foreign body (Petkova, Björnsdotter, Gentile, Jonsson, Li, & Ehrsson, 2011). Similar stimulation to the participant’s face and a foreign face results in changes in the mental representation of one’s face-identity (Tajadura-Jiménez, Grehl, & Tsakiris, 2012a; Tsakiris, 2008). These consistent results demonstrate the dominant influence that exteroception (i.e., the perception of the body from the outside) exerts on two key elements of self-awareness, that is, the feeling that this body is mine (i.e., body-ownership) and the ability to recognize one’s self as distinct from other people. An alternative influential model of the self emphasizes the role of interoception (i.e., the perception of the body from within) as a vital type of information-processing necessary for self-awareness (Craig, 2009; Damasio, 2010). For example, the remapping of interoceptive signals in the cortex has been proposed to underpin not only a primary form of self-awareness but also to participate in higher forms of self-awareness such as the distinction between self-other required for efficient social interactions. Interestingly, the insular cortex, one brain area among an extended self-related brain network, is activated during interoceptive tasks, multisensory induced changes in body-ownership and self-face recognition (Craig, 2009), suggesting that in this brain area exteroceptive and interoceptive signals converge to globally represent the material self. While the dual perception of the self from the outside and from within is a fundamental aspect of human experience and a key idea in the history of psychology (James, 1890), few studies have looked at the interaction between these two modes of self-perception. Recent research has found that exteroceptively-induced body-ownership depends on (Kammers, Rose, & Haggard, 2011) and affects autonomic processes (Barnsley, McAuley, Mohan, Dey, Thomas, & Moseley, 2011), and, further, that sensitivity to autonomic states modulates the effects of exteroceptive stimulation on illusory ownership of body-parts (Tsakiris, Tajadura-Jiménez, & Costantini, 2011). These results evidence the modulatory effect of interoceptive sensitivity, that is, the body as perceived from within, on the malleability of the representation of body-parts as perceived from the outside. However, these studies have focused on the sense of body-ownership that taps on processes that allow the distinction between external objects that may or may not be experienced as part of my body. Notwithstanding the importance of ownership of body-parts for self-awareness, such bodily illusions have been criticized for not capturing the more global awareness of one’s whole body (Blanke & Metzinger, 2008), and the social dimension of embodiment as a means of distinguishing between individuals (Sforza, Bufalari, Haggard, & Aglioti, 2010) and not simply body-parts.
Thus, while the aforementioned studies demonstrate the role of interoception for body-ownership, which is a fundamental aspect of self-awareness, the extent to which interoceptive awareness might also influence the distinction between self and other remains unknown. To address this question we focused on another fundamental aspect of self-awareness, namely, the mental representation of one’s own face. Our face is the most distinctive feature of our physical appearance, and one of the key ways by which we become known as individuals, both to ourselves and to others. Given that the ability to recognize one’s own face is considered a hallmark of self-awareness in both phylogeny and ontogeny (Povinelli & Simon, 1988), the investigation of how the interaction between interoceptive and exteroceptive signals underpins self-recognition and the distinction between self and other can provide novel insights on the nature of self-awareness. To that end, we sought to contrast the exteroceptive and interoceptive models of the self and investigate the hitherto unexplored interaction between the perception of the self from the outside and from within.
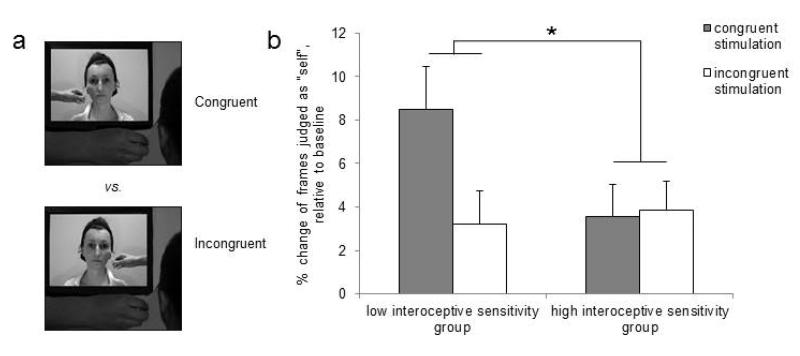
Multisensory stimulation between self and other was used to induce controlled changes in the representation of one’s identity. We took advantage of a multisensory illusion that affects self-face recognition (the enfacement illusion; Sforza et al., 2010; Tajadura-Jiménez et al., 2012a; Tsakiris, 2008) to quantify the contribution of exteroceptive information on distinguishing between self and others. Participants were stroked on the left side of their face while seeing the face of an unfamiliar other person being stroked in synchrony. Importantly, the strokes on the other’s face were delivered either in a specularly congruent location, as if the participant was looking at a mirror, or at the specularly incongruent side of the face (see Fig. 1a). This manipulation of interpersonal multisensory stimulation (IMS) ensured synchrony of visuo-tactile stimulation across conditions, while the stimulation location was used to control for the induction of the enfacement illusion.
Figure 1. (a) The specularly congruent and incongruent interpersonal multisensory stimulation (IMS) and (b) mean change in % (+ SEM) of frames judged as “self” across conditions and interoceptive sensitivity groups in Experiment 1 (point zero indicates the % of frames judged as “self” in pre-test).
Asterisks denote significant differences between means (* denotes p < .05, 2-tailed).
Interoceptive sensitivity (IS) was quantified by people’s accuracy in mentally tracking and counting their heartbeats. In addition, empathetic traits were quantified, because they have been shown to correlate positively with the enfacement illusion (Sforza et al., 2010). Across two experiments, we explored whether interoceptive sensitivity and empathetic traits are significant predictors of the changes in self-processing during the enfacement illusion, quantified by introspective and behavioral measures of self-other boundaries in Experiment 1, and by measuring autonomic arousal in response to threat to the other person after IMS in Experiment 2.
We predicted that levels of interoceptive sensitivity would modulate the behavioral and physiological effects on self-other distinction following the enfacement illusion. In particular, based on recent hypotheses about the role of interoception on processing exteroceptive signals (Tsakiris et al., 2011), and its role for the sense of presence and self-awareness (Seth, Suzuki, & Critchley, 2011), we hypothesized that lower interoceptive sensitivity would result in larger behavioral and physiological effects on self-other merging.
2. Method
2.1. Participants
The same 28 female paid-volunteers (mean age = 25 years, SD = 5.6) participated in two experiments, after informed consent to participate was gained. This sample size was pre-determined in a Power Sample Analysis (SPSS Power Sample 3) in which we used the difference between high and low IS groups on the subjective ratings of the enfacement illusion reported in Tajadura-Jiménez, Longo, Coleman, & Tsakiris (2012b) as the basis of the expected behavioral effect size in the present study. Participants were students or staff members of Royal Holloway, University of London. The study was approved the Departmental Ethics Committees.
2.2. Materials
A photograph of the participant’s face with a neutral facial expression was mirrored and converted to gray scale (Keenan, McCutcheon, Freund, Gallup, Sanders, & Pascual-Leone, 1999). Non-facial attributes were removed with a black template. Subsequently, a computerized morphing procedure was implemented (Abrasoft Fantamorph) to produce a 100 sec-“face-morphing” movie in which the face of a female individual, unfamiliar to the participant (~20 years old), morphed into the participant’s face in 1% morphing transitions. Two “face-morphing” movies were produced, each showing a different unfamiliar face transforming into the participant’s face.
Four 120 sec “induction” movies, displaying each model being touched on the right or the left cheek with a cotton-bud approximately at .33 Hz, each stroke covering a distance of approximately 2 cm from the zygomatic bone downwards, were produced and presented with a 20″ LCD-screen, 50 cm away from participants. In Experiment 2, participants’ electrodermal activity (EDA) was monitored with bipolar finger electrodes. After Experiment 2 was completed, participants’ IS was quantified by monitoring their heart signal with a piezo-electric finger pulse transducer. Physiological signals were amplified and sampled (1 kHz for heart signal, 250 Hz for EDA signal; PowerLab 26T, AD Instruments, UK).
2.3. Procedure
2.3.1. Experiment 1: Behavioral and introspective measures of self-other boundaries
Each experimental block contained three phases: pre-stimulation test (pre-test), interpersonal multisensory stimulation (IMS) phase and post-stimulation test (post-test). In the pre-test, a self-recognition baseline measure was taken. Participants were presented with a “face-morphing” movie depicting the model’s face being morphed into the participant’s own face, and were required to stop the movie with a key-press when they felt that the face looked more like self than like other (Keenan et al., 1999; Tajadura-Jiménez et al., 2012a). The movie was only presented once, and participants were not allowed to adjust their decision. Next, participants were exposed to the IMS phase. For 120 sec, participants were stroked on the left side of their face while they were seeing the face of an unfamiliar other person being stroked in synchrony either in a specularly congruent location, as if the participant was looking at a mirror, or at the specularly incongruent side of the face, on different blocks (see Fig. 1a). The specularly incongruent condition served as a control condition in which no enfacement illusion was expected. We considered this to be a superior control than the specularly congruent asynchronous condition used in previous studies on the enfacement illusion (Sforza et al., 2010; Tajadura-Jiménez et al., 2012a; Tsakiris, 2008), since it controlled for the general effect of increased attention during the synchronous IMS. To that end, the synchrony of stimulation across both conditions ensured comparable levels of attention, while the side of stimulation (i.e., specularly congruent or incongruent) was used to induce the enfacement illusion or not. In the post-test, a second self-face recognition measure quantified the effect of stimulation on face recognition. At the end of each block, the subjective experience of participants during IMS was assessed with five statements, for which participants rated their level of agreement using a visual analog scale ranging from “strongly agree” to “strongly disagree” (see Tab. 1). The statements were adopted from Tajadura-Jiménez et al. (2012a) and were presented in a random order. Participants completed two experimental blocks, one “congruent” and one “incongruent”. The block order and the models viewed in each block were counterbalanced across participants. A different model was used in each block in order to avoid any familiarity effect with the shown face.
Table 1. Mean ratings (± SD) for each questionnaire item for each interoceptive sensitivity group of participants (HIGH vs. LOW) and experimental condition (congruent vs. incongruent) in Experiment 1.
Participants rated their level of agreement with the statements using a scale ranging from −3 (“strongly disagree”) to +3 (“strongly agree”).
| LOW | HIGH | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Question | Cong. | Incon. | t(13) | Cong. | Incon. | t(13) |
| Q1. It seemed like I felt the touch of the cotton bud delivered in the other’s face |
.24 (1.2) | −.29 (.7) | 1.39 | .49 (1.9) | .14 (1.6) | .58 |
|
| ||||||
| Q2. I felt like the other’s face was my face |
−.3 (.9) | .09 (1.5) | −1.06 | .04 (1.5) | .77 (1.5) | −1.35 |
|
| ||||||
| Q3. It seemed like I was looking at my own reflection in a mirror rather than at the other’s face |
.17 (.9) | −.71 (1.1) | 4.11* | .03 (1.6) | .13 (1.3) | −.23 |
|
| ||||||
| Q4. It seemed like the other’s face resembled my own face |
−.3 (.7) | .24 (1.7) | −.16 | −.49 (1.3) | .31 (1.5) | −2.19 |
|
| ||||||
| Q5. It seemed like my own face resembled the other person’s face |
.16 (1.2) | −.14 (1.3) | 1.06 | −.41 (1.7) | −.23 (1.4) | −.33 |
p = .001, 2-tailed, corrected for multiple comparisons
For all statistical tests alpha level was set at .05, 2-tailed.
2.3.2. Experiment 2: Autonomic arousal in response to threat
A recurring worry with experiments based on illusions of body-ownership has to do with demand characteristics when using explicit measures of self-recognition (for a discussion see Tajadura-Jiménez et al., 2012a). In order to overcome this potential confound, the self-other boundaries were assessed physiologically by measuring EDA in response to threatening or non-threatening stimuli that touched the other’s face following IMS. When people experience ownership over a foreign body, as a result of multisensory stimulation, they also exhibit increased arousal responses to threatening stimuli approaching this newly owned body (Armel & Ramachandran, 2003), and arousing stimuli are usually followed by an increase in EDA (Boucsein, 1992).
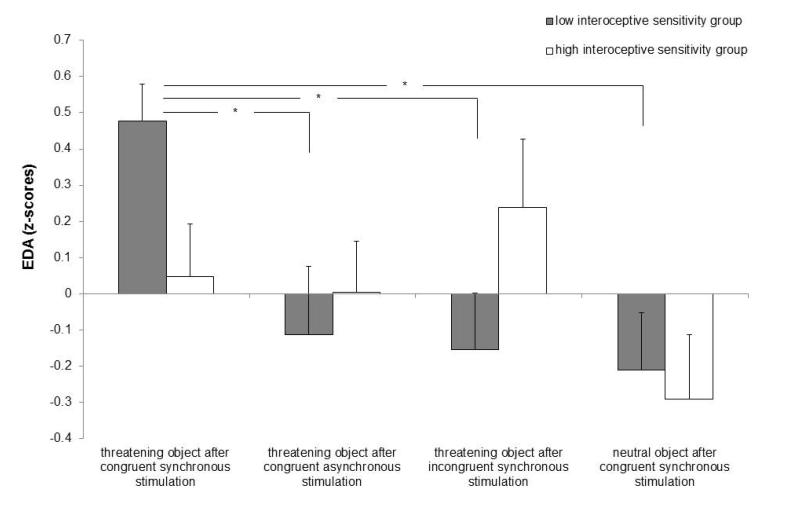
IMS was delivered to participants as in Experiment 1. Importantly, at the end of the IMS, participants observed an object appearing from the side of the screen and making contact with the model’s cheek. In the test condition (‘threat’), participants were exposed to synchronous and congruent IMS, and at the end of the IMS a threatening object (i.e., a blade) appeared from the left side of the screen and made contact with the model’s right cheek approximately 1 sec after, with the touch lasting about 1 sec and covering a distance of approximately 2 cm from the zygomatic bone downwards. In order to make this movie more realistic, the blade painted a path of fake blood onto the participant’s cheek (see Supplementary material). We included three control conditions. A first control condition, ‘incongruent’, in which synchronous IMS was delivered to the two faces in specularly incongruent locations, controlled for a general effect of increased attention due to the synchronous IMS. As before, the ‘blade’ appeared at the end of the stimulation, but this time in the specularly incongruent side of the face. A second control condition, ‘asynchronous’, was similar to the ‘threat’ condition except that during IMS the cotton-bud touches to the participant were presented in asynchrony of 1.5 sec with the touches displayed in the movie. Finally, an additional control condition, ‘non-threat’, similar to the ‘threat’ condition except that the object displayed was a ‘cotton ball’ instead of a ‘blade’ and white paste substituted the fake blood, controlled for a general effect of seeing an object approaching the face.
Each condition was presented in a random order and twice, for 90 sec and 105 sec, to ensure that participants could not anticipate the appearance of the object. Participants viewed one model in the synchronous congruent IMS conditions (i.e., ‘threat’ and ‘non-threat’ conditions), and another model in the ‘incongruent’ and ‘asynchronous’ conditions, in order to avoid any familiarity effect with the shown face. The block order and the models viewed in each pair of conditions were counterbalanced across participants.
2.3.3. Interoceptive Sensitivity and Interpersonal Reactivity Index
After Experiment 2 was completed, participants’ interoceptive sensitivity (IS) and Interpersonal Reactivity Index (IRI) were measured. IS was measured by using the Mental Tracking Method (Schandry, 1981). While monitoring participants’ heartbeat, and in four trials of different length (25, 35, 45 and 100 sec), participants were asked to concentrate and silently count their own heart beats. Participants were not allowed to take their own pulse, did not receive any feedback on their performance and were not informed of the length of the trial. An audiovisual cue marked the start and the stop of the trial.
Participants completed the IRI questionnaire (Davis, 1983), comprised by four different scales of trait reactivity to others: perspective-taking scale (PT), fantasy scale (FS), empathic concern scale (EC) and personal distress scale (PD). PT and EC have been shown to correlate positively with the enfacement illusion (Sforza et al., 2010). We therefore included these sub-scales as covariates in our statistical analyses with the aim of controlling for any individual differences in IRI scores and ensuring that any effect of levels of IS on enfacement cannot be accounted for by differences in IRI scores.
3. Results
3.1. Interoceptive Sensitivity and Interpersonal Reactivity Index
IS was calculated from the four heartbeat detection trials, according to the following formula (Schandry, 1981):
Higher IS scores indicate higher accuracy of the participants in counting their heartbeats (i.e., higher IS). The participants’ IS median score (Median = .67, SD = .17) was used to split them into two groups of high IS (HIGH group, mean IS score = .82, SD = .08; N = 14) and low IS (LOW group, mean IS score = .53, SD = .1; N = 14; see Tsakiris et al., 2011). This allowed us to investigate the effect of the High IS and Low IS groups on the behavioral, introspective and physiological results, in addition to the regression analyses that we report for which IS scores were entered as a continuous variable reflecting the individual differences of interest.
The mean PT for the HIGH group was 18.4 (SD = 5.1) and for the LOW group was 15.9 (SD = 5.3); the mean FS for the HIGH group was 18.6 (SD = 3.8) and for the LOW group was 15.6 (SD = 7.4); the mean EC for the HIGH group was 19.8 (SD = 4.4) and for the LOW group was 19.6 (SD = 4.9); and the mean PD for the HIGH group was 11.4 (SD = 4.7) and for the LOW group was 14.7 (SD = 4.2). No significant differences between the HIGH and LOW groups were observed neither for any of the IRI scales (PT: t(26) = 1.23, p = .23, Cohen’s d = .48; FS: t(26) = 1.32, p = .2, Cohen’s d = .52; EC: t(26) = .08, p = .94, Cohen’s d = .03; PD: t(26) = 1.94, p = .06, Cohen’s d = .76) nor for the total IRI score (i.e., the mean of the 4 scales: t(26) = .43, p = .67, Cohen’s d = .16). Moreover, correlation analyses between the IRI scores and the IS scores, which were entered as a continuous variable, showed that none of the IRI scores correlated with IS (PT: r = .24, p = .2; FS: r = .33, p = .08; EC: r = .04, p = .8; PD: r = −.33, p = .08; see Supplementary Table 1). In addition, the PT and EC scores were included as covariates in the subsequently reported analyses of variance (ANOVAs).
3.2. Experiment 1: Behavioral and introspective measures of self-other boundaries
3.2.1. Behavioral measure
The points at which participants stopped the “face-morphing” movies were used to calculate the percentage of frames judged as belonging more to the participants’ own face than to the other person’s face. These percentages were submitted in a mixed ANOVA with timing of the test (i.e., pre-test vs. post-test) and type of stimulation (i.e., congruent vs. incongruent) as within-subject factors, and IS group (i.e., HIGH vs. LOW) as between-subjects factor, while the effect of the PT and EC scores was covaried-out. The main effect of the timing of the test was significant (F(1, 25) = 4.93, p = .036, η2 = .165), while no other significant effects or double interactions were observed (type of stimulation: F(1, 25) = 1.5, p = .23, η2 = .057; type of stimulation by IS group: F(1, 25) = 1.38, p = .25, η2 = .052; timing of the test by IS group: F(1, 25) = 1.81, p = .19, η2 = .067; type of stimulation by timing of the test: F(1, 25) = .32, p = .57, η2 = .013). Importantly, the three-way interaction was significant (F(1, 25) = 4.39, p = .046, η2 = .15; see Fig. 1b). An independent samples t-test between the two groups revealed that the change in self-recognition performance post-stimulation relative to pre-stimulation between congruent and incongruent stimulation was significantly different between the two groups (t(26) = 2.18, p = .038, Cohen’s d = .82). A further ANOVA with the pre-test mean values and type of stimulation as within-subject factor, and IS group as between-subject factor, revealed neither significant effects (type of stimulation: F(1, 26) = .43, p = .52, η2 = .016; type of stimulation by IS group: F(1, 26) = 3.39, p = .077, η2 = .12), nor main effect of IS group (F(1,26) = .65, p = .43, η2 = .02), thus validating the choice of the pre-test values as an appropriate baseline.
A multiple regression analysis explored the predictive roles of IS, PT and EC on the changes in self-recognition between post-test and pre-test in the congruent stimulation condition. Only IS was a significant predictor (IS: R2 = .25; β= .5; t(25) = 2.92, p = .007, Cohen’s f2 = .33; PT-EC: β= −.06; t(25) = −.32, p = .75) with lower IS predicting larger changes in self-recognition. Moreover, correlation analyses between the IRI scores, IS scores and the changes in self-recognition between post-test and pre-test in the congruent stimulation, showed that none of the IRI scores correlated with the changes in self-recognition, while IS correlated significantly (see Supplementary Table 1).
Overall, participants with lower IS judged 8.5% more frames as depicting the self-face following congruent IMS. This suggests a change in self-face recognition which reflects the influence of IS and which cannot be explained simply by the synchronicity of stimuli, nor by a general task-specific bias, or a general visual adaptation to the other’s face (Leopold, Rhodes, Müller, & Jeffery, 2005), because across both conditions stimulation was synchronous and exposure to the other’s face was equal.
3.2.2. Introspective measure
The answers to the statements assessing the subjective experience of participants during the congruent and incongruent IMS conditions were translated into a scale ranging from −3 (“strongly disagree”) to +3 (“strongly agree”) and submitted in a mixed ANOVA with type of IMS (i.e., congruent vs. incongruent) as within-subjects factor, IS group (HIGH vs. LOW) as between-subjects factor, and the five statements (Q1-Q5) as dependent variables, while the effect of the PT and EC scores was covaried-out. PT and EC scores effects were not significant and were therefore removed from subsequent analysis. A significant interaction of condition by group (F(1, 26) = 4.18, p = .05, η2 = .14) was observed in the subjective ratings to the third statement (“It seemed like I was looking at my own reflection in a mirror rather than at the other’s face”), suggesting that similarly to the behavioral results, the LOW IS group agreed more with this statement. A significant difference (t(13) = 4.11, p = .001, Cohen’s d = .88, corrected for multiple comparisons) between congruent and incongruent condition was found in the LOW group only (for the HIGH IS group t(13) = .23, p = .82, Cohen’s d = .07), while no other significant differences were found (see Tab. 1).
These results suggest that, only for the LOW IS group, the congruent IMS condition felt closer to the experience of looking at one’s own face into a mirror, as compared to the incongruent IMS condition.
3.3. Experiment 2: Autonomic arousal in response to threat
EDA change scores were calculated for each trial by subtracting the maximum response from the minimum response (Petkova & Ehrsson, 2008) during the period 1 to 7 sec following object – blade or cotton ball - onset (i.e., the period ended approximately 5 sec after object offset). This interval was chosen to be the region of interest, because changes in EDA are not immediate but they normally occur between 1 and 2 sec after stimulus onset, although the response can be delayed up to 5 sec (Edelberg, 1967). Change scores were log-transformed and individually z-scored to control for variation in responsiveness (Boucsein, 1992; Venables & Christie, 1973, 1980).
No difference was found across the different duration, 90 sec and 105 sec, conditions (‘threat’: t(27) = 1.62, p = .12, Cohen’s d = .51, ‘non-threat’: t(27) = −.59, p = .56, Cohen’s d = .17, ‘incongruent’: t(27) = −.8, p = .43, Cohen’s d = .22, ‘asynchronous’: t(27) = −.38, p = .7, Cohen’s d = .1), therefore we averaged the data from those conditions. Non-parametric statistical tests were used to analyze the data (two-tailed Wilcoxon Signed Ranks Test and Holm–Bonferroni for multiple comparisons) because of violation of the normality Shaphiro-Wilk test.
Higher EDA responses were observed in the test ‘threat’ condition relative to the three control conditions only for the LOW IS group (‘non-threat’: Z = −2.54, p = .011, r = .68; ‘incongruent’: Z = −2.54, p = .011, r = .68; ‘asynchronous’: Z = −2.1, p = .035, r = .56; see Fig. 2). We also performed linear multiple regression analyses using Hierarchical Models where IS, PT and EC were entered as predictors of the difference in EDA between the ‘threat’ condition and the ‘asynchronous’ condition. IS (β= −.39, p = .048) explained 14% of the variance (R2 = .14, F(1,26) = 4.08, p = .05), while PT and EC were not significant predictors (β= .12; t(25) = .66, p = .5). A similar Hierarchical Model on the difference in EDA between the ‘threat’ condition and the ‘incongruent’ condition indicated that IS (β= −.53, p = .005) explained 26% of the variance (R2 = .26, F(1,26) = 9.18, p = .005), while PT and EC were not significant predictors (β= .1; t(25) = .61, p = .55). Moreover, correlation analyses between the IRI scores, IS scores and the difference in EDA between the ‘threat’ condition and the ‘incongruent’ condition, as well as between the ‘threat’ condition and the ‘asynchronous’ condition, showed that none of the IRI scores correlated with the changes in EDA, while IS correlated significantly (see Supplementary Table 1).
Figure 2. Mean z-scored changes (+ SEM) in electrodermal activity (EDA) across conditions and interoceptive sensitivity groups in Experiment 2 (point zero indicates the minimum EDA in response to stimuli).
At the end of the IMS participants observed an object appearing from the side of the screen and making contact with the model’s cheek. In the test condition (‘threat’), participants were exposed to synchronous and congruent IMS, and at the end of the IMS a threatening object (i.e., a blade) appeared from the left side of the screen and made contact with the model’s right cheek approximately 1 sec after. We included three control conditions: the blade appeared at the end of asynchronous and congruent IMS (‘asynchronous’); the blade appeared at the end of synchronous and incongruent IMS (‘incongruent’); and a neutral object appeared at the end of synchronous and congruent IMS (‘non-threat’). Higher EDA responses in response to seeing this object were observed in the test ‘threat’ condition relative to the three control conditions only for the LOW interoceptive sensitivity group. Asterisks denote significant differences between means (* denotes p < .05, 2-tailed).
Thus, consistent with the behavioral pattern, participants with low IS displayed higher arousal to the threat perceived on the other’s face. These results are consistent with the pattern of arousal previously observed in response to threatening objects approaching body-parts that come to be experienced as one’s own (Petkova & Ehrsson, 2008), suggesting that the increased EDA in the present study can be considered as an index of identification with the other’s face, but only for the LOW IS group and following specularly congruent stimulation.
4. Discussion
The present study investigated the role of interoceptive sensitivity in the sensory-driven malleability of self-other boundaries. We measured people’s interoceptive sensitivity using an established interoceptive task and quantified changes in self-other boundaries using the experimental paradigm of the enfacement illusion. Taken together, the results reveal how interoceptive sensitivity modulates the effects of exteroceptive signals on self-representations as measured explicitly, in the post-stimulation self-recognition test, and by assessing the subjective experience of participants, as well as physiologically, by quantifying changes in arousal. Importantly, we found no effects of interoceptive sensitivity on the baseline self-recognition performance. Instead, lower levels of interoceptive sensitivity resulted in larger changes in self-other boundaries caused by multisensory stimulation. Furthermore, the use of synchronous multisensory stimulation across conditions provided a better control for overall differences in attention between conditions than the classic manipulation of synchrony versus asynchrony, which can lead to potential attentional confounds, as synchronous events might result in stronger perceptual binding as compared to asynchronous events. By using synchronous stimulation throughout, we aimed at eliciting comparable levels of perceptual binding between felt and seen stimuli, while selectively manipulating the location of stimulation (i.e., congruency) to induce the enfacement illusion.
One potential study limitation was the use of only one measure of interoceptive sensitivity, the Mental Tracking Method (Schandry, 1981), which although is widely used and is comparable to other heartbeat perception tasks (e.g., the Whitehead method, see Knoll & Hodapp, 1992; but see Schulz, Lass-Hennemann, Sütterlin, Schächinger, & Vögele, 2013, for conflicting results when performance in the two tasks was compared under stress-inducing conditions), suffers from certain methodological confounds such as a priori knowledge of resting heart rate, as well as insensitivity of the task to changes in heart rate, and possible confounding with ability to retain a count in working memory (Khalsa, Rudrauf, Damasio, Davidson, Lutz, & Tranel, 2008; Khalsa, Rudrauf, Sandesara, Olshansky & Tranel, 2009; Windmann, Schonecke, Fröhlig, & Maldener, 1999). However, other studies (e.g., Dunn, Galton, Morgan, Evans, Oliver, Meyer et al., 2010) that have carefully controlled for such confounding factors have demonstrated the validity of the heartbeat tracking method as a proxy of interoceptive awareness, and its close link to performance in other interoceptive tasks such as the waterload task (Herbert, Muth, Pollatos, & Herbert, 2012). Thus, while the consideration of the specific confounds of the Schandry methods and its interocorrelation with other interoceptive tasks does not invalidate heartbeat tracking as a measure of interoception, future studies would benefit by using additional proxies of interoceptive sensitivity. Another potential study limitation was that only female participants took part. Our sample was opportunistic as the population of the undergraduate students in UK social science departments is largely female. However, it should be noted that in a previous study in which we investigated the effect of gender on the enfacement illusion (Tajadura-Jiménez et al., 2012b), we did not find any significant effect of gender on the strength of the enfacement illusion.
Given that previous studies had reported correlations between components of the Interpersonal Reactivity Index (IRI, in particular perspective-taking and empathetic concern; Sforza et al., 2010) and the strength of bodily illusions, we felt inclined to include this measure, with the aim of controlling for any individual differences in IRI scores. Thus, our focus here was not on studying the link between IRI and strength of the enfacement illusion per se, but rather to ensure that any effect of IS levels on enfacement are not confounded by differences in IRI. There are suggestions in the literature that insofar interoceptive processing is crucially involved in affective processing, there has to be a link between IS and empathy (e.g., Ernst, Northoff, Böker, Seifritz, & Grimm, 2012). However, no direct links between interoceptive sensitivity as measured by the Heartbeat Tracking Method (or the Whitehead method) and the IRI have been reported as far as we know, despite evidence of a link between interoception and empathy in terms of brain activity in the insular cortex (Craig, 2009; Ernst et al., 2012; Singer, Critchley, & Preuschoff, 2009). In terms of the relation between empathy and the self-processing model that we propose here, we suggest that future studies should first distinguish clearly between different types of empathetic processing (e.g., affective, somatic, cognitive) and develop appropriate tasks that can then be linked to levels of IS. We hypothesize that different levels of IS might be positively correlated with different types of empathy. For example, based on the idea of more malleable self-other boundaries in people with low IS, we might expect that these people would show enhanced somatic empathy (e.g., measured by tasks similar to the ones reported in Avenanti, Sirigu, & Aglioti, 2010), while people with high IS might be better at more cognitive types of empathy because they can co-represent self and other.
Exteroceptive models of the self rely on multimodal cues such as vision, touch and proprioception, to produce a coherent percept (Ernst & Bülthoff, 2004), such as the awareness of one’s body (Blanke & Metzinger, 2008; Lenggenhager et al., 2007). Synchronous exteroceptive information, such as seen and felt touch, establishes strong statistical correlations that are harvested by the brain to create a sense of self. In the enfacement illusion, as well as in other bodily illusions, the available multisensory evidence is interpreted as self-related sensory events. Interestingly, exteroceptively-induced body-ownership affects autonomic processes (Barnsley et al., 2011), and awareness of autonomic states (i.e., interoceptive sensitivity) modulates the effects of exteroceptive stimulation on illusory ownership of body-parts (Tsakiris et al., 2011). However, while previous studies have shown that interoceptive sensitivity might modulate the incorporeability of external objects such as body-parts (Tsakiris et al., 2011), the present study shows how awareness of internal states might be essential in regulating self-other boundaries and therefore play a role in social cognition. Given the importance of one’s face for representing one’s personal and social identity and the effects of the enfacement illusion, not only on the mental representation of how we look like but also on social cognition processes (Paladino, Mazzurega, Pavani & Schubert, 2010), the induced changes in the mental representation of one’s face seem to rely on neurocognitive processes that link a primarily bodily sense of self (e.g., how I look like) and a more narrative sense of self (i.e., how does the self relates to others). We here show that sensitivity to interoceptive signals participate as an additional cue used by a self-recognition system to distinguish between self and other. This significantly adds to previous results on body-awareness, given the different processes recruited by self-face recognition (Slaughter, Stone, & Reed, 2004), and provides novel insights into the nature of self-awareness, given that the ability to recognize one’s own face is considered the hallmark of self-awareness (Povinelli & Simon, 1988).
Self-perception is characterized by a strong affective element, experienced as the feeling of being or seeing “me” (Kircher, Senior, Phillips, Rabe-Hesketh, Benson, Bullmore, et al., 2001). According to recent models of self-awareness and conscious presence (Seth et al., 2011), high interoceptive sensitivity would provide precise predictions about how it feels to see and recognize oneself or not. The sensitivity to such feelings is weighted during the combination of multimodal cues that may or may not prime self-identification (e.g., different patterns of multisensory stimulation). When seeing another face being touched in synchrony with one’s face, the visuo-tactile signals prime a sense of self, but the interoceptive prediction of how it feels to see oneself is in conflict with what the exteroceptive evidence suggests. This view is consistent with the recently suggested interoceptive predictive coding model (Seth et al., 2011; Critchley & Seth, 2012) used to monitor and assign the sources of sensory input either to the self or to others. Brain areas, such as the anterior insular cortex, that function as a convergence zone of interoceptive and exteroceptive information, might exert top-down modulations on unisensory brain areas that register prediction errors between the expected and actual sensory feedback. Given the involvement of the anterior insular cortex in interoceptive feelings (Craig, 2009), body-ownership (Tsakiris, Hesse, Boy, Haggard, & Fink, 2007) and self-processing (Enzi, de Greck, Prösch, Tempelmann, & Northoff, 2009; Modinos, Ormel, & Aleman, 2007; for meta-analyses see Denny, Kober, Wager, & Ochsner, 2012; and Northoff, Qin, & Feinberg, 2006), we propose that this brain area might maintain predictions about how self-related sensory processing, such as seeing oneself, feels like. On this view, the self-recognition mechanism might be computing in a Bayesian manner the stimuli that are most likely to be “me”. Both interoceptive and exteroceptive information are integrated from hierarchically organized unimodal sensory systems into higher-level multimodal areas such as the anterior insula, that can in turn exert top-down influences to minimize prediction errors across the unimodal brain areas (Apps & Tsakiris, 2013).
The distinction between self and other is essential for the awareness of the self as distinct from other agents (Decety & Sommerville, 2003). The findings of the present study are in line with recent suggestions that the representation of internal body states is perhaps the most fundamental element of self-awareness (Craig, 2009), because the interoceptive system is used to remap in the cerebral cortex the most stable aspects of one’s environment, that is, the organism’s body (Damasio, 2010). At the same time, this interoceptive system is crucially involved in mediating and facilitating self-other interactions. For example, empathy, that at least to a certain extent makes us overcome self-other boundaries and merge with the other, requires the contribution of both affective and sensorimotor aspects of the interoceptive system (Lamm & Singer, 2010). While some previous studies have shown the link between the enfacement illusion and social cognition (i.e., Paladino et al., 2010; Mazzurega, Pavani, Paladino, & Schubert, 2011), our study is the first to highlight the role that different degrees of interoceptive sensitivity have in modulating self-other boundaries during sensory experiences that are shared between individuals. These findings pave the way for a more systematic investigation of the interplay between interoceptive awareness and different kinds of social interactions. Given the implication of interoceptive awareness (Herbert & Pollatos, 2012) in several clinical disorders on one hand (e.g., eating disorders; see also Eshkevari, Rieger, Longo, Haggard, & Treasure, 2012) and recent advances in our understanding of normal and abnormal social cognition on the other (Lieberman, 2007), studying the link between interoceptive awareness and self-other boundaries can bridge the gap between the “inner” and the “outer” self, and shed light on how ourselves interact with others.
Supplementary Material
Acknowledgements
ESRC First Grant RES-061-25-0233, and European Research Council (ERC-2010-StG-262853) under the FP7 (to M.T.).
References
- Apps MAJ, Tsakiris M. The Free-energy self: A predictive coding account of self-recognition. Neuroscience and Biobehavioural Reviews. 2013 doi: 10.1016/j.neubiorev.2013.01.029. doi:10.1016/j.neubiorev.2013.01.029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armel KC, Ramachandran VS. Projecting sensations to external objects: evidence from skin conductance response. Proceedings in Biological Sciences. 2003;270:1499–1506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Current Biology. 2010;20:1018–1022. doi: 10.1016/j.cub.2010.03.071. [DOI] [PubMed] [Google Scholar]
- Barnsley N, McAuley JH, Mohan R, Dey A, Thomas P, Moseley GL. The rubber hand illusion increases histamine reactivity in the real arm. Current Biology. 2011;21:R945–R946. doi: 10.1016/j.cub.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Blanke O, Metzinger T. Full-body illusions and minimal phenomenal selfhood. Trends in Cognitive Sciences. 2008;13:7–13. doi: 10.1016/j.tics.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. Plenum press; New York: 1992. [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley H, Seth A. Will studies of macaque insula reveal the neural mechanisms of self-awareness? Neuron. 2012;74:423–426. doi: 10.1016/j.neuron.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Damasio A. Self comes to mind: Constructing the conscious brain. Pantheon; New York: 2010. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences. 2003;7:527–33. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, et al. Listening to your heart: How interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010;21(12):1835–1844. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Edelberg R. Electrical properties of the skin. In: Brown CC, editor. Methods in psychophysiology. Williams and Wilkins; Baltimore: 1967. pp. 1–53. [Google Scholar]
- Enzi B, de Greck M, Prösch U, Tempelmann C, Northoff G. Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS ONE. 2009;4(12):e8429. doi: 10.1371/journal.pone.0008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends in Cognitive Sciences. 2004;8:162–169. doi: 10.1016/j.tics.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Human Brain Mapping. 2012 Feb 22; doi: 10.1002/hbm.22014. 2012. doi:10.1002/hbm.22014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshkevari E, Rieger E, Longo MR, Haggard P, Treasure J. Increased plasticity of the bodily self in eating disorders. Psychological Medicine. 2012;42(04):819–828. doi: 10.1017/S0033291711002091. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: On the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS ONE. 2012;7(5):e36646. doi: 10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O. The body in the mind: On the relationship between interoception and embodiment. Topics in Cognitive Science. 2012;4:692–704. doi: 10.1111/j.1756-8765.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. Harvard University Press; Cambridge: 1890. [Google Scholar]
- Kammers MPM, Rose K, Haggard P. Feeling numb: Temperature, but not thermal pain, modulates feeling of body ownership. Neuropsychologia. 2011;49:1316–1321. doi: 10.1016/j.neuropsychologia.2011.02.039. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, Tranel D. Interoceptive awareness in experienced meditators. Psychophysiology. 2008;45(4):671–677. doi: 10.1111/j.1469-8986.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. Psychophysiology. 2009;72(1):34–45. doi: 10.1016/j.ijpsycho.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan JP, McCutcheon B, Freund S, Gallup GG, Jr., Sanders G, Pascual-Leone A. Left hand advantage in a self-face recognition task. Neuropsychologia. 1999;37:1421–1425. doi: 10.1016/s0028-3932(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Rabe-Hesketh S, Benson PJ, Bullmore ET, David AS. Recognizing one’s own face. Cognition. 2001;78:B1–B15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Knoll JF, Hodapp V. A comparison between two methods for assessing heartbeat perception. Psychophysiology. 1992;29:218–222. doi: 10.1111/j.1469-8986.1992.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Tadi T, Metzinger T, Blanke O. Video ergo sum: Manipulation of bodily self consciousness. Science. 2007;317:1096–1099. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Rhodes G, Müller K-M, Jeffery L. The dynamics of visual adaptation to faces. Proceedings of the Royal Society Series B: Biological Sciences. 2005;272:897–904. doi: 10.1098/rspb.2004.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Mazzurega M, Pavani F, Paladino M-P, Schubert TW. It is a matter of time: Self-other bodily merging in the context of synchronous but arbitrary related multisensory inputs. Experimental Brain Research. 2011;213:213–221. doi: 10.1007/s00221-011-2744-6. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Activation of anterior insula during self-reflection. PLoS ONE. 2009;4(2):e4618. doi: 10.1371/journal.pone.0004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self – Conceptual, anatomical and methodological issues. Consciousness and Cognition. 2011;20:52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Paladino M-P, Mazzurega M, Pavani F, Schubert TW. Synchronous multisensory stimulation blurs self-other boundaries. Psychological Science. 2010;21:1202–1207. doi: 10.1177/0956797610379234. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Ehrsson HH. If I Were You: Perceptual Illusion of Body Swapping. PLoS ONE. 2008;3:e3832. doi: 10.1371/journal.pone.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova VI, Björnsdotter M, Gentile G, Jonsson T, Li TQ, Ehrsson HH. From part to whole-body ownership in the multisensory brain. Current Biology. 2011;21:1118–1122. doi: 10.1016/j.cub.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Simon BB. Young children’s understanding of briefly versus extremely delayed images of the self: Emergence of the autobiography stance. Developmental Psychology. 1998;34:188–194. doi: 10.1037/0012-1649.34.1.188. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schulz A, Lass-Hennemann J, Sütterlin S, Schächinger H, Vögele C. Cold pressor stress induces opposite effects on cardioceptive accuracy dependent on assessment paradigm. Biological psychology. 2013;93:167–174. doi: 10.1016/j.biopsycho.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Frontiers in Psychology. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Science. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sforza A, Bufalari I, Haggard P, Aglioti SM. My face in yours: Visuo-tactile facial stimulation influences sense of identity. Social Neuroscience. 2010;5:148–162. doi: 10.1080/17470910903205503. [DOI] [PubMed] [Google Scholar]
- Slaughter V, Stone VE, Reed C. Perception of faces and bodies. Similar or different? Current Directions in Psychological Science. 2004;13:219–223. [Google Scholar]
- Tajadura-Jiménez A, Grehl S, Tsakiris M. The other in me: Interpersonal multisensory stimulation changes the mental representation of the self. PLoS ONE. 2012a;7:e40682. doi: 10.1371/journal.pone.0040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajadura-Jiménez A, Longo M, Coleman R, Tsakiris M. The person in the mirror: using the enfacement illusion to investigate the experiential structure of self-identification. Consciousness & Cognition. 2012b;21(4):1725–38. doi: 10.1016/j.concog.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Hesse M, Boy C, Haggard P, Fink GR. Neural correlates of body-ownership: a sensory network for bodily self-consciousness. Cerebral Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. Looking for myself: Current multisensory input alters self-face recognition. PLoS ONE. 2008;3:e4040. doi: 10.1371/journal.pone.0004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Tajadura-Jiménez A, Costantini M. Just a heartbeat away from one’s body: interoceptive sensitivity predicts malleability of body-representations. Proceedings of the Royal Society Series B: Biological Sciences. 2011;278:2470–2476. doi: 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S, Schonecke OW, Fröhlig G, Maldener G. Dissociating beliefs about heart rates and actual heart rates in patients with cardiac pacemakers. Psychophysiology. 1999;36(3):339–42. doi: 10.1017/s0048577299980381. [DOI] [PubMed] [Google Scholar]
- Venables PH, Christie MJ. Mechanisms, instrumentation, recording techniques, and quantification of responses. In: Prokasy WF, Raskin DC, editors. Electrodermal activity in psychological research. Academic Press; New York: 1973. pp. 1–124. [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques in psychophysiology. Wiley; Chichester: 1980. pp. 3–67. [Google Scholar]
- Zaki J, Davis ID, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.