Abstract
Background
The ParS/ParR two component regulatory system plays critical roles for multidrug resistance in Pseudomonas aeruginosa. It was demonstrated that in the presence of antimicrobials, ParR enhances bacterial survival by distinct mechanisms including activation of the mexXY efflux genes, enhancement of lipopolysaccharide modification through the arn operon, and reduction of the expression of oprD porin.
Results
In this study, we report on transcriptomic analyses of P. aeruginosa PAO1 wild type and parS and parR mutants growing in a defined minimal medium. Our transcriptomic analysis provides the first estimates of transcript abundance for the 5570 coding genes in P. aeruginosa PAO1. Comparative transcriptomics of P. aeruginosa PAO1 and par mutants identified a total of 464 genes regulated by ParS and ParR. Results also showed that mutations in the parS/parR system abolished expression of the mexEF-oprN operon by down-regulating the regulatory gene mexS. In addition to the known effects on drug resistance genes, transcript abundances of the quorum sensing genes (rhlIR and pqsABCDE-phnAB) were higher in both parS and parR mutants. In accordance with these results, a significant portion of the ParS/ParR regulated genes belonged to the MexEF-OprN and quorum sensing regulons. Deletion of the par genes also led to increased phenazine production and swarming motility, consistent with the up-regulation of the phenazine and rhamnolipid biosynthetic genes, respectively.
Conclusion
Our results link the ParS/ParR two component signal transduction system to MexEF-OprN and quorum sensing systems in P. aeruginosa. These results expand our understanding of the roles of the ParS/ParR system in the regulation of gene expression in P. aeruginosa, especially in the absence of antimicrobials.
Keywords: Pseudomonas, Two component signal transduction, parS/parR, mexEF-oprN, Quorum sensing
Background
Pseudomonas aeruginosa is a Gram-negative, metabolically versatile and environmentally ubiquitous bacterial species that is capable of surviving in a variety of animal and plant hosts and causing opportunistic infections in humans. It is responsible for serious chronic and often fatal lung infections in patients with cystic fibrosis and acute infections in patients that are immune compromised or have serious burns [1]. Infections caused by P. aeruginosa often are difficult to treat due to their intrinsic resistance to diverse antibiotics and their capacity for adaptive resistance [2-4]. In P. aeruginosa, major mechanisms of multidrug resistance include the production of enzymes that inactivate β-lactamases and aminoglycosides through modification, alterations in topoisomerases, reduced expression of genes encoding outer membrane proteins such as OprD, and increased expression of genes encoding efflux pumps [5,6]. Additionally P. aeruginosa can exhibit adaptive resistance, whereby sub-inhibitory concentrations of antibiotics transiently increase resistance to lethal doses. This adaption occurs largely as a result of expression of the mexXY-oprM efflux [7] and arnBCADTEF-ugd lipopolysaccharide modification operons [8,9].
The versatility of P. aeruginosa in adapting to different environments has been attributed in part to the complex regulatory networks that coordinate the control of genes involved in adaptation, including coordination of two-component signal transduction (TCST) systems and quorum sensing [10]. The P. aeruginosa genome appears to be especially rich in two-component signal transduction (TCST) systems, which use phosphorylation as a mechanism for responding to specific environmental cues [11]. Annotations of P. aeruginosa genomes have identified 123 potential TCSTs, most of which have not been characterized functionally [10]. The ParS/ParR TCST is a key regulatory component for intrinsic and adaptive multidrug resistance in P. aeruginosa [8,9]. As is typical of TCST systems, the ParS/ParR system consists of a membrane-bound histidine sensor kinase (ParS) and a cytoplasmic response regulator (ParR). Mutations in parR result in susceptibility to a wide range of antibiotics including polymyxin B, gentamycin and tobramycin [8]. Previous microarray analyses identified over 100 genes controlled by the ParS/ParR system in the presence of antimicrobial agents [8,9]. Among them are genes encoding the outer membrane porin protein OprD, the RND efflux pump MexXY-OprM, and the arnBCADTEF-ugd lipopolysaccharide modification operon.
The P. aeruginosa genome also contains a diversity of quorum-sensing (QS) systems. QS gene regulation has been described as a method of cell-cell communication used by bacteria to synchronize gene expression within a population [12]. In P. aeruginosa, QS depends on the autoinducer synthases LasI, RhlI and PqsABCDH/PhnAB as well as their cognate transcriptional regulators LasR, RhlR and PqsR (MvfR), respectively [13]. LasI and RhlI synthesize the canonical autoinducers 3-oxo-dodecanoyl-homoserine lactone (3-oxo-C12-HSL) and butanoyl-homoserine lactone (C4-HSL) respectively, which cause transcriptional responses by interacting with LasR and RhlR. In contrast, PqsABCDH/PhnAB catalyzes the synthesis of 2-heptyl-3-hydroxy-4-quinolone (PQS), which in turn regulates gene expression through the PqsR protein [14]. The three QS systems function in a hierarchical manner whereby the LasR/I system positively regulates the RhlR/I system, and PQS is considered the terminal signal [15]. This interlinked QS network controls the expression of multiple virulence factors including exoenzymes, toxins, and secondary metabolites (e.g. chitinase, elastase, protease, exotoxin A, hydrogen cyanide, phenazines, pyoverdin, rhamnolipid) as well as the ability to form biofilms [16]. Indeed, activation of the QS signaling systems in P. aeruginosa causes significant transcriptional changes. For instance, transcriptome analysis using microarrays identified 315 QS-induced and 38 QS-repressed genes, representing about 6% of the P. aeruginosa genome [17].
RND efflux systems (such as MexXY-OprM, MexAB-OprM, MexCD-OprJ, MexEF-OprN) are important not only for intrinsic and/or adaptive resistance to antimicrobial compounds in P. aeruginosa, but they affect the transport of QS signals and precursors and thus QS-dependent phenotypes [18]. Each RND efflux system typically consists of a cytoplasmic membrane component that functions as a transporter (e.g. MexY), an outer membrane component presumed to form channels (e.g. OprM), and a protein presumed to link the two membrane proteins (e.g. MexX) [18]. The RND efflux systems differ somewhat in substrate specificities. For example, MexXY-OprM is capable of excreting aminoglycosides and certain unrelated antibiotics (including macrolides and tetracyclines), whereas MexAB-OprM and MexCD-OprJ are responsible for excreting other antibiotics including quinolones and β-lactams [19]. The MexEF-OprN RND efflux pump transports fluoroquinolones, trimethoprim, as well as chloramphenicol [20,21]. In addition to its role in resistance to antibiotics, the MexAB-OprM efflux pump has been shown to play a role in the selective transport of quorum sensing signals [22,23]. The MexEF-OprN efflux pump also exports the PQS precursor 4-hydroxy-2-heptylquinoline (HHQ) and affects many QS-dependent virulence phenotypes [24]. Indeed, 40% of the genes (102 out of 254) regulated by MexEF-OprN belong to the QS regulon [25].
To date most of what is known about the linkage between TCST regulation, especially via the ParS/ParR operon, quorum sensing, and RND efflux pumps in the control of adaptation and virulence traits comes from studies comparing mutants to wild-type in the presence of antimicrobials. Interestingly, quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis revealed that ParR-dependent genes such as arnBCADTEF-ugd, pmrB, pagL and PA1797 are not induced by ParR in the absence of indolicidin, suggesting that the ParS/ParR system regulates gene expression in an environment-dependent manner [8]. The objectives of the current study were to identify, using RNA-seq whole transcriptome analysis, genes differentially regulated by the ParS/ParR system in the absence of antimicrobials. We discuss the hierarchical relationship of the regulatory elements and the suites of traits controlled by each.
Results
Growth dependent expression of parS and parR in wild type P. aeruginosa PAO1
Transcriptomic analysis revealed that in P. aeruginosa PAO1, the parS (1287 bp) and parR (708 bp) genes are located in a single operon, as indicated by the absence of non-coding nucleotides between the two genes. To determine the cell density at which expression of parS and parR are optimal for transcriptomic analysis, qRT-PCR was conducted using wild-type PAO1 at six different cell densities. The transcript abundances of parS and parR were similar to each other over time and the highest values were observed at an OD600 of 1.2 (5 × 109 cfu/ml, mid log phase) (Additional file 1: Figure S1A, B). Moreover, the qRT-PCR results showed that the transcript abundance of parS and parR (at OD600 = 1.2) was 3–6 fold higher when grown in AB minimal medium + 2% casamino acids (CAA) as compared to LB medium. Thus, subsequent work was performed using P. aeruginosa grown in AB minimal medium + 2% CAA at OD600 ~ 1.2.
Quantitative analysis of the wild type P. aeruginosa PAO1 transcriptome
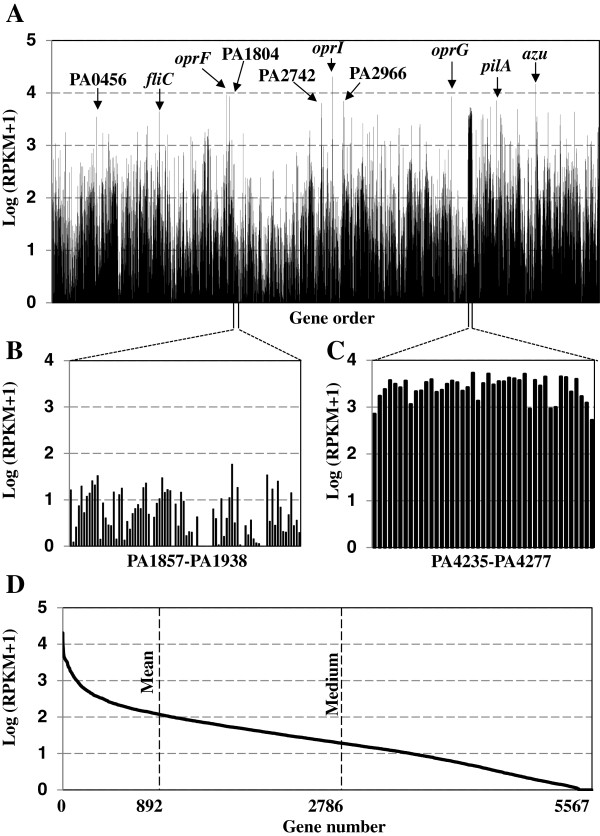
RNA-seq data representing the alignment of sequences (short reads) to coding sequences (CDS) were quantified as reads per kilobase CDS length per million reads analyzed (RPKM), as described previously [26]. The RNA-seq analysis provided a gene expression map showing log (RPKM + 1) values for all 5570 annotated genes in the PAO1 genome (Figure 1A). The mean and medium values were 2.14 and 1.32, respectively, indicting a pronounced skew toward highly expressed genes (Figure 1D). A total of 892 genes were expressed higher than 2.14, whereas the remaining 4678 genes were expressed at lower levels than the mean value (Figure 1D). Genes within large genomic loci (40–90 genes) showed similar patterns in transcript abundance. For example, genes within the region between PA1857 and PA1938 (abundant in hypothetical proteins) were expressed at a relatively low level compared with the more highly expressed genes in the chromosomal segment from PA4235 TO PA4277 (composed of ribosomal proteins, RNA polymerase and elongation factors) (Figure 1B, C).
Figure 1.
Quantification of P. aeruginosa PAO1 gene expression. A. Gene expression profile across the entire P. aeruginosa PAO1 genome (6.3 Mb). Each column represents one of the 5570 annotated genes in the PAO1 genome, with the x-axis showing gene order (from the DNA replication origin), and the y-axis showing the log10 of transcript abundance (RPKM values) for each gene. The arrows point to a few of the genes or gene clusters that are highly expressed. PA0456: probable cold-shock protein; PA1804: DNA-binding protein HU; PA2742: 50S ribosomal protein L35; PA2966: acyl carrier protein; conserved hypothetical protein; fliC: flagellin type B; pilA: type 4 fimbrial precursor; oprF, oprG, oprI: outer membrane proteins; azu: azurin precursor. B. 56.6 kb genome region (PA1857 to PA1938) that shows low levels of gene expression (log (RPKM + 1) < 2). C. 33.8 kb genome region (PA4235 to PA4277) that exhibits high levels of gene expression (log (RPKM + 1) > 2). D. Distribution of the transcript abundance of the 5570 annotated genes in the PAO1 genome, with the x-axis showing gene number (sorted from high to low values), and the y-axis showing the log10 of transcript abundance for each gene in the wild type. The dashed lines indicate gene numbers where mean and medium values are first detected.
The top 20 highly expressed genes (excluding genes encoding ribosomal proteins) are included in Additional file 2: Table S2 and many are indicated in Figure 1A. The gene with the greatest transcript abundance in the wild type strain under our experimental conditions was PA2853 (outer membrane lipoprotein oprI), having a log (RPKM + 1) value of 4.3 (Figure 1A). Two other membrane protein genes (oprF and oprG) also had high transcript levels [log (RPKM + 1) values of 3.97 and 3.93, respectively] (Figure 1A). Consistently, the protein levels of OprI, OprF and OprG were shown previously to be highly abundant in P. aeruginosa[27,28].
Genes in the top twenty include virulence-related genes such as pilA [29], fliC[30], azu [31], oprF [32], fabF [33], capB [33], lon [33] and sodB [34]. In contrast, the transcripts of 139 genes were not detected [log (RPKM + 1) = 0] suggesting these genes are barely expressed under the conditions tested. As expected, among the genes with RPKM + 1 values of 0 were 110 genes annotated as encoding hypothetical proteins or proteins with unknown functions.
The relative transcript abundance of the 123 genes annotated as being part of TCST systems (e.g. 64 putative sensor and 59 putative regulator genes including parS and parR) are provided in Additional file 3: Table S3. The average of the log (RPKM + 1) values for the TCST genes was 1.37, which is relatively low compared to the mean value of 2.14 for all genes. In fact, none of the TCST gene had a log (RPKM + 1) value above 3. The parS and parR genes were expressed at medium levels [e.g. log (RPKM + 1) values of 1.46 and 1.32, respectively]. Overall, response regulator genes were expressed at higher levels compared with the sensor kinase genes. For instance, among the top twenty highly expressed TCST system genes [log (RPKM + 1) > 1.97], sixteen were response regulator genes. In contrast, sixteen sensor kinase genes were found among the twenty least expressed TCST system genes under these growth conditions [log (RPKM + 1) < 0.8].
ParS and ParR regulated genes
In order to identify genes regulated by the ParS/ParR system in PAO1, mean RPKM values for both parS and parR mutants were compared with the wild type. The ratios of RPKM values (mutant to wild type) were log-transformed to better illustrate genes that were differentially expressed in parS and parR mutants. As shown in Figure 2, mutations in parS or parR caused similar changes in the PAO1 transcriptome at the mid log phase. The transcript abundance of a total of 257 and 331 genes were changed in the parS and parR mutants compared to wild type, respectively (Additional file 4: Table S4). Of these genes, 124 genes were differentially expressed in both parS and parR mutants (e.g. the transcript abundance of 100 and 24 genes were higher or lower than wild type, respectively) (Additional file 4: Table S4). These results suggest that mutations in parS and parR have both common and differential influences on the bacterial transcriptome.
Figure 2.

Differential gene expression profile between wild-type P. aeruginosa PAO1 and a parS (A) and a parR (B) mutant. Each point represents one of the 5570 annotated genes in the PAO1 genome, with the x-axis showing gene order (from the DNA replication origin), and the y-axis showing the log2 of transcript abundance for each gene in the parS or parR mutant relative to the wild-type (WT) strain. The arrows point to a few genes or gene clusters that are differentially expressed in both parS and parR mutants. pqsABCDE: PQS signal biosynthetic genes. phzA2: phenazine biosynthetic gene; mexEF-oprN: RND efflux pump operon; PA1970, PA3229, PA4881: hypothetical proteins; PA3326-PA3336: A 16.6 kb region including clpP2 (ATP-dependent Clp protease), fabH2 (3-oxoacyl-[acyl-carrier-protein] synthase III) and many hypothetical genes.
Among the 24 genes activated by both ParS and ParR, only 4 genes (mexE, mexF, mexS and oprN) have been functionally characterized in P. aeruginosa (Table 1) and further discussion of these genes appears below. Among the 100 genes repressed by both ParS and ParR were genes encoding enzymes (e.g. chitinase, elastase, and protease), genes involved in secondary metabolite biosynthesis (e.g. hydrogen cyanide, phenazine, and rhamnolipid synthesis), and genes involved nitrous oxide reduction (Table 1). To validate the expression profiles obtained by RNA-Seq, qRT-PCR was performed on 18 genes; these included genes encoding for components of the quorum sensing systems, enzymes involved in chitin degradation, phenazine and rhamnolipid synthesis, and proteins previously shown to respond to toxic compounds. The data (e.g. fold differences in mutant versus wild type transcript abundances) from the qRT-PCR analysis were comparable to those obtained by the RNA-seq analysis for all selected genes (Additional file 5: Figure S2), thus verifying the RNA-seq data.
Table 1.
Selected differentially regulated genes in the parS and parR mutants compared to WT
| Locus | Gene | Log foldab ∆ parS /WT | Log foldab ∆ parR /WT | Protein description | |||
|---|---|---|---|---|---|---|---|
|
Down-regulated genes |
|
|
|
|
|
|
|
| PA2491 |
mexS |
−3.51 |
−2.39 |
Oxidoreductase |
|
|
|
| PA2493 |
mexE |
−8.21 |
−7.30 |
Multidrug efflux membrane fusion protein |
|
|
|
| PA2494 |
mexF |
−8.75 |
−9.36 |
Multidrug efflux transporter |
|
|
|
| PA2495 |
oprN |
−7.11 |
−8.53 |
Multidrug efflux outer membrane protein |
|
|
|
| PA2811 |
… |
−2.05 |
−0.45 |
Probable permease of ABC transporter |
|
|
|
| PA2812 |
… |
−2.62 |
−0.86 |
Probable ABC transporter |
|
|
|
| PA2813 |
… |
−2.44 |
−1.36 |
Probable glutathione S-transferase |
|
|
|
| PA3229 |
… |
−7.4 |
−5.2 |
Hypothetical protein |
|
|
|
| PA4354 |
… |
−3.07 |
−1.47 |
Conserved hypothetical protein |
|
|
|
| PA4356 |
… |
−3.55 |
−2.94 |
Xenobiotic reductase |
|
|
|
| PA4623 |
… |
−4.55 |
−4.91 |
Hypothetical protein |
|
|
|
| PA4661 |
pagL |
−1.01 |
−0.72 |
Lipid A 3-O-deacylase |
|
|
|
| PA4881 |
… |
−10.68 |
−9.13 |
Hypothetical protein |
|
|
|
|
Up-regulated genes |
|
|
|
|
|
|
|
| PA0051 |
phzH |
3.56 |
1.40 |
Phenazine-modifying enzyme |
|
|
|
| PA0441 |
… |
1.86 |
1.53 |
Dihydropyrimidinase |
|
|
|
| PA0523 |
norC |
3.61 |
2.52 |
Nitric-oxide reductase subunit C |
|
|
|
| PA0524 |
norB |
3.24 |
1.84 |
Nitric-oxide reductase subunit B |
|
|
|
| PA0525 |
norD |
2.56 |
1.32 |
Probable dinitrification protein D |
|
|
|
| PA1130 |
rhlC |
3.07 |
2.42 |
Rhamnosyltransferase 2 |
|
|
|
| PA1671 |
stk1 |
2.78 |
2.99 |
Serine-threonine kinase |
|
|
|
| PA1707 |
pcrH |
2.09 |
2.23 |
Regulatory protein |
|
|
|
| PA1778 |
cobA |
1.92 |
2.14 |
Methyltransferase |
|
|
|
| PA2193 |
hcnA |
3.86 |
5.95 |
Hydrogen cyanide synthase |
|
|
|
| PA2300 |
chiC |
5.52 |
2.27 |
Chitinase |
|
|
|
| PA2303 |
ambD |
0.92 |
1.53 |
Taurine catabolism dioxygenase |
|
|
|
| PA1899 |
phzA2 |
12.66 |
11.33 |
Phenazine biosynthesis protein |
|
|
|
| PA1900 |
phzB2 |
5.26 |
1.79 |
Phenazine biosynthesis protein |
|
|
|
| PA2593 |
qteE |
3.18 |
2.53 |
Quorum threshold expression element |
|
|
|
| PA3326 |
clpP2 |
2.80 |
3.33 |
Protease |
|
|
|
| PA3331 |
… |
5.82 |
5.56 |
Cytochrome P450 |
|
|
|
| PA3333 |
fabH2 |
6.82 |
6.36 |
3-oxoacyl-[acyl-carrier-protein] synthase III |
|
|
|
| PA3478 |
rhlB |
3.68 |
2.50 |
Rhamnosyltransferase chain B |
|
|
|
| PA3479 |
rhlA |
4.37 |
3.31 |
Rhamnosyltransferase chain A |
|
|
|
| PA3724 |
lasB |
4.29 |
3.32 |
Elastase |
|
|
|
| PA3757 |
nagR |
1.75 |
3.48 |
Transcriptional regulator |
|
|
|
| PA3974 |
ladS |
1.52 |
1.40 |
Sensor protein |
|
|
|
| PA4133 |
… |
4.07 |
4.47 |
Cytochrome C oxidase subunit |
|
|
|
| PA4209 |
phzM |
4.22 |
1.94 |
Phenazine-specific methyltransferase |
|
|
|
| PA4211 |
phzB1 |
5.72 |
8.13 |
Phenazine biosynthesis protein |
|
|
|
| PA4217 | phzS | 6.04 | 5.95 | Flavin-containing monooxygenase |
aLog expression ratio ≥1.0 indicates genes are up-regulated in mutants and ≤ −1 indicates genes are down-regulated in mutants.
bThe number in bold with P value very close to 0.05. All others P value < 0.05.
The ParS/ParR system activates the mexEF-oprN operon through mexS
The sequence reads from the wild type and parR mutant were mapped to the genome sequence of the mexEF-oprN region and displayed using Artemis and Bamview (Figure 3A). Compared to the wild type strain, which had abundant reads over the mexEF-oprN operon, few reads were detected across this region for the parR mutant. The parS mutant showed a similar expression pattern to that of the parR mutant (data not shown). Consistently, the log (RPKM + 1) values were around 2.5 for mexE, mexF and oprN, for the wild type, whereas deletion of parS or parR reduced the values to less than 0.8 (Figure 3B). qRT-PCR also confirmed that the expression of mexF and oprN was highly down-regulated by log (fold changes) of 7.6 ± 0.2 and 6.6 ± 1.8 in the parR mutant and by 8.6 ± 1.4 and 7.6 ± 1.6 in the parS mutant compared with wild type, consistent with the RNA-seq data (Additional file 6: Figure S3).
Figure 3.
Expression of mexT and mexEF-oprN genes in wild type PAO1, parS and parR mutants. A. RNA-seq profile showing sequence reads across the mexT and mexEF-oprN region. RNA-seq results were visualized using the Artemis (http://www.sanger.ac.uk/resources/software/artemis/) genome browser. B. Transcript abundance (log10 of RPKM values) of mexS, mexT and mexEF-oprN genes in wild type PAO1, parS and parR mutants. Data points represent means of three replicates ± standard deviations.
As reported previously, expression of the mexEF-oprN operon is under the positive control of the DNA-binding protein MvaT [35], the oxidoreductase MexS [36] and the LysR family protein MexT [37]. Transcripts of mvaT were expressed at similar high levels in the wild type and mutant strains: log (RPKM + 1) values for wild type, parS and parR mutants were 3.29 ± 0.04, 3.30 ± 0.15 and 3.35 ± 0.02, respectively. Although the expression of mexT was not appreciably altered, mutation in parS/parR reduced the transcript levels of mexS (Figure 3A, B; Additional file 5: Figure S2). Since regulation of MexEF-OprN by MexS depends on MexT [36], these results suggest that the ParS/ParR system activates the mexEF-oprN operon through the MexS-MexT pathway.
The ParS/ParR system negatively controls quorum sensing
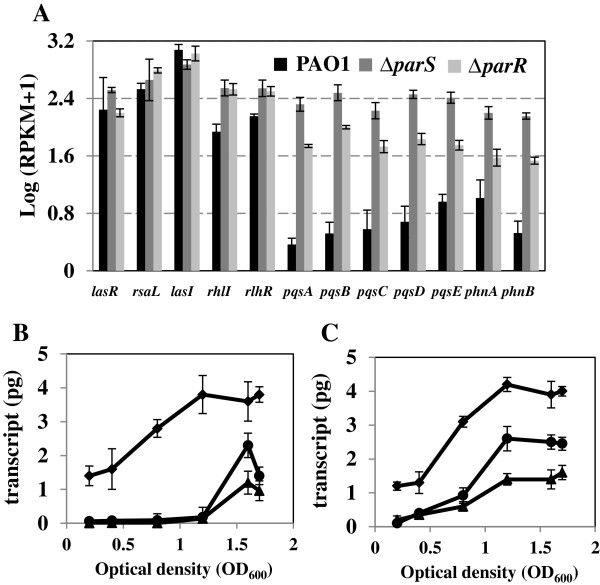
It was shown previously that the MexEF-OprN efflux pump interferes with quorum sensing by extruding HHQ and kynurenine in P. aeruginosa[24,25]. Moreover, a quorum sensing regulatory gene encoding the quorum threshold expression element QteE [38] also was differentially expressed in parS and parR mutants (Table 1). Therefore, the ParS/ParR system has regulatory effects on QS in P. aeruginosa. Indeed, transcripts of rhlI and rhlR were elevated in parS and parR mutants compared with the wild type (Figure 4A). Although the expression levels of lasI and lasR were not altered, the rsaL gene was slightly increased in the parR mutant. The pqsABCDE and phnAB operons also were expressed at higher levels in the mutant strains. The pqsABCDE-phnAB cluster is known to be positively controlled by the cognate regulator PqsR, also named MvfR [39]. Interestingly, pqsR was expressed at similar levels in the wild type [log (RPKM + 1) = 2.77 ± 0.15] and mutant strains [log (RPKM + 1) = 2.66 ± 0.21 for ∆parS and 2.80 ± 0.13 for ∆parR] suggesting that the ParS/ParR system regulates the level of pqsABCDE-phnAB independently of pqsR.
Figure 4.
Impact of the ParS/ParR system on quorum sensing. A. Transcript abundance (log10 of RPKM values) of QS systems in wild type PAO1, parS and parR mutants. Data points represent means of three replicates ± standard deviations. Expression levels of lasI, rhlI and pqsA in cultures of wild type PAO1 (B.) and ΔparR(C.) at different cell densities. Bacteria were grown in AB minimal medium + 2% casamino acids and RNA was isolated from cells harvested at six different growth stages (OD600). The relative abundance of lasI (♦), rhlI (●) and pqsA (▲) was estimated based on rpoD transcript quantity in cDNA samples determined by qRT-PCR.
To better understand how ParS-ParR regulates the three QS systems in P. aeruginosa, the transcript abundances of lasI, rhlI and pqsA were monitored at six different cell densities in the wild type and parR mutant (Figure 4B, C). The lasI gene appeared to be constitutively expressed and reached the highest level at an OD600 of 1.2 in both wild type and parR mutant. In contrast, wild type and the parR mutant exhibited different patterns in the expression of the rhlI and pqsA genes. First, the expression levels of both rhlI and pqsA were not detectable until cultures reached an OD600 of 0.8 in the wild type, whereas these genes were detected at OD600 of 0.4 in the mutant strains. Secondly, the transcripts of rhlI and pqsA genes reached their highest levels at an OD600 of 1.6 for the wild type, but at an OD600 of 1.2 for the parR mutant. Together, these results confirm a role of the ParS/ParR system in controlling the timing of QS gene expression in P. aeruginosa.
Genes known to be activated by ParS/ParR system in the presence of antimicrobials
In the presence of antimicrobial agents such as indolicidin, the ParR protein promotes drug resistance through several known, distinct mechanisms including: activating the mexXY efflux genes, suppressing the expression of oprD porin, and enhancing lipopolysaccharide modification through the arn genes (9). The RNA-seq analysis showed that in the absence of antimicrobials the expression of oprD increased by 3.1 and 6.9 fold in parS and parR mutants, respectively (Table 1), similar to the effect of mutations observed in the presence of antimicrobials. However, the transcript levels of mexXY-oprM and arnBCADTE-ugd were not appreciably different suggesting that the ParS/ParR system does not have a strong influence on these two operons in defined minimal medium. One possible reason is that the arnBCADTE-ugd and mexXY operons were expressed at low levels in the wild type strain under our growth conditions. Indeed, the log (RPKM + 1) values of arnBCADTE-ugd and mexXY were 0.4-1.7 and 0.5-0.7, respectively.
Negative impact of the ParS/ParR system on phenazine production and motility
We noticed previously that the ParS/ParR system controls the production of the phenazine pyocyanin. Especially in pigment-production medium (PPMD), the parS and parR mutants produce more pyocyanin (green color) than the wild-type (Figure 5A). The impact of the deletion of parS or parR on phenazine production was quantified using chloroform extraction of cultures grown in PPMD medium followed by spectrophotometric assays as described previously [40]. The amount of phenazine produced by the parS and parR mutants was greater than two fold the amount produced by the wild-type strain (Figure 5B). Complementation of the mutants by introducing parS-parR in trans on a medium-copy-number vector reduced phenazine production below wild type levels, confirming its negative regulatory role in phenazine biosynthesis.
Figure 5.
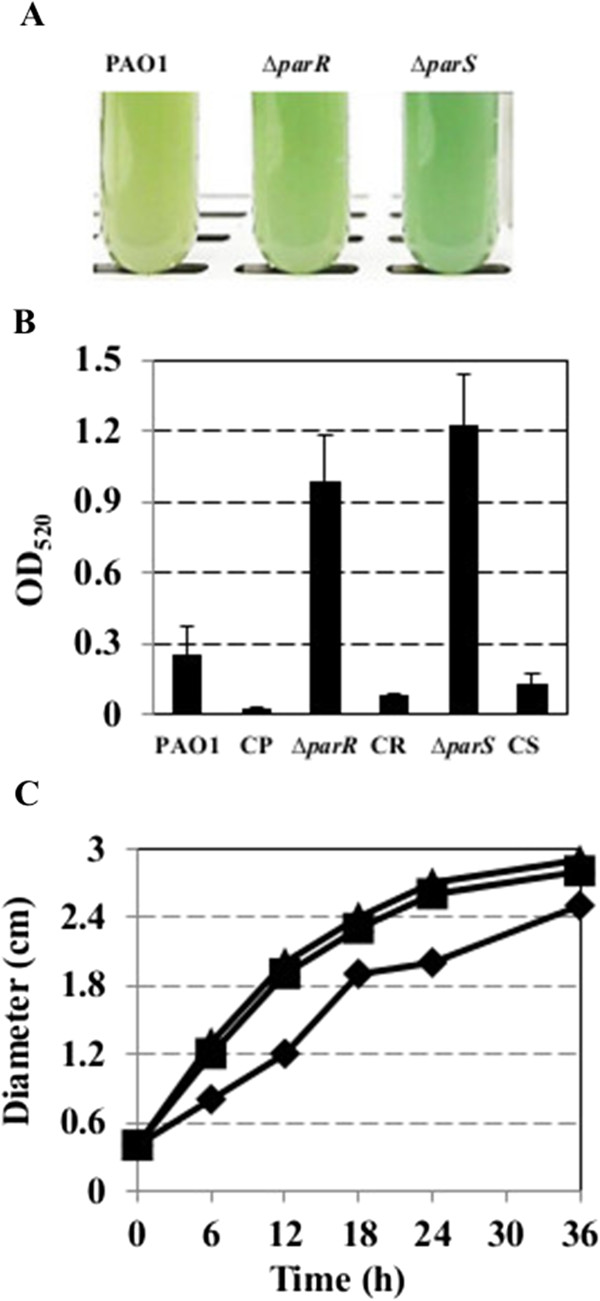
The ParS/ParR system negatively controls phenazine production and motility. A. Pyocyanin production of wild-type (PAO1), and mutant strains in AB medium + 2% CAA 24 h post-inoculation. B. Pyocyanin production by different strains in vitro. Bacterial strains were grown in AB medium +2% CAA for 24 h at 37°C with shaking. Pyocyanin was extracted and quantified at OD520. Data points represent the means of three replicates ± standard deviations. Similar results were obtained in at least two independent experiments. CP, CR and CS indicate the wild type, parR and parS mutants harboring the complementation plasmid pKT2-parSR, respectively. C. Comparison of motility among wild-type, parS and parR mutants. Cells were inoculated on the surface of a motility plate containing 0.4% agar as described previously. Plates were incubated at 28°C for 36 h, during which the displacement (diameter) of the outermost edge of the movement was measured. The experiment was performed three times in triplicate. Errors bars were smaller than the symbols.
Rhamnolipids are an essential component for P. aeruginosa swarming motility [41]. To determine whether the increased expression of rhamnolipid biosynthetic genes (rhlA, rhlB and rhlC) resulted in increased motility in the mutant strains, swarming motility was assessed by inoculating bacterial cells on a motility plate (0.4% agar) and measuring the diameter of the circle covered by bacterial cells for up to 36 h as described previously [42,43]. Both parS and parR mutants exhibited enhanced motility compared with that of the wild type strain (Figure 5C). These results suggest that the ParS/ParR system is a negative regulator of bacterial motility in P. aeruginosa.
qRT-PCR was used to verify phenazine- and motility-related gene expression in these bacterial strains, including phzA1, phzA2, phzM, rhlA and rhlB, in AB medium + 2% CAA at mid-logarithmic phase. Expression of phzA1, phzA2, phzM, rhlA and rhlB in the parS and parR mutants was up-regulated by 2–6 log (fold change), as compared to that of the wild type (Additional file 5: Figure S2). These results further confirmed the regulatory roles of ParS/ParR on phenazine and motility genes.
Discussion
In this study, we determined the transcript levels of all 5570 of the annotated coding sequences within the P. aeruginosa PAO1 genome. To the best of our knowledge, this is the first quantitative transcriptomic atlas of P. aeruginosa PAO1. RNA-seq analysis also identified genes regulated by ParS and ParR and linked the ParS/ParR TCST system to the well characterized MexEF-OprN operon and the three quorum sensing systems. This linkage was not identified previously by transcriptomic analyses using microarrays to compare mutants to wild type under different growth conditions (e.g. in the presence of antibiotics). Hence, these results provide important clues toward understanding the complexity of the regulatory roles mediated by ParS/ParR in controlling drug resistance.
Previous microarray studies identified 114 genes controlled by ParR in the presence of 4 μg/ml indolicidin [8] and 17 genes controlled by a point mutation (M59I) in the ParR protein [9]. The two microarray studies shared 14 common genes including arnABCDEF and 8 genes (PA1559, PA1660, PA1797, PA2358, PA2655, PA4773, PA4774 and PA4775) encoding hypothetical proteins (Additional file 6: Figure S3A). The agreement between these studies in identifying genes within the arnBCADTEF-ugd operon confirms the importance of ParR in the regulation of lipopolysaccharide modification genes in the presence of antimicrobials. Two of those genes arnF and PA4773 also were identified as being ParS/ParR regulated in our study (Additional file 4: Table S4). Since arnF and PA4773 belong to the arnBCADTEF-ugd and PA4473-PA4475 operons, these data indicate that the influence of ParS and ParR on the two operons is weaker in the absence of antimicrobials. Interestingly, data from all three studies indicate that ParS/ParR are responsible for the repression of the oprD gene (basic amino acid and carbapenem permeable porin) (Additional file 4: Table S4, Additional file 6: Figure S3A).
The 24 commonly regulated genes identified by our study and the indolicidin treatment studies included 19 genes that were suppressed by ParR in both studies (Table 1; Additional file 4: Table S4). These included are phzA2, phzB2, phzS (phenazine biosynthetic genes), norBCD (nitric oxide reductase), chiC (chitinase), lecB (fucose-binding lectin), PA4133 (cytochrome c oxidase) and 10 genes encoding hypothetical proteins. One gene pagL encoding a lipid A 3-O-deacylase was down-regulated in both studies. Interestingly, 4 genes including PA0282-83 (sulfate transporter genes), PA4443 (ATP sulfurylase small subunit) and PA4773 (hypothetical protein) that were over-expressed in the parR mutant in this study, were down-regulated in the study using indolicidin. These results indicate that induction of a portion of ParR-regulated genes depends on the environmental conditions.
It was reported that the MexEF-OprN efflux pump produces specific transcriptional changes in P. aeruginosa regulatory networks [25]. Since both the MexEF-OprN efflux pump and QS systems were regulated by the ParS/ParR system, we compared our transcriptome data with those of the two studies that have contributed to define the MexEF-OprN and QS regulons in P. aeruginosa[17,25]. The comparison revealed that approximately 16% and 22% of the genes (74 and 98 out of 464) that were differentially regulated by ParS/ParR belong to the MexEF-OprN and QS regulons, respectively (Additional file 6: Figure S3B). A total of 41 genes were commonly regulated by the three systems (Additional file 7: Table S5). Half of these genes (22) were classified as hypothetical or functionally unknown. Other genes of interest included pqsABCDE-phnAB, hcnAC (hydrogen cyanide synthase), phzB1 (phenazine biosynthesis), chiC (chitinase), lecB (fucose-binding lectin), clpP2 (ATP-dependent protease), lptF (lipotoxin), mexH-opmD (multidrug efflux pump). Another interesting feature was that all 41 genes were over-expressed in the parS/parR mutant. Together, these results suggest that the ParS/ParR regulatory effects are partially mediated by the MexEF-OprN and QS systems.
A total of 33 genes were specifically regulated by ParS/ParR and MexEF-OprN, but not QS (Additional file 6: Figure S3B; Additional file 8: Table S6). Among them, 24 genes were under-expressed in the parS/parR mutant including pagL, pncB1 (PA4919, nicotinate phosphoribosyltransferase), xenB (PA4356, xenobiotic reductase), idh (PA2624, isocitrate dehydrogenase). PncB1 and XenB are enzymes involved in the degradation of nicotinate and trinitrotoluene (TNT), respectively [44,45]. Another interestingly gene cluster was PA2811-PA2813 encoding two ABC transporter and a glutathione S-transferase (GST). GSTs constitute a large family of enzymes that catalyze the addition of glutathione to many toxic exogenous compounds [46]. The 9 genes that were over-expressed included oprD, nosFY (PA3394-95 nitrous oxide reductases), hpcB (PA4124, xenobiotic reductase), hpaA (PA4091, 4-hydroxyphenylacetate 3-monooxygenase large chain), PA3951 (molybdopterin biosynthetic protein B1), narK1 (PA3877, nitrite extrusion protein 1) and PA1875 (probable outer membrane protein). Since the expression of mexEF-oprN was activated by ParS/ParR; whereas the expression of parS or parR was not affected by MexEF-OprN [25], it is reasonable to speculate that the ParS/ParR system functions upstream of MexEF-OprN. These results also suggest that the ParS/ParR system may control membrane permeability and detoxification genes through the positive control of the MexEF-OprN efflux pump, but not through QS. A total of 57 genes were controlled by ParS/ParR and the QS systems, but not the MexEF-OprN operon (Additional file 6: Figure S3B; Additional file 9: Table S7). Consistent with the up-regulation of QS-controlled genes, the expression of rhlI and rhlR was increased 2–5 fold in parS and parR mutants. The QS regulatory gene qteE was also in this group. Some other noteworthy members of this group were genes encoding RND efflux transporters (PA3676-77 and triC), secondary metabolism genes (rhlABC, aprAE and lasB), cytochrome c oxidases (PA0105-08, PA1556) and regulatory genes (PA2591 and PA3347). These results suggest the possibility that the impact of ParS and ParR on QS is partially mediated by the RhlR/I system.
The ladS gene encoding a two component sensor protein was expressed at high levels in both parS and parR mutants compared with the wild type (Table 1). Previous studies showed that the LadS protein activates P. aeruginosa QS expression through the GacS/GacA two component system and the regulatory RNA RsmZ [47]. This observation linked the ParS/ParR system with the well-characterized GacS/GacA regulatory system. Another gene, phzH encoding a phenazine terminal modifying enzyme, was also negatively regulated by the ParS/ParR system (Table 1). PhzH is a unique transamidase involved in the conversion of phenazine-1-carboxylic acid (PCA) to phenazine-1-carboxamide (PCN) [48]. Unlike phzM and phzS (phenazine modifying enzymes), which were positively controlled by QS, phzH was not regulated by the QS and MexEF-OprN systems [17,25]. Five genes (PA3095, PA3096, PA3099, PA3102 and PA3105) annotated as encoding components of a type II secretion system (T2SS) also were over-expressed (2 to 6-fold) in the parS and parR mutants relative to the wild type, suggesting positive control by ParS and ParR (Additional file 4: Table S4). The T2SS is a secretory pathway for most extracellular proteins. Although, these genes were not identified as targets for QS by the transcriptomic studies [17], transcriptional fusion assays showed that their expression was influenced by the Rhl and Las QS systems [49].
Conclusion
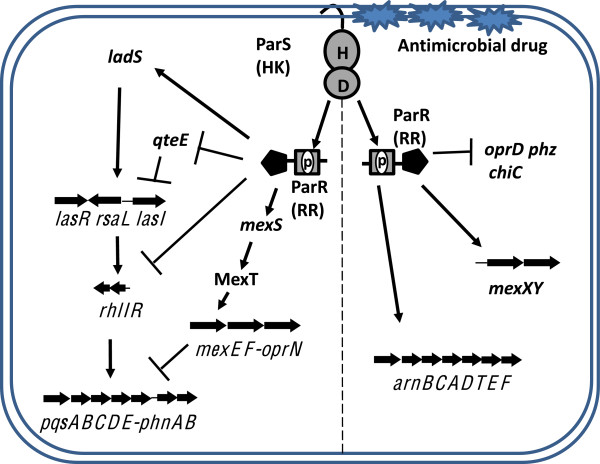
Previous microarray studies demonstrated that the ParS/ParR TCST system controls the expression of the lipopolysaccharide modifying (arnBCADTEF-ugd), efflux (mexXY), porin (oprD), chitinase (chiC) and phenazine biosynthetic genes (phzA2B2) when exposed to subinhibitory concentrations of antimicrobial reagents [8,9]. In this study, the major components of the ParS/ParR signal transduction pathway in P. aeruginosa PAO1 were identified in bacteria grown without antimicrobials (Figure 6). Significantly, we showed that the three QS regulatory genes, the gene cluster including the mexEF-oprN efflux system, the ladS sensor kinase and the quorum threshold expression element qteE were differentially regulated by the ParS/ParR system. The sensor kinase LadS is an activator of the GacS/GacA two component system and it positively regulates the levels of Las QS system through the titration of the translational regulatory protein RsmA [50]. In contrast, expression of the QteE protein reduces LasR protein stability without affecting its expression [38]. Finally, the MexEF-OprN efflux pump is a transporter of the PQS precursor HHQ and negatively controls the expression of the pqsABCDE-phnAB cluster [24,25]. These results indicate that the ParS/ParR system regulates QS at both transcriptional and translational levels through multiple mechanisms. Since the expression of parS and parR is not controlled by QS or MexEF-OprN, whereas both QS and MexEF-OprN gene are regulated by ParS and ParR, we conclude that the ParS/ParR system is on the top of this hierarchical regulatory cascade.
Figure 6.
ParS/ParR hierarchical control in P. aeruginosa. The model shows the predicted position of the ParS/ParR system in relation to other known regulators of QS. ParS is a transmembrane protein according to in silico predictions based on amino acid sequences. Amino acids H (histidine) and D (aspartate) involved in phosphorylation are indicated. The solid area of ParR indicates the DNA-binding domain. Solid arrows and blunt lines point to genes (or processes) that are positively or negatively affected, respectively. ParR controls the expression of mexEF-oprN, qteE and ladS, which in turn regulate QS through different mechanisms. HK: histidine kinase; RR: response regulator; P: phosphoryl group.
Methods
Bacterial strains and growth conditions
The wild type strain PAO1 and its parS and parR mutants were obtained from the P. aeruginosa PAO1 transposon mutant library [51] (Table 2). Analysis of RNA transcript abundance indicated that the insertions did not cause polar effects since the expression of PA1797, the ORF immediately downstream of parS and parR, was not affected by either insertion (data not shown). Liquid LB medium, pigment production medium (PPMD) or AB minimal medium supplemented with 2% casamino acids (AB + 2% CAA) (Difco, Becton Dickinson and Company, Franklin Lakes, NJ) were used for culturing P. aeruginosa as described previously [52]. The following antibiotics were added to the medium when necessary: ampicillin (Ap) 100 μg ml-1, kanamycin (Km) 50 μg ml-1, and gentamicin (Gn) 30 μg ml-1.
Table 2.
Bacterial strains and plasmids used in this study
| Strains and plasmids | Relevant charactersa | Reference or source |
|---|---|---|
|
P. aeruginosa |
|
|
| PAO1 |
wild-type (WT) |
[51] |
| ∆parS |
PAO1 Tn5 mutant, insertion at bp 402 in parS, GnR |
[51] |
| ∆parR |
PAO1 Tn5 mutant, insertion at bp 299 in parR, GnR |
[51] |
|
E. coli |
|
|
| DH5α |
F-recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 Δ (argF-lacZYA)I169 ϕ 80lacZΔM15λ- |
GIBCO-BRL |
|
Plasmids |
|
|
| pPROBE-KT2 |
KmR, GFP based promoter trap vector containing a promoter-less gfp gene |
[58] |
| pKT2-parSR | 2.3 kb DNA fragment containing parS and parR genes in pPROBE-KT2 | This study |
aKmR and GnR = kanamycin and gentamycin resistance, respectively.
DNA manipulation and sequence analysis
Standard procedures were used for plasmid isolation, cloning, restriction enzyme digestion and T4 DNA ligation [53]. Polymerase chain reaction (PCR) was carried out using Invitrogen Taq DNA polymerase (Life Technologies, Carlsbad, CA) at 95°C for 5 min, followed by 30 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 90 sec, and a final elongation step of 70°C for 10 min. DNA sequencing was performed at the Genome Technology Lab (GTL) within the Texas A&M University Institute for Plant Genomics & Biotechnology.
RNA preparation
Three biological replicates of every strain were started from single colonies located on three separate plates containing AB + 2% CAA and then transferred to 10 ml AB + 2% CAA broth. All cultures were grown at 37°C with shaking (200 rpm) to an approximate OD600 = 1.2. Cell cultures collected at OD600 = 1.2 were diluted to OD600 = 0.3 with AB + 2% CAA broth. RNA extraction was performed as described previously [54-56] with one exception: contaminating genomic DNA was removed off-column with Turbo DNA-free DNase (Life Technologies, Carlsbad, CA). Elimination of contaminating DNA was confirmed via qPCR amplification of the rpoD gene with SYBR green® dye on an ABI 9400HT PCR machine (Life Technologies, Carlsbad, CA). RNA samples were ethanol-precipitated and resuspended in 0.1% diethylpyrocarbonate (DEPC). RNA quantification was performed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) at the Texas A&M GTL.
RNA-seq analysis
RNA-seq was performed as described previously [26]. Briefly, ribosomal RNA (rRNA) was depleted from ~9 μg of total RNA using the RiboZero rRNA depletion kit (for Gram-negative bacteria, Epicentre Biotechnologies, Madison, WI). Strand-specific cDNA libraries were constructed using the SOLiD Total RNA-Seq kit. Paired-end sequencing was conducted by the University of Texas Genomic Sequencing and Analysis Facility on a Life Technologies SOLiD 5500xl sequencing system with a targeted sequencing depth of six-million paired-end reads per sample. Filtering and alignment of the SOLiD 4 paired-end data was performed at the UTGSAF using the AB SOLiD BioScope Whole Transcriptome pipeline (v1.3), for whole-transcriptome RNA-seq analysis. Mapped reads were visualized using BamView in Artemis 13.2.0 [57].
To determine RNA transcriptional abundance for each gene, the number of reads that mapped within each annotated coding sequence (CDS) was determined. The number of reads per kb of transcript per million mapped reads (RPKM) was used to normalize the raw data [26], and mean RPKM values were determined for the three biological replicates. The complete dataset including raw and processed data has been deposited at the National Center for Biotechnology Information (NCBI), Accession No. GSE44681. Comparisons were performed using a modified t-test [26]. A ratio of the mean RPKM values (mutant/WT) was determined for each gene. Ratios over 2 or below 0.5 and p-value < 0.05 were considered differentially expressed [26].
qPCR methods and analysis
qPCR was performed at the Texas A&M GTL using a previously described method [26]. RNA was reverse-transcribed using random primers (Invitrogen) and Superscript III (Invitrogen) at 50°C for 1 h and inactivated at 75°C for 15 min. SYBR Green reactions were performed using the ABI 7900 HT Fast System (Applied Biosystems, Foster City, CA) in 384 well optical reaction plates. Aliquots (1 μl) of cDNA (2 ng/reaction) or water (no-template control) were used as template for qPCR reactions with Fast SYBR Green PCR Master Mix (Applied Biosystems) and primers (500 nM final concentration). Primer pairs parSRT1-parSRT2, parRRT1-parRRT2, lasIRT1-lasIRT2, lasRRT1-lasRRT2, rhlIRT1-rhlIRT2, rhlRRT1-rhlRRT2, pqsART1-pqsART2, pqsCRT1-pqsCRT2, pqsDRT1-pqsDRT2, pqsRRT1-pqsRBRT2, qteERT1-qteERT2, mexSRT1-mexSRT2, mexFRT1-mexFRT2, oprNRT1-oprNRT2, chiCRT1-chiCRT2, phzA1RT1-phzA1RT2, phzA2RT1-phzA2RT2, phzMRT1-phzMRT2, rhlART1-rhlART2, rhlBRT1-rhlBRT2 and rpoDRT1-rpoDRT2 were used to detect the expression of parS, parR, lasI, lasR, rhlI, rhlR, pqsA, pqsC, pqsD, pqsR, qteE, mexS, mexF, oprN, chiC, phzA1, phzA2, phzM, rhlA, rhlB and rpoD genes, respectively (Additional file 10: Table S1). qPCR amplifications were carried out at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min, and a final dissociation curve analysis step from 65°C to 95°C. Two technical replicates of each of three biological replicates were used for each experiment. Amplification specificity for each reaction was confirmed by the dissociation curve analysis. Ct values determined by the software were then used for further ∆∆Ct analysis. The rpoD gene was used as the reference gene to normalize samples and a relative quantification (RQ) value was calculated for each gene with the control group as a reference [26]. For quantification of transcript abundance, a standard curve was generated using purified rpoD PCR product over a dilution range of known concentrations and rpoD transcript quantity in cDNA samples determined by quantitative real-time PCR was used to estimate the relative amount of template concentrations of the experimental genes.
Cloning of the parR-parS operon
In order to determine whether complementation of the ΔparR and ΔparS mutants restored normal phenazine production, the parR-parS flanking sequences were used to design primers (par1-par2) to amplify the two genes and their promoter sequence. Following amplification, the PCR product was directly cloned into pTOPO 2.1 (Invitrogen). Transformants were selected on LB plates supplemented with 100 μg ml-1 Ap. The pTOPO-parRS construct and pKT2 vector [58] were digested by EcoRI and BamHI and ligated resulting in plasmid pKT-parRS (Table 2). The plasmid was introduced into P. aeruginosa strains by electroporation as described previously [52]. Transformants were selected on LB plates supplemented with 50 μg ml-1 Km. To confirm transformation, the genotype was confirmed by both enzymatic digestion and sequencing.
Quantification of phenazine production
P. aeruginosa strains were grown with aeration at 37°C in PPMD for 24 h. Phenazines were extracted and quantified by UV-visible light spectroscopy as described previously [40]. Briefly, phenazines were extracted with chloroform from culture supernatants and then extracted with an equal volume of HCl (0.2 N); optical density was measured at OD520 nm. The absorbance for each sample was normalized to the total absorbance of the 10-ml culture.
Bacterial swarming motility assays
For P. aeruginosa PAO1, parS and parR mutants, bacterial cell suspensions were grown overnight in LB broth. Five μl of the bacterial suspensions were plated onto the center of motility agar plates (10 g tryptone, 5 g NaCl, 3.5 g agar per liter distilled water) as described previously [42,43]. Diameters were determined following incubation at 28°C for 36 h. The experiments were repeated at least three times.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Experiments conceived and designed by: EAP LSP DPW CS. Experiments were performed and analyzed by: DPW CS EAP LSP. Contributed reagents/materials/analysis tools: EAP LSP. Wrote the paper: DPW CS LSP EAP. All authors read and approved the final manuscript.
Supplementary Material
Transcript abundance of parS and parR mutants at different cell densities. A. Growth of PAO1 in AB minimal medium + 2% CAA assessed as OD600 (●) or Log cfu/ml (○). he arrow indicates the time at which cells were harvested for RNA-seq analysis. B. PA01 was grown in AB minimal medium + 2% CAA and RNA was isolated from cells harvested at six different growing stages (OD600). A standard curve was generated using purified rpoD PCR product over a dilution range of known concentrations and the relative abundance of parS and parR was estimated based on rpoD transcript quantity in cDNA samples determined by qRT-PCR.
Top twenty highly expressed genes in P. aeruginosa PAO1.
Transcript levels of the 123 TCST genes in P. aeruginosa PAO1.
Differentially expressed genes in parS and parR mutant compared to wild type strain PAO1. Up- and down-regulated genes (ratios) are indicated by red and green, respectively. Genes with p-values < 0.05 are highlighted in yellow.
Validation of RNA-seq results by qRT-PCR. Relative gene expression levels in the parS and parR mutants compared to the wild type strain. Bacterial strains were grown in 5 mL AB medium + 2% CAA. Relative expression of 16 selected genes, normalized to the expression value of the rpoD gene, was determined by qRT-PCR after 16 h growth (OD600 at 1.2). Data points represent means ± SD of three replicates. These experiments were repeated at least twice and similar results were obtained.
A. Comparison of ParS/ParR-regulated genes with ParR-regulated genes in the presence of 4 μg/ml indolicidin (indicated as parR1; [8]) and with genes differentially regulated by a ParR point mutation (indicated as parR2; [9]). B. Venn diagram comparing the number of genes regulated by the three regulons: ParS/ParR (this study), QS [17] and MexEF-OprN [25].
Genes commonly regulated by ParS/ParR, MexEF-OprN and QS.
Genes commonly regulated by ParS/ParR and MexEF-OprN, but not QS.
Genes commonly regulated by ParS/ParR and QS, but not MexEF-OprN.
Oligonucleotides used for gene cloning and qRT-PCR.
Contributor Information
Dongping Wang, Email: dwang22@lanl.gov.
Candace Seeve, Email: Candace_Seeve@baylor.edu.
Leland S Pierson, III, Email: lspierson@tamu.edu.
Elizabeth A Pierson, Email: eapierson@tamu.edu.
Acknowledgements
This project was supported by the NSF Integrated Organismal Biology Project no. IOS-1035157. We thank Scott Hunicke-Smith and members of the University of Texas Genomic Sequencing and Analysis Facility for assistance with many aspects of the RNA-seq analysis. We also thank Julien Levy and Jun Myoung Yu for technical help with various aspects of the project.
References
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/S1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- Alonso A, Campanario E, Martinez JL. Emergence of multidrug-resistant mutants is increased under antibiotic selective pressure in Pseudomonas aeruginosa. Microbiology. 1999;145:2857–2862. doi: 10.1099/00221287-145-10-2857. [DOI] [PubMed] [Google Scholar]
- Beinlich KL, Chuanchuen R, Schweizer HP. Contribution of multidrug efflux pumps to multiple antibiotic resistance in veterinary clinical isolates of Pseudomonas aeruginosa. FEMS Microbiol Lett. 2001;198:129–134. doi: 10.1111/j.1574-6968.2001.tb10631.x. [DOI] [PubMed] [Google Scholar]
- Chen HY, Yuan M, Livermore DM. Mechanisms of resistance to beta-lactam antibiotics amongst Pseudomonas aeruginosa isolates collected in the UK in 1993. J Med Microbiol. 1995;43:300–309. doi: 10.1099/00222615-43-4-300. [DOI] [PubMed] [Google Scholar]
- Bonomo RA, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43:S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- Tomas M, Doumith M, Warner M, Turton FF, Beceiro A, Bou G, Livermore DM, Woodford N. Efflux pumps, OprD porin, AmpC β-lactamase, and multiresistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob Agents Chemother. 2010;54(5):2219–2224. doi: 10.1128/AAC.00816-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocquet D, Vogne C, El Garch F, Vejux A, Gotoh N, Lee A, Lomovskaya O, Plésiat P. MexXY-OprM efflux pump is necessary for adaptive resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 2003;47:1371–1375. doi: 10.1128/AAC.47.4.1371-1375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock REW. Adaptive resistance to the ‘Last Hope’ antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob Agents Ch. 2010;54:3372–3382. doi: 10.1128/AAC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Plesiat P, Jeannot K. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and beta-lactams in Pseudomonas aeruginosa. Antimicrob Agents Ch. 2011;55:1211–1221. doi: 10.1128/AAC.01252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Chang HY, Lu CL, Peng HL. Evolutionary analysis of the two-component systems in Pseudomonas aeruginosa PAO1. J Mol Evol. 2004;59:725–737. doi: 10.1007/s00239-004-2663-2. [DOI] [PubMed] [Google Scholar]
- Parkinson JS. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-T. [DOI] [PubMed] [Google Scholar]
- Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P, Camara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol. 2009;12:182–191. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS. et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signaling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;261:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- Balasubramanian D, Schneper L, Kumari H, Mathee K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucl Acids Res. 2013;41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JM, Piddock LJ. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr Opin Microbiol. 2009;12:512–519. doi: 10.1016/j.mib.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Substrate Specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM Efflux Pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:3322–3327. doi: 10.1128/AAC.44.12.3322-3327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler T, Epp SF, Curty LK, Pechère JC. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J Bacteriol. 1999;181:6300–6305. doi: 10.1128/jb.181.20.6300-6305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty LK, Pechere JC. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- Evans K, Passador L, Srikumar R, Tsang E, Nezezon J, Poole K. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1998;180:5443–5447. doi: 10.1128/jb.180.20.5443-5447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa S, Inami H, Kato T, Sawada S, Yasuki T, Miyairi S, Horikawa M, Okuda J, Gotoh N. RND type efflux pump system MexAB-OprM of Pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol. 2012;12:70. doi: 10.1186/1471-2180-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche MG, Deziel E. MexEF-OprN efflux pump exports the Pseudomonas quinolone signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline) PLoS ONE. 2011;6:e24310. doi: 10.1371/journal.pone.0024310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares J, Alvarez-Ortega C, Linares JF, Rojo F, Köhler T, Martínez JL. Overproduction of the multidrug efflux pump MexEF-OprN does not impair Pseudomonas aeruginosa fitness in competition tests, but produces specific changes in bacterial regulatory networks. Environ Microbiol. 2012;14:1968–1981. doi: 10.1111/j.1462-2920.2012.02727.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Lee SH, Seeve C, Yu J, Pierson LS, Pierson EA. Roles of the Gac-Rsm pathway in the regulation of phenazine biosynthesis in Pseudomonas chlororaphis 30–84. Microbiology Open. 2013;2:505–524. doi: 10.1002/mbo3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchene M, Barron C, Schweizer A, Vonspecht BU, Domdey H. Pseudomonas aeruginosa outer-membrane lipoprotein-I gene - molecular-cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989;171:4130–4137. doi: 10.1128/jb.171.8.4130-4137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee JB, Tamber S, Bains M, Maier E, Gellatly S, Lo A. et al. The major outer membrane protein OprG of Pseudomonas aeruginosa contributes to cytotoxicity and forms an anaerobically regulated, cation-selective channel. FEMS Microbiol Lett. 2009;296:241–247. doi: 10.1111/j.1574-6968.2009.01651.x. [DOI] [PubMed] [Google Scholar]
- Hahn HP. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene. 1997;192:99–108. doi: 10.1016/S0378-1119(97)00116-9. [DOI] [PubMed] [Google Scholar]
- Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Goto M, Punj V, Zaborina O, Kimbara K, Das Gupta TK, Chakrabarty AM. Bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect Immun. 2002;70:7054–7062. doi: 10.1128/IAI.70.12.7054-7062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fito-Boncompte L, Chapalain A, Bouffartigues E, Chaker H. et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect Immun. 2011;79:1176–1186. doi: 10.1128/IAI.00850-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinbaum RL, Urbach JM, Liberati NT, Djonovic S, Adonizio A. et al. Genome-Wide Identification of Pseudomonas aeruginosa Virulence-Related Genes Using a Caenorhabditis elegans Infection Model. PLoS Pathog. 2012;8:e1002813. doi: 10.1371/journal.ppat.1002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyama K, Chieda Y, Lee JM, Kusakabe T, Yasunaga-Aoki C, Shimizu S. Effect of superoxide dismutase gene inactivation on virulence of Pseudomonas aeruginosa PAO1 toward the silkworm Bombyx mori. Appl Environ Microbiol. 2007;73:1569–1575. doi: 10.1128/AEM.00981-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall LW, Carty NL, Layland N, Kuan P, Colmer-Hamood JA. et al. mvaT mutation modifies the expression of the Pseudomonas aeruginosa multidrug efflux operon mexEF-oprN. FEMS Microbiol Lett. 2006;255:247–254. doi: 10.1111/j.1574-6968.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- Sobel ML, Neshat S, Poole K. Mutations in PA2491 (mexS) promote MexT-dependent mexEF-oprN expression and multidrug resistance in a clinical strain of Pseudomonas aeruginosa. J Bacteriol. 2005;187:1246–1253. doi: 10.1128/JB.187.4.1246-1253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maseda H, Saito K, Nakajima A, Nakae T. Variation of the mexT gene, a regulator of the MexEF-OprN efflux pump expression in wild-type strains of Pseudomonas aeruginosa. FEMS Microbiol Lett. 2000;192:107–112. doi: 10.1111/j.1574-6968.2000.tb09367.x. [DOI] [PubMed] [Google Scholar]
- Siehnel R, Traxler B, An DD, Parsek MR, Schaefer AL, Singh PK. A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc Natl Acad Sci. 2010;107:7916–7921. doi: 10.1073/pnas.0908511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deziel E, Gopalan S, Tampakaki AP, Lepine F, Padfield KE, Saucier M. et al. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl--homoserine lactone. Mol Microbiol. 2005;55:998–1014. doi: 10.1111/j.1365-2958.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- Kesarwani M, Hazan R, He J, Que YA, Apidianakis Y, Lesic B, Xiao G, Dekimpe V, Milot S. et al. A quorum sensing regulated small volatile molecule reduces acute virulence and promotes chronic infection phenotypes. PLoS Pathog. 2011;7:e1002192. doi: 10.1371/journal.ppat.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déziel E, Lépine F, Milot S, Villemur R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology. 2003;149:2005–2013. doi: 10.1099/mic.0.26154-0. [DOI] [PubMed] [Google Scholar]
- Wang D, Calla B, Vimolmangkang S, Wu X, Korban SS, Huber SC, Clough SJ, Zhao Y. The orphan gene ybjN conveys pleiotropic effects on multicellular behavior and survival of Escherichia coli. PLoS ONE. 2011;6(9):e25293. doi: 10.1371/journal.pone.0025293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Korban SS, Zhao YF. The Rcs phosphorelay system is essential for pathogenicity in Erwinia amylovora. Mol Plant Pathol. 2009;10:277–290. doi: 10.1111/j.1364-3703.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak JW, Knoke KL, Noguera DR, Fox BG, Chambliss GH. Transformation of 2,4,6-trinitrotoluene by purified xenobiotic reductase B from Pseudomonas fluorescens. Appl Environ Microbiol. 2000;66:4742–4750. doi: 10.1128/AEM.66.11.4742-4750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinitsky A, Teng H, Grubmeyer CT. Cloning and nucleic acid sequence of the Salmonella typhimurium pncB gene and structure of nicotinate phosphoribosyltransferase. J Bacteriol. 1991;173:536–540. doi: 10.1128/jb.173.2.536-540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtiazi GT, Saleh M, Hassanshahian E. The effect of bacterial glutathione S-transferase on morpholine degradation. Biotechnol J. 2009;10:202–205. doi: 10.1002/biot.200800238. [DOI] [PubMed] [Google Scholar]
- Kay E, Humair B, Dénervaud V, Riedel K, Spahr S, Eberl L. et al. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J Bacteriol. 2006;188:6026–6033. doi: 10.1128/JB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-A-Woeng TFC, Thomas-Oates JE, Lugtenberg BJJ, Bloemberg GV. Introduction of the phzH gene of Pseudomonas chlororaphis PCL1391 extends the range of biocontrol ability of phenazine-1-carboxylic acid-producing Pseudomonas spp strains. Mol Plant Microbe Interact. 2001;14:1006–1015. doi: 10.1094/MPMI.2001.14.8.1006. [DOI] [PubMed] [Google Scholar]
- Chapon-Hervé V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:1169–1178. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- Coggan KA, Wolfgang MC. Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr Issues Mol Biol. 2012;14:47–70. [PMC free article] [PubMed] [Google Scholar]
- Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S. et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yu J, Pierson LS, Pierson EA. Differential regulation of phenazine biosynthesis by RpeA and RpeB in Pseudomonas chlororaphis strain 30–84. Microbiology. 2012;158:1745–1757. doi: 10.1099/mic.0.059352-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular cloning: a laboratory manual. 3. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Wang D. Regulation of amylovoran biosynthesis in Erwinia amylovora. University of Illinois: URI; 2011. https://www.ideals.illinois.edu/handle/2142/24468. [Google Scholar]
- Wang D, Korban SS, Pusey PL, Zhao Y. AmyR Is a Novel Negative Regulator of Amylovoran Production in Erwinia amylovora. PLoS ONE. 2012;7(9):e45038. doi: 10.1371/journal.pone.0045038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Qi M, Calla B, Korban SS, Clough SJ, Sundin GW, Toth I, Zhao Y. Genome-wide identification of genes regulated by the Rcs phosphorelay system in Erwinia amylovora. Mol Plant-Microbe Interact. 2012;25:6–17. doi: 10.1094/MPMI-08-11-0207. [DOI] [PubMed] [Google Scholar]
- Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- Miller WG, Leveau JH, Lindow SE. Improved gfp and inaZ Broad-host-range promoter-probe vectors. Mol Plant Microbe Interact. 2000;13(11):1243–1250. doi: 10.1094/MPMI.2000.13.11.1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transcript abundance of parS and parR mutants at different cell densities. A. Growth of PAO1 in AB minimal medium + 2% CAA assessed as OD600 (●) or Log cfu/ml (○). he arrow indicates the time at which cells were harvested for RNA-seq analysis. B. PA01 was grown in AB minimal medium + 2% CAA and RNA was isolated from cells harvested at six different growing stages (OD600). A standard curve was generated using purified rpoD PCR product over a dilution range of known concentrations and the relative abundance of parS and parR was estimated based on rpoD transcript quantity in cDNA samples determined by qRT-PCR.
Top twenty highly expressed genes in P. aeruginosa PAO1.
Transcript levels of the 123 TCST genes in P. aeruginosa PAO1.
Differentially expressed genes in parS and parR mutant compared to wild type strain PAO1. Up- and down-regulated genes (ratios) are indicated by red and green, respectively. Genes with p-values < 0.05 are highlighted in yellow.
Validation of RNA-seq results by qRT-PCR. Relative gene expression levels in the parS and parR mutants compared to the wild type strain. Bacterial strains were grown in 5 mL AB medium + 2% CAA. Relative expression of 16 selected genes, normalized to the expression value of the rpoD gene, was determined by qRT-PCR after 16 h growth (OD600 at 1.2). Data points represent means ± SD of three replicates. These experiments were repeated at least twice and similar results were obtained.
A. Comparison of ParS/ParR-regulated genes with ParR-regulated genes in the presence of 4 μg/ml indolicidin (indicated as parR1; [8]) and with genes differentially regulated by a ParR point mutation (indicated as parR2; [9]). B. Venn diagram comparing the number of genes regulated by the three regulons: ParS/ParR (this study), QS [17] and MexEF-OprN [25].
Genes commonly regulated by ParS/ParR, MexEF-OprN and QS.
Genes commonly regulated by ParS/ParR and MexEF-OprN, but not QS.
Genes commonly regulated by ParS/ParR and QS, but not MexEF-OprN.
Oligonucleotides used for gene cloning and qRT-PCR.