Abstract
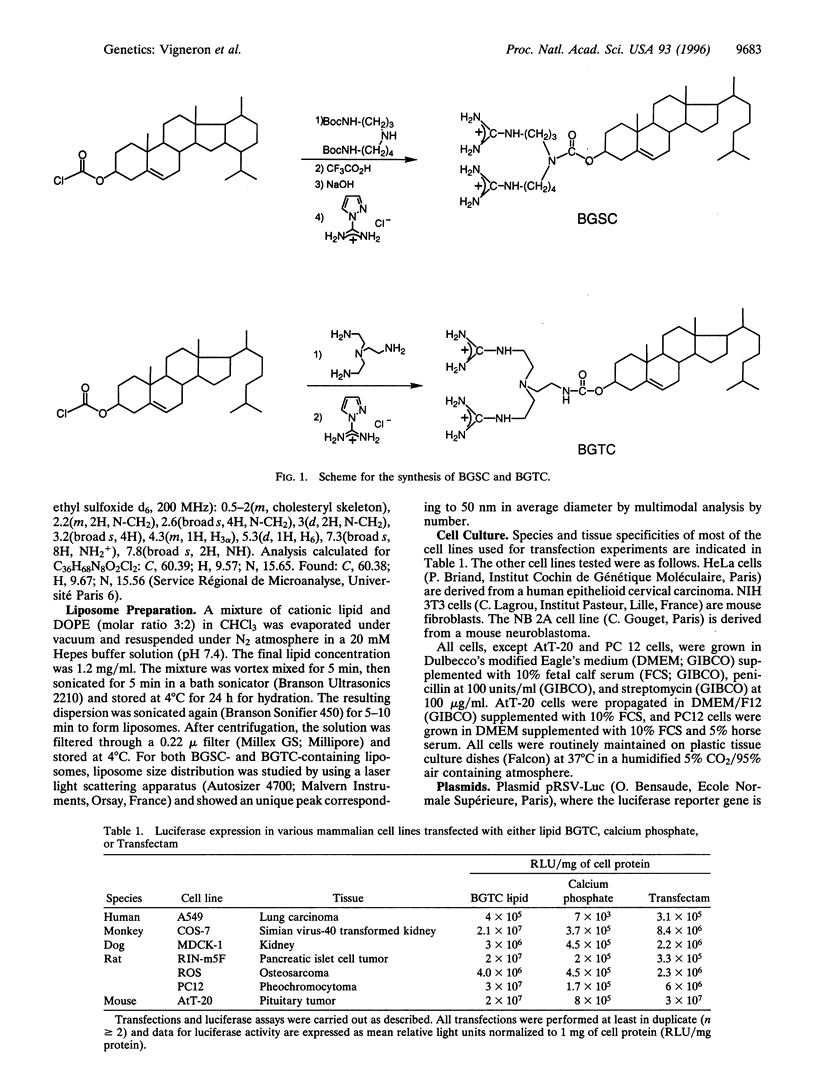
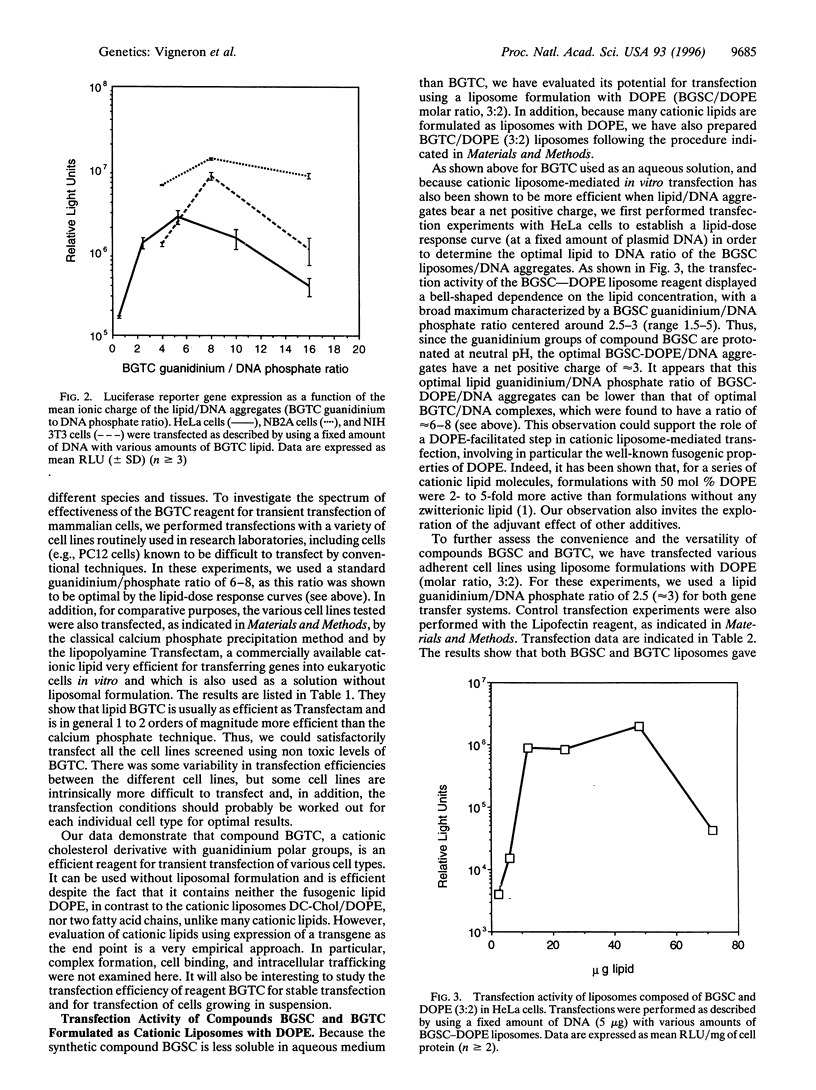
Two cationic lipids, bis-guanidinium-spermidine-cholesterol (BGSC) and bis-guanidinium-trencholesterol (BGTC)-cholesterol derivatives bearing two guanidinium groups-have been synthesized and tested as artificial vectors for gene transfer. They combine the membrane compatible features of the cholesterol subunit and the favorable structural and high pKa features of the guanidinium functions for binding DNA via its phosphate groups. Reagent BGTC is very efficient for transfection into a variety of mammalian cell lines when used as a micellar solution. In addition, both BGTC and BGSC present also a high transfection activity when formulated as liposomes with the neutral phospholipid dioleoylphosphatidyl ethanolamine. These results reveal the usefulness of cholesterol derivatives bearing guanidinium groups for gene transfer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthel F., Remy J. S., Loeffler J. P., Behr J. P. Gene transfer optimization with lipospermine-coated DNA. DNA Cell Biol. 1993 Jul-Aug;12(6):553–560. doi: 10.1089/dna.1993.12.553. [DOI] [PubMed] [Google Scholar]
- Behr J. P., Demeneix B., Loeffler J. P., Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor N., Prokai L., Wu W. M., Farag H., Jonalagadda S., Kawamura M., Simpkins J. A strategy for delivering peptides into the central nervous system by sequential metabolism. Science. 1992 Sep 18;257(5077):1698–1700. doi: 10.1126/science.1529356. [DOI] [PubMed] [Google Scholar]
- Boussif O., Lezoualc'h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutorin A. S., Gus'kova L. V., Ivanova E. M., Kobetz N. D., Zarytova V. F., Ryte A. S., Yurchenko L. V., Vlassov V. V. Synthesis of alkylating oligonucleotide derivatives containing cholesterol or phenazinium residues at their 3'-terminus and their interaction with DNA within mammalian cells. FEBS Lett. 1989 Aug 28;254(1-2):129–132. doi: 10.1016/0014-5793(89)81023-3. [DOI] [PubMed] [Google Scholar]
- Caplen N. J., Alton E. W., Middleton P. G., Dorin J. R., Stevenson B. J., Gao X., Durham S. R., Jeffery P. K., Hodson M. E., Coutelle C. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995 Jan;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- Cotton F. A., Day V. W., Hazen E. E., Jr, Larsen S. Structure of methylguanidinium dihydrogenorthophosphate. A model compound for arginine-phosphate hydrogen bonding. J Am Chem Soc. 1973 Jul 25;95(15):4834–4840. doi: 10.1021/ja00796a012. [DOI] [PubMed] [Google Scholar]
- Felgner J. H., Kumar R., Sridhar C. N., Wheeler C. J., Tsai Y. J., Border R., Ramsey P., Martin M., Felgner P. L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994 Jan 28;269(4):2550–2561. [PubMed] [Google Scholar]
- Gao X., Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991 Aug 30;179(1):280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- Keown W. A., Campbell C. R., Kucherlapati R. S. Methods for introducing DNA into mammalian cells. Methods Enzymol. 1990;185:527–537. doi: 10.1016/0076-6879(90)85043-n. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Zhang G. R., Sun D. K., Ikeuchi T., Sarin P. S. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G. J., Nabel E. G., Yang Z. Y., Fox B. A., Plautz G. E., Gao X., Huang L., Shu S., Gordon D., Chang A. E. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi J. D., Tan R., Calnan B. J., Frankel A. D., Williamson J. R. Conformation of the TAR RNA-arginine complex by NMR spectroscopy. Science. 1992 Jul 3;257(5066):76–80. doi: 10.1126/science.1621097. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Steitz T. A. Structural basis of anticodon loop recognition by glutaminyl-tRNA synthetase. Nature. 1991 Jul 18;352(6332):213–218. doi: 10.1038/352213a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]



