Abstract
The extracellular matrix (ECM) is the physical scaffold where cells are organized into tissues and organs. The ECM may be modified during cancer to allow and promote proliferation, invasion, and metastasis. The family of lysyl oxidase (LOX) enzymes cross-links collagens and elastin and, therefore, is a central player in ECM deposition and maturation. Extensive research has revealed how the LOX proteins participate in every stage of cancer progression, and two family members, LOX and LOX-like 2, have been linked to metastasis, the final stage of cancer responsible for over 90% of cancer patient deaths. However, LOX biosynthesis results in by-product with antiproliferative properties in certain cancers, and LOX enzymes may have different effects depending on the molecular network in which they are active. Therefore, the design of therapies targeting the LOX family needs to be guided by the molecular makeup of the individual disease and will probably require other agents to act on both the LOX enzymes and their associated network.
Keywords: cancer, extracellular matrix, lysyl oxidase, metastasis
Extracellular matrix in cancer
Cancer cells in a tumor proliferate amongst stromal cells and vessels supported by the interstitial extracellular matrix (ECM). The ECM is a material formed by macromolecules that gives structure and anatomy to every tissue, but during cancer it also supplies proinflammatory signals associated with invasion, intravasation, and metastasis. These biological functions seem to be related to the physical properties of the ECM; cancer-associated ECM is stiffer and denser and suffers simultaneous processes of deposition and degradation that liberate growth factors embedded in it, while trapping more stromal and circulating cancer cells.1,2 Lysyl oxidase (LOX) has been proven to be a crucial mediator in remodeling of the cancer-associated ECM, metastasis, and the premetastatic niche.3 This evidence points to the LOX family as a potential target in the prevention and treatment of metastatic disease. However, extracellular LOX has been shown to have antitumor activity in certain cancers,4 whereas the by-product of LOX biosynthesis has antioncogenic activity. This suggests that: 1) an anti-LOX treatment for cancer must be limited to tumors where LOX enhances progression, which possibly implies the need for molecular diagnostic tools; and 2) there may be a role for LOX or LOX by-products as anticancer agents. Here we will review the current knowledge on the extracellular LOX enzymes and their potential role in cancer therapy.
The LOX family
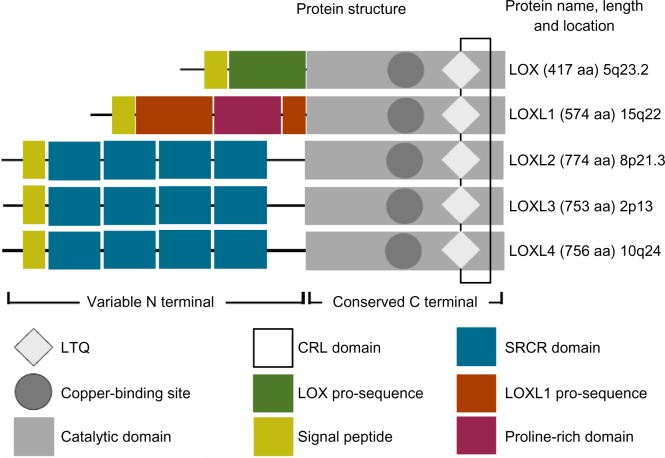
LOX is an enzyme that mediates the cross-linking of collagens and elastin; two basic components of the ECM. LOX was first isolated from bovine aorta and some insight into function was obtained.5 Initial research of LOX in cancer found it was downregulated in epithelial tumors6 and had oncogene-suppressing actions.7 The LOX family has five members; LOX and LOX-like 1 to 4 (LOXL2, LOXL3, LOXL4, respectively) (Figure 1). The family shares a highly conserved amino acid sequence that includes a cytokine receptor-like domain, residues for carbonyl cofactor formation, and a copper-binding site. An atom of copper tightly bound to the active site is essential for protein conformation and, therefore, for the catalytic activity of the enzymes. The N-terminal of every member differs, but, in LOX-like 2, 3, and 4, it is formed of scavenger receptor cysteine-rich domains.
Figure 1.
The lysyl oxidase family.
Notes: The lysyl oxidase family shares a highly conserved catalytic domain that includes a copper-binding motif, a lysyl-tyrosine-quinone cofactor and a cytokine receptor-like domain. These are necessary for protein conformation and enzymatic activity. LOX and LOXL1 contain pro-sequences which are cleaved by bone morphogenetic protein 1 before their secretion into the extracellular space. LOXL2 to 4 form a subfamily defined by its scavenger receptor cysteine rich domains thought to mediate cell adhesion and protein-protein interactions.
Abbreviations: LOX, lysyl oxidase; LOXL, lysyl oxidase-like; LTQ, lysyl-tyrosine-quinone; SRCR, scavenger receptor cysteine rich; CRL, cytokine receptor-like.
LOX is synthesized as a preproenzyme that is first cleaved in the endoplasmic reticulum before the N-terminal propeptide is glycosylated and the C-terminal is folded to acquire three disulphide bonds. The catalytic site incorporates copper and the proenzyme is then released to the extracellular space. The glycosylated N-terminal is cleaved by bone morphogenetic protein 1 (BMP1) (or procollagen C proteinase [PCP])8 resulting in release of the active form of the enzyme and of the LOX propeptide (LOX-PP). LOXL1 is also synthesized as a proenzyme; however, less is known about its secretion and activation, and nothing is known about that of the other family members. While LOX is necessary for ECM maintenance, LOX-PP is biologically active and suppresses gene expression.4
The first substrates described for LOX and later for the other LOXL proteins were collagens and elastin.5 LOX cross-links fibers of collagen and elastin by oxidizing lysine residues into an aldehyde that spontaneously condenses with other aldehydes or peptidyl lysines to form covalent unions among fibers. These unions insolubilize, stabilize, and harden the ECM. The other LOX family members are thought to act in the same way, which is highly likely given the close homology of their catalytic domains.9,10
The LOX family’s role in ECM building and maintenance implies its involvement in pathological conditions. LOX has been showed to be an agent of chronic hepatic fibrosis, arterial fibrosis, and Adriamycin-induced kidney fibrosis in human patients,11 and liver and lung fibrosis in mouse models.12 Lack of LOX is also related to vascular, respiratory, and cutaneous fragility; LOX−/− mice have defective collagen and elastin cross-linking, which translates into impaired development of the upper and lower airways, lungs, vessels, and skin.13 These mice die at birth; thus, LOX function cannot be substituted by any of the other family members. Reduced LOX activity has been detected in two inherited human syndromes: Menkes disease and occipital horn syndrome.11 Both are caused by mutations that limit copper availability, therefore affecting LOX-mediated catalysis. Patients diagnosed with these syndromes have a shortened lifespan, defective connective tissues, and neurological impairment. LOX overexpression as a result of copper deficiency is associated with Ehlers Danlos syndrome (EDS), where patients have over-elasticated skin,14 and a mutation of the LOX gene is related to EDS type VI, which has as its main clinical characteristics neonatal kyphoscoliosis, joint laxity, skin fragility, and abnormal muscle tone.15 LOX has been also linked to neurodegenerative disorders in animal models of amyotrophic lateral sclerosis, Alzheimer’s disease, and dementia.16,17 This suggests that LOX may contribute to diseases not only related to abnormal connective tissue.
LOX−/− mice are viable, but the females have defective elastin renewal in the pelvic floor, which leads to postpartum pelvic prolapse.18 In humans, the lack of LOXL1 is associated with pseudoexfoliation syndrome; a pathology of elastic fibres that degenerates in fragile vascularity, including brain vessels. Currently, a clinical trial for the detection of LOXL1 polymorphism is ongoing.19 Other mutations of LOXL1 produce abnormal elastic fibers in the eyes, heart, lungs, liver, kidneys, and other tissues.20
LOXL2 acts by oxidizing collagens and elastin.21 It promotes chondrocyte differentiation and cartilage ECM maturation.22 It is overexpressed in the stroma of pathologically fibrotic tissues, such as cirrhotic liver and Wilson’s disease, and confers susceptibility to brain aneurysms.23 Antibody targeting of LOXL2 decreases lung and liver fibrosis in mouse models and prevents the activation of ECM-deposing cells such as fibroblasts and endothelial cells.24 Use of a LOXL2-targeting monoclonal antibody24 is currently in Phase II clinical trials for fibrosis patients.
LOXL3 has been less studied than the other family members. Its anatomical pattern of expression overlaps partially with other members of the LOX family and shares substrates (collagens and elastin).25 It has an enzymatically active subvariant that lacks the scavenger domains 1, 2, and 3. LOXL3 overexpression is related to ventricular stiffness and T lymphocyte function in cardiac remodeling.26
LOXL4 was first described as necessary for cartilage maturation and cartilage ECM deposition.27 It is widely expressed in human tissues, notably by endothelial cells;10 Transforming growth factor beta 1-activated LOXL4 contributes to vascular basement membrane and vascular ECM.28 LOXL4 is also overexpressed during the proliferative phase of the human reproductive cycle and has been associated, by one population study, to endometriosis.29
The LOX family as cancer therapy targets
The discovery of the prominent role played by the LOX family in the ECM coincided with the growing interest in the tumor microenvironment, and today the LOX family is considered a potential cancer therapy target; however, early research proposed LOX as a tumor suppressor. LOX was identified as a Ras rescission gene with reduced expression in Ras transformed fibroblasts, but highly expressed in spontaneous revertants.7,30 The case for the tumor suppressor role of LOX was reinforced by studies showing its epigenetic inactivation in a wide diversity of human gastric cancer cell lines and in gastric tract tumors.31 Later research attributed the antitumor activity of LOX to the peptide cleaved by BMP1 from preproLOX.32 This inhibits the activation of NF-kB and may also repress the transcription of the BCL2 oncogene. On the other hand, LOX and other family members (notably LOXL2) induce epithelial-to-mesenchymal transition (EMT) and enhance invasion.33 Hypoxia, a common condition in tumors, is associated with EMT. Hypoxia induces tumor expression of LOX through hypoxia-inducible factor-1 (HIF-1) to enhance cell-matrix adhesion, migration, invasion, and metastasis.34 Hypoxia also induces stromal expression of LOX, which produces a linearization of collagen I, increases the stiffness of the ECM, and induces a loss of epithelial phenotype in cancer cells, enhancing tumor cell invasion through ECM remodeling, intravasation and metastasis.35,39 In addition, tumor-derived LOX stabilizes Snail1 through a regulatory loop with Notch and HIF-1alpha and is, therefore, directly involved in the regulatory mechanisms of EMT36 and likely participates in the opposite mesenchymal-to-epithelial transition; a step thought to be necessary for target organ colonization.
Head and neck cancer patients with high LOX have a higher probability of metastatic invasion and shorter survival.34 Similarly, LOX expression is associated with increased staging and metastasis in colon cancer and renal cancer patients.37,38 LOX-mediated ECM remodeling seems to be essential for the creation of the “cancer niche”; a location that provides proliferative signals, protection from immune attack, and vascular supply (Figure 2).39 This mediation seems to be triggered by hypoxia and HIF-1.40 A provocative result obtained along with these findings is the elimination of metastasis by LOX inhibition.34
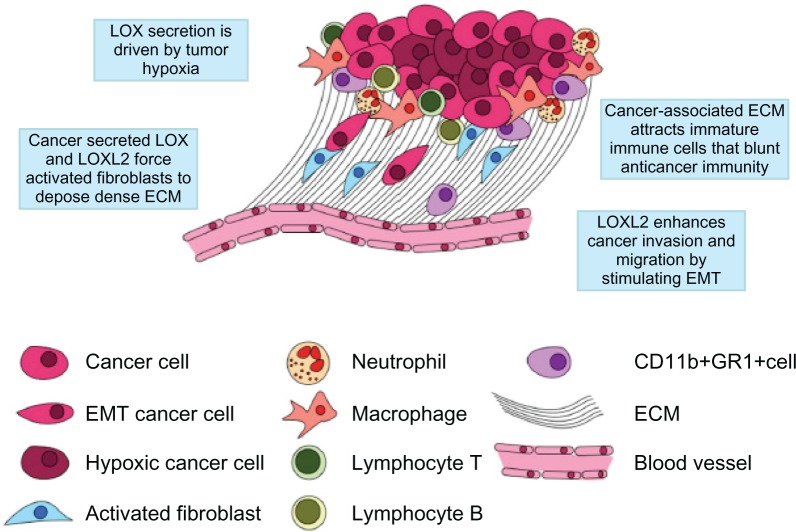
Figure 2.
The cancer niche.
Notes: Just as bone marrow cells require a “niche” to maintain their character and complete their development, cancer cells require a specialized microenvironment to thrive. The cancer niche typically is backed-up by a densely woven ECM formed mainly of collagen I, collagen IV, fibronectin, laminin, and tenascin. This ECM serves as a physical scaffold for the tumor and as a path for stromal cells, such as immune cells and fibroblasts, to migrate along to the tumor mass and, inversely, as a way for tumor cells to escape from the tumor and enter circulation. LOX and LOXL2 expression is induced by tumor hypoxia and mediates the cross-linking of collagen. The LOX family is a central player in cancer related fibrosis, which results in the constant recruitment of protumor stromal cells (ie, cancer-associated inflammation). Additionally, LOXL2 induces epithelial mesenchymal transition, a process that allows tumor cells to acquire fibroblast-like mobility.
Abbreviations: ECM, extracellular matrix; EMT, epithelial mesenchymal transition; LOX, lysyl oxidase; LOXL2, lysyl oxidase-like 2.
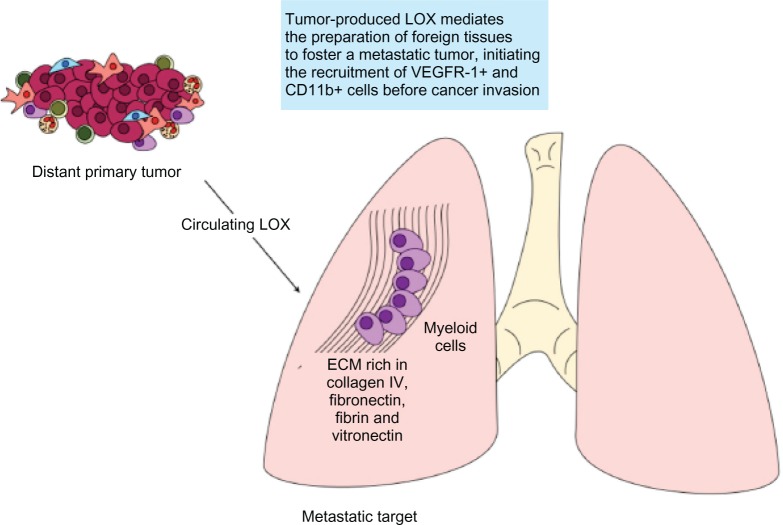
If a cancer niche is necessary for primary tumor success, then it follows that another is even more necessary to shield metastatic cells conquering a foreign organ. An elegant series of experiments later pushed this idea further by showing the “pre-metastatic niche” (Figure 3).41,42 These studies demonstrate that primary tumors are able to modify there targets at a distance to foster progression. Both the metastatic and pre-metastatic niches use the mediation of LOX to cross-link collagen I and IV, components of cancer-associated ECM, and targets of the LOX family.43 A dense ECM buttressed by collagens attracts and traps myeloid cells that conform a chemoattractive area located in places usually invaded by circulating cancer cells.44
Figure 3.
The premetastatic niche.
Notes: Hypoxic cancer cells produce high levels of secreted LOX that mediates the remodeling of ECM at distant sites of future metastasis, resulting in the attraction of CD11b+ myeloid cells. These stromal cells produce matrix metalloproteinases, urokinase, kallikrein-related peptidases, and cathepsins, which can cleave collagen and other ECM proteins initiating a typical cancer-associated process of ECM destruction and deposition. These alterations create a chemoattractive niche for circulating cancer cells and the ECM remodeling enhances metastatic efficiency by enhancing cell-matrix adhesion and tumor cell proliferation. One unresolved question is the anatomic specificity of LOX mediated premetastatic niche formation. This may be explained by different extracellular matrix components present in organs and different tumor tissues; cancers of the lung, breast, ovary, colon, and liver, among others, are all different regarding ECM components such as fibrin, collagen (different types), vitronectin, fibronectin, laminin, etc.
Abbreviations: ECM, extracellular matrix; KLK, kallikrein-related peptidases; LOX, lysyl oxidase; VEGFR, vascular endothelial growth factor receptors.
LOXL2 maintains the tumor stroma and the stroma of fibrotic conditions, activating cancer-associated fibroblasts and vasculature in mice and humans.24 Antibody blocking of LOXL2 in mice decreases tumor-induced and chemically-induced fibrosis, improving survival and hepatic function.
There are currently no clinical trials testing for LOX family members against cancer. However, it has been proven that these are inhibitable and “druggable” factors as active site antagonist beta-aminopropionitrile or copper chelator D-penicillamine are able to block the catalytic activity of LOX and LOXL2, albeit nonselectively, and function-blocking antibodies against LOX and LOXL2 have been made.24,35 It must be remembered, however, that extracellular LOX is composed of two parts: the enzyme and its propeptide (PP; LOX-PP). It has been shown that LOX-PP inhibits the extracellular signal-regulated kinase (ERK)-mitogen-activated protein kinase (MAPK) pathway and other pathways associated with cell division during Ewing’s sarcoma4 and has antiproliferative properties, while the active enzyme simultaneously fulfils its protumor functions, as described above. Therefore, anti-LOX therapies must target the cleaved, active enzyme (not its pro-form proLOX or LOX-PP) or, if this is not feasible, then LOX-PP may be used as an additional antitumor agent.
The LOX family as part of a network
One important message from the vast literature on the LOX family is that the lysyl oxidases may induce contradictory effects depending on the tissue studied and on the pathways to which they are associated. Another message is that the same catalytic action, such as the cross-linking of collagen, may not be associated to pathological conditions, but be part of a reaction needed to repair or strengthen a tissue.
A LOX (PP)-Harvey rat sarcoma viral oncogene homolog (HRAS)-nuclear factor kappa B (NFKB) pathway in gastric cancers is associated with tumor suppression,30 but a LOX-FAK-SRC increases cell motility and cell-ECM adhesion,35 which is necessary for local invasion and metastasis. Data from our group suggests that the inhibition of extracellular LOXL2 may hamper or enhance metastasis, depending on the tumor type treated. We are only beginning to scratch at the surface of understanding LOX family function, and further research about the usefulness of the LOX family in aneurysms and vascular defects is needed.
A targeted attack on LOX family synthesis will conceivably affect its antitumoral as well as its protumoral characteristics, and, given the importance of the ECM for cellular behavior, altering LOX family function is potentially fraught with secondary effects. Using LOX as an anti-HRAS agent could slow primary tumor growth, but boost metastasis (a situation similar to that found with other targeted drugs in humans). However, as the tumor suppressor functions of LOX have been shown to be independent of its enzymatic function, selective inhibitors of LOX catalytic activity should leave any tumor suppressor function intact. However, development of such compounds is complicated by the identical nature of the LOX family active sites and by the lack of available crystal structures. Nevertheless, the overexpression of LOX early during in situ breast cancer followed by its repression during invasive carcinoma suggests that it may rewire its molecular connections over time, pushed by dynamic microenvironmental changes.44,45 Thus, much more needs to be learnt about LOX function and dynamics, in both healthy and disease contexts, before effective targeting can begin.
Network medicine may provide a way to tackle these problems.46 It has been demonstrated that a systems biology simulation of epidermal growth factor receptor signaling variation in vitro will produce a combined therapy cycle that maximizes effect and minimizes the emergence of resistance.47 The simulation predicts the rewiring of molecular networks associated with cancer proliferation, allowing for therapy that prevents resistance. However, predicting and attacking networks involved in the running of the cancer microenvironment is an unmet challenge. Knowledge may be gained by using omics approaches to understand how LOX connects to molecules produced locally and how molecules in the primary tumor interact with those in metastatic target organs. Targeting molecular networks involving LOX family members may allow for dissection and attack of their protumoral activities, while keeping side-effects and resistance at a minimum.
Conclusion
While it is clear from the extensive research that the LOX family are potent anticancer targets, strategies for combination therapies and for preventing resistance have not been investigated. Despite this, clinical trials in cancer patients are due to begin with the LOXL2 monoclonal antibody, and several LOX/LOXL2 drug development programs are underway, quickly approaching clinical trials in patients.
Acknowledgments
AMG is supported by funding from the Association for International Cancer Research and the Danish Cancer Society. JTE is supported by a Hallas Møller Stipend from the Novo Nordisk Foundation, and Biotech Research and Innovation Centre (BRIC).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol. 2012;226(2):185–199. doi: 10.1002/path.3031. [DOI] [PubMed] [Google Scholar]
- 3.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nature Cancer Rev. 2012;12(8):540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 4.Agra N, Cidre F, García-García L, de la Parra J, Alonso J. Lysysl oxidase is downregulated by the EWS/FLI1 oncoprotein and its propeptide domain displys tumor suppressor activities in ewing sarcoma cells. PLoS One. 2013;8(6):e66281. doi: 10.1371/journal.pone.0066281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinnell SR, Martin GR. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide lynkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamalainen ER, Kemppainen R, Kuivaniemi H, et al. Quatitative polymerase chain reaction of lysil oxidase mRNA in malignantly transformed human cell liness demonstrates that their low lysyl oxidase activity is due to low quantities of its mRNA and low levels of transcription of the respective gene. J Biol Chem. 1995;270(37):21590–21593. doi: 10.1074/jbc.270.37.21590. [DOI] [PubMed] [Google Scholar]
- 7.Contente S, Kenyon K, Rimoldi D, Friedman RM. Expression of gene rrg is associated with reversion of NIH 3T3 transformed by LTR-c-H-ras. Science. 1990;249(4970):796–798. doi: 10.1126/science.1697103. [DOI] [PubMed] [Google Scholar]
- 8.Uzel MI, Scott IC, Babakhanlou-Chase H, et al. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem. 2001;276(25):22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- 9.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 10.Molnar J, Fong KS, He QP, et al. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta. 2003;1647(1–2):220–224. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 11.Mäki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24(5):651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- 12.Cox TR, Bird D, Baker AM, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73(6):1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibres in various tissues. Am J Pathol. 2005;167(4):927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuivaniemi H, Peltonen L, Kivirikko KI. Type IX Ehlers-Danlos syndrome and Menkes syndrome: the decrease in lysyl oxidase activity is associated with a corresponding deficiency in the enzyme protein. Am J Human Genet. 1985;37(4):798–808. [PMC free article] [PubMed] [Google Scholar]
- 15.Yeowell HN, Walker LC. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers–Danlos syndrome type VI. Mol Genet Metab. 2000;71(1–2):212–224. doi: 10.1006/mgme.2000.3076. [DOI] [PubMed] [Google Scholar]
- 16.Li PA, He Q, Cao T, et al. Up-regulation and altered distribution of lysyl oxidase in the centrak nervous system of mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Brain Res Mol Brain Res. 2004;120(2):115–122. doi: 10.1016/j.molbrainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Gilad GM, Kagan HM, Gilad VH. Evidence for increased lysyl oxidase, the extracellular matrix-forming enzyme, in Alzheimer’s disease brain. Neurosci Lett. 2005;376(3):210–214. doi: 10.1016/j.neulet.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis leads to pelvic floor disorders. Nat Genet. 2004;36(2):178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 19.Samsung Medical Center LOXL1 polymorphism in pseudoexfoliation syndrome Available from: http://clinicaltrials.gov/show/NCT01515735. NLM identifier: NCT01515735Accessed July 1, 2013
- 20.Tarkanen A, Reunanen A, Kvelä T. Frequency of systemic vascular diseases in patients with primary open angle glaucoma and exfoliation glaucoma. Acta Ophtamol. 2008;86(6):598–602. doi: 10.1111/j.1600-0420.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim YM, Kim E, Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Bio Rep. 2011;38(1):145–149. doi: 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 22.Iftikhar M, Hurtado P, Bais MV, et al. lysyl oxidase-like-2 (loxl2) is a major isoform in chondrocytes and is critically required for differentiation. J Biol Chem. 2011;286:909–918. doi: 10.1074/jbc.M110.155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadasz Z, Kessler O, Akiri G, et al. Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like 2. J Hepatol. 2005;43(3):499–507. doi: 10.1016/j.jhep.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 24.Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 25.Lee JE, Kim Y. A Tissue-specific variant of the human lysyl oxidase-like protein 3 (LOXL3) functions as an amine oxidase with substrate specificity. J Biol Chem. 2006;281(49):37282–37290. doi: 10.1074/jbc.M600977200. [DOI] [PubMed] [Google Scholar]
- 26.Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodelling. Hypertension. 2006;48(1):98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Akiyama H, Iguchi H, et al. Molecular cloning and biological activity of a novel lysyl oxidase-related gene expressed in cartilage. J Biol Chem. 2001;276(26):24023–24029. doi: 10.1074/jbc.M100861200. [DOI] [PubMed] [Google Scholar]
- 28.Busnadiego O, González-Santamaría J, Lagares D, et al. LOXL4 is induced by transforming growth factor β1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling. Mol Cell Biol. 2013;33(12):2388–2401. doi: 10.1128/MCB.00036-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Ruiz, Dutil J, Ruiz A, et al. Single-nucleotide polymorphisms in the lysyl oxidase-like protein 4 and complement component 3 genes are associated with increased risk for endometriosis and endometriosis-associated infertility. Fertil Steril. 2011;96(2):512–515. doi: 10.1016/j.fertnstert.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palamakumbura AH, Jeay S, Guo Y, et al. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279(39):40593–40600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- 31.Kaneda A, Wakazono K, Tsukamoto T, et al. Lysyl oxidase is a tumor suppressor gene inactivated by methylation and loss of heterozygosity in human gastric cancers. Cancer Res. 2004;64(18):6410–6415. doi: 10.1158/0008-5472.CAN-04-1543. [DOI] [PubMed] [Google Scholar]
- 32.Wu M, Min C, Wang X, et al. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67(13):6278–6285. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 33.Cano A, Santamaría PG, Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. 2012;8(9):1095–1108. doi: 10.2217/fon.12.105. [DOI] [PubMed] [Google Scholar]
- 34.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 35.Pickup MW, Laklai H, Acerbi I, et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas. Cancer Res. 2013;73(17):5336–5346. doi: 10.1158/0008-5472.CAN-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105(17):6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker AM, Cox TR, Bird D, et al. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103(5):407–424. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 38.Kirschmann DA, Seftor EA, Fong SF, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62(15):4478–4483. [PubMed] [Google Scholar]
- 39.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signalling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schietke R, Warnecke C, Wacker I, et al. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: insights into cellular transformation processes mediated by HIF-1. J Biol Chem. 2010;285(9):6658–6669. doi: 10.1074/jbc.M109.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong CC, Gilkes DM, Zhang H, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A. 2011;108(39):16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decitre M, Gleyzal C, Raccurt M, et al. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stroma reaction of ductal breast carcinomas. Lab Invest. 1998;78(2):143–151. [PubMed] [Google Scholar]
- 45.Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol. 1997;150(2):497–507. [PMC free article] [PubMed] [Google Scholar]
- 46.Pawson T, Linding R. Network Medicine. FEBS Lett. 2008;582(8):1266–1270. doi: 10.1016/j.febslet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Lee MJ, Ye AS, Gardino AK, et al. Sequential et al. Cell. 2012;49(4):780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]