Abstract
Stem cells hold remarkable promise for applications in disease modeling, cancer therapy and regenerative medicine. Despite the significant progress made during the last decade, designing materials to control stem cell fate remains challenging. As an alternative, materials microarray technology has received great attention because it allows for high throughput materials synthesis and screening at a reasonable cost. Here, we discuss recent developments in materials microarray technology and their applications in stem cell engineering. Future opportunities in the field will also be reviewed.
Stem cells are defined by two fundamental characteristics: They can replicate themselves (i.e., self-renew), and they can differentiate into a variety of specific cell types.1–6 These properties offer remarkable promise for applications in disease modeling, cancer therapy and regenerative medicine.7–11 To realize the full potential of stem cells, it is essential to have a set of tools to strictly control stem cell growth and differentiation. In vivo (e.g., inside the body), the fate of stem cells is tightly regulated by their microenvironment (Fig 1), which is composed of three major components: neighboring cells, soluble factors (e.g., growth factors) and insoluble factors (e.g., extracellular matrix proteins).12,13 During the last decade, potent soluble factors (growth factors, genetic factors and small molecules) have been identified and utilized to modulate specific target(s) in signaling pathways and epigenetic mechanisms to manipulate stem cells.14–19 In particular, drug-like small molecules have received enormous attention because they can be synthesized in large quantity with high purity and low batch-to-batch variation. Further, they can be added and removed from culture media in a temporally defined manner to provide flexible modulation of signaling pathways.16,17 This progress has made it possible to culture and differentiate stem cells in animal product free (i.e., xeno-free), chemically defined media (i.e., soluble components for cell culture), an essential requirement for clinical applications.18,20–23
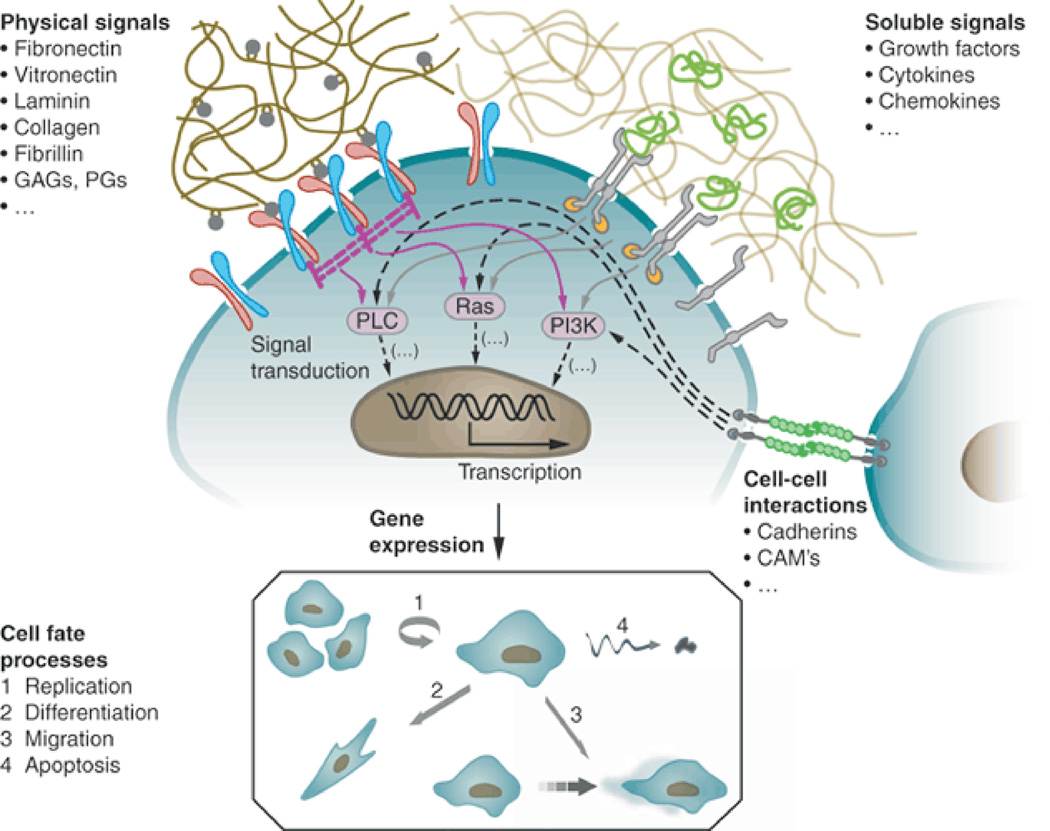
Figure 1.
The behavior of stem cells in vivo is regulated by intricate reciprocal molecular interactions between cells and their surroundings. This extracellular microenvironment is a hydrated protein and proteoglycan-based gel network comprising soluble and physically bound signals and signals arising from cell-cell interactions. Adapted from ref. 13. Specific binding of these signaling cues with cell-surface receptors induces complex intracellular signaling cascades that converge to regulate gene expression, establish cell phenotype and direct tissue formation, homeostasis and regeneration. Ellipsis (…) indicates that the lists of signals are not intended to be complete. PLC, phospholipase C; GAGs, glycosaminoglycans; PGs, proteoglycans; CAMs, cell adhesion molecules. (Figure from reference [13] with permission).
In addition to soluble factors, insoluble factors such as substrates on which cell grow have increasingly been shown to have controlling effects on stem cells. In contrast to (water soluble) drug-like small molecules, which can interact with a specific protein in signaling cascades to control stem cell fate; (water insoluble) materials simultaneously elicit an array of signals, such as topographical, mechanical and biochemical cues to influence stem cells.20,24–26 Despite the last decade’s significant progress in understanding interactions between stem cells and biomaterials, designing materials to direct stem cells remains challenging. As an alterative, high throughput technology has emerged as a powerful approach to rapidly identify suitable materials for stem cell applications because of its capacity to rapidly synthesize and test a large number of materials.23,27–29
Over the last ten years, numerous high throughput technologies have been developed, and they have dramatically accelerated materials discovery for biomedical applications.22,30–33 Microarray based materials development has received a great deal of attention because it allows us to place a large collection of materials onto two-dimensional substrates (e.g., glass slides) in a spatially numbered matrix22,34, which permits computer assisted, fully/semi-automatic characterization of chemical, physical and biological properties of materials.20,35- 38 The large data set could enable development of a global understanding of relationships between materials structure and biological functions,20,36,39 which is critical to develop materials capable of controlling stem cells.
It is well known that chemical structures of materials can have controlling effects on stem cells. Physical properties of materials, in particular elastic modulus and surface topography, have recently been shown to be potent factors to modulate stem cell behavior. In this review, we will first discuss microarrays developed for screening the effects of materials chemical structures on stem cells (Section 1). It is noteworthy that changes in materials chemical structures can lead to changes in materials physical properties, which also could have effects on stem cell behavior. We then will describe microarrays designed for assessing effects of materials physical properties with a focus on elastic modulus and surface topography of materials (Section 2). In the last example, microarrays capable of examining combinatorial effects of materials chemical structures and physical properties will be reviewed (Section 3). We will also discuss design considerations for microarrayed materials synthesis and testing, and we will conclude with outlook in the field. This review is not intended to be comprehensive. Rather, we will focus on conceptual points, major challenges and future opportunities in the field.
Section 1: Screening effects of materials chemical structures on stem cells
Combinatorial polymer microarrays
Pluripotent human stem cells include human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hIPSCs). Unlike other kinds of stem cell, these cells can replicate indefinitely in culture and can differentiate into all human cell types. Thus, they have the potential to provide the large number of cells (>109) necessary for cell replacement therapy.40,41 However, hESCs and hIPSCs are currently cultivated on a layer of feeder cells from mouse origin or on Matrigel™, an undefined mixture of extracellular matrix (ECM) proteins secreted by mouse carcinoma cells. The introduction of cells and proteins of mouse origin leads to a cell population unsuitable for cell replacement therapy.20,21,42 Notably, fully chemically refined, xeno-free media (soluble components) for hESC and hIPSC are commercially available.43,44 Thus, the lack of synthetic substrates (insoluble components) to promote pluripotence has been limiting cultivation of hESCs and hIPSCs in a xeno-free, chemically defined condition, which includes both soluble components (media) and insoluble components (substrates).
To rapidly identify synthetic substrates for hESC and hIPSC culture, we employed a microarray technology to fabricate thousands of microscale substrates with diverse properties. By exploiting robotic liquid handling technology and kinetics of UV photopolymerization, 22 acrylate monomers were used to construct polymer arrays containing 496 different materials. Material properties including surface wettability, indentation elastic modulus, surface roughness and surface chemistry of all polymeric substrates were quantified using high throughput methods, and they were used to establish structure-function relationships between material properties and biological performance.20 The results showed surface chemistry had controlling effects on hES cell undifferentiated growth while indentation elastic modulus or roughness had less pronounced effects on growth. (Fig 2) Esters and hydrocarbons were identified as key chemical moieties on materials surface to promote hES cell self-renewal. The optimal (“hit”) surface was found to effectively enhance adsorption of vitronectin, which can engage with cell-surface receptors, integrin αvβ3 and αvβ5, to promote robust self-renewal of hESCs and hIPSCs.
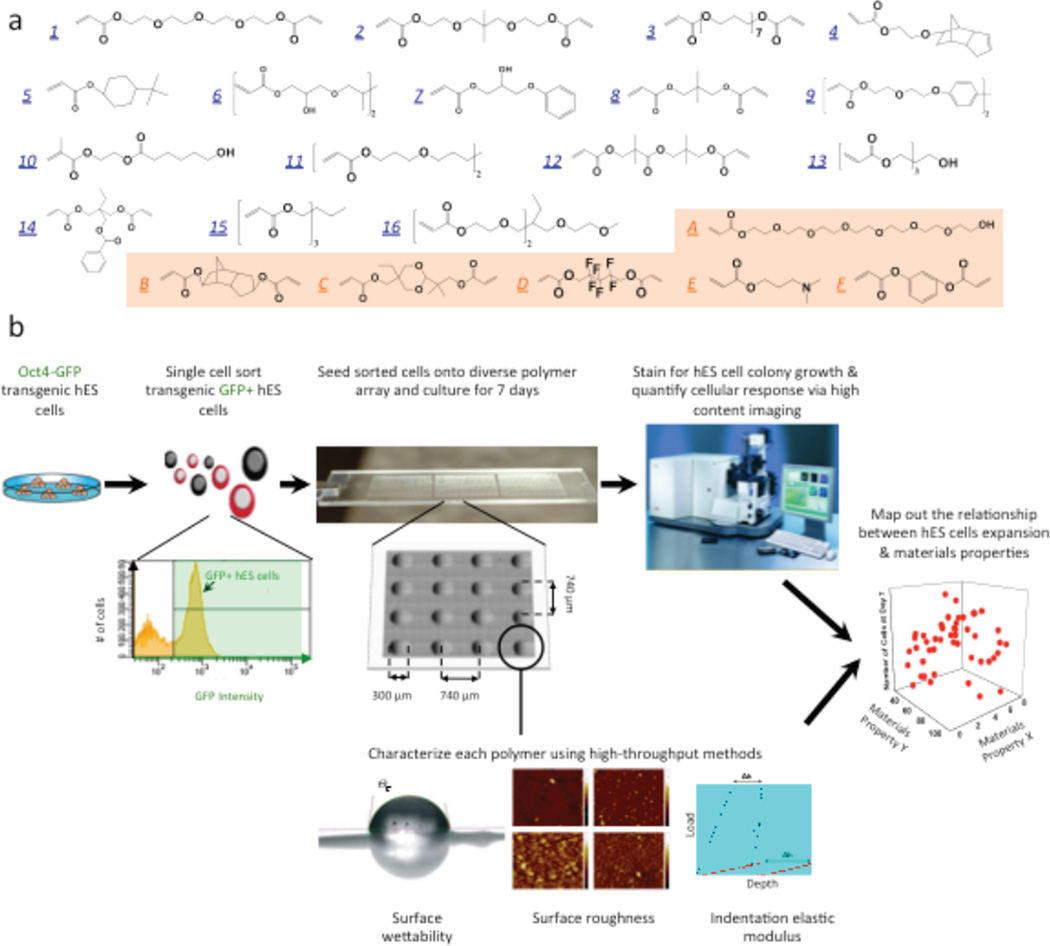
Figure 2.
High-throughput screening of biomaterials for clonal growth. a, Monomers used for array synthesis were classified into two categories: “major” monomers that constitute >50% of the reactant mixture and “minor” monomers that constitute <50% of the mixture. Sixteen major monomers were named numerically (blue), and six minor monomers were labeled alphabetically (orange). b, Schematic diagram of the screen. First, transgenic Oct4–GFP hES cells were maintained on mEFs. Then flow cytometry enabled the isolation of high purity undifferentiated hES cells from the completely dissociated coculture of hES cells and mEFs. A flow cytometry histogram during a representative cell sort is shown. GFP+ cells (right of the black gate) were seeded onto the arrays, whereas the differentiated cells and mEFs (GFP−, left of the black gate) were not used. A photograph of the polymer microarray with 16 polymer spots is shown to illustrate dimensions and separation. Each polymer was also characterized using high-throughput methods to characterize its surface roughness, indentation elastic modulus, wettability (water contact angle, °C) and surface chemistry. Finally, the cellular response on the polymer array was quantified using laser-scanning cytometry, and structure-function relationships were determined by numerical analysis of both the cellular response and materials characterization data. (Figure from reference [20] with permission).
In light of this information, we have developed a culture system based on ultraviolet/ozone radiation modification of polystyrene (PS), a typical cell culture plastic, to produce a favorable surface environment for hESC and hIPSC culture. By exposing PS to an optimized dose of short wavelength UV, key chemical moieties (hydrocarbons and ester/carboxylic acid), which were identified to promote hESC self-renewal from the polymer array, can be introduced onto the surface of PS.45 (Fig 3) PS surfaces treated with the optimal dose of UV (UVPS) can support more than three times more cells per area than traditional mouse embryonic fibroblast (mEF) feeder cells, the current gold standard. As “hit” polymers, UVPS can promote adsorption of vitronectin and engagement with integrin αvβ3 and αvβ5 to support self-renewal of hESCs and hIPSCs.
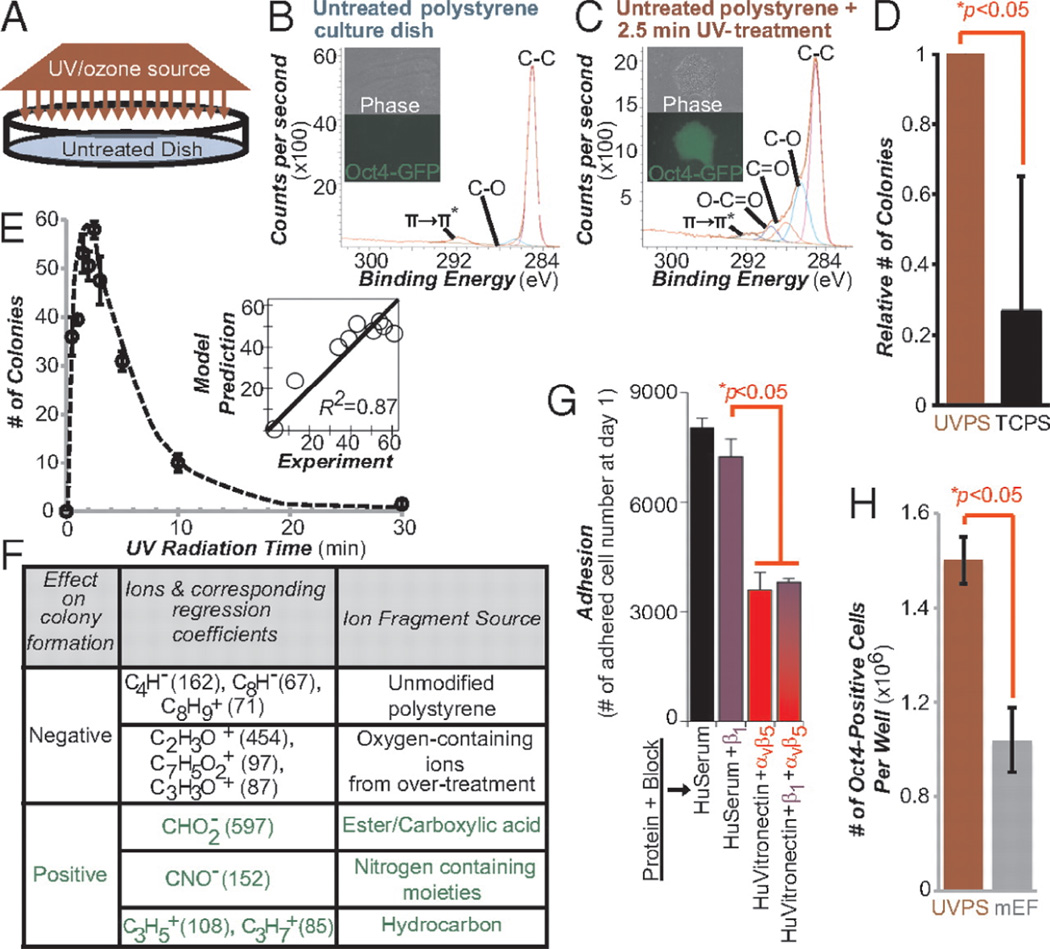
Figure 3.
More undifferentiated human pluripotent stem cells grow on chemically optimized substrates than on feeder containing substrates. (A) Schematic diagram of UV treatment. XPS spectra of surface chemical functionality of an untreated “virgin” polystyrene culture dish (B) and after 2.5 min of UV treatment (C) are shown. Phase-contrast and fluorescence images of transgenic Oct4-GFP hESCs on these surfaces are shown. Bright green fluorescence on the UV treated areas indicates strong expression of the pluripotency marker Oct4. (D) Relative number of hESC colonies on UVPS treated for 2.5 min vs. conventional TCPS on Day 7 after cell seeding. (E) Colony formation on virgin polystyrene treated with various UV doses. The y axis lists the number of colonies formed on Day 7. (Inset) PLS model prediction of the number of colonies plotted against the experimental results. For A–E, surfaces were precoated with 20%(vol/vol) bovine serum and cells were seeded in ROCK inhibitor for the first 8–12 h. (F) Using the PLS model of the ToF-SIMS data, surface ions with high positive or negative regression coefficients were identified as supporting or inhibiting hESC colony formation (G) Number of adhered cells after 24 h of culture on UVPS coated with either human serum (HuSerum) or recombinant human vitronectin (HuVitronectin) and with the specified integrin-blocking antibody. Blocking with αvβ5 reduced adhesion, whereas blocking with β1 had minimal effect, either alone or in combination with αvβ5 blocking. (H) Number of undifferentiated Oct4-GFP-positive hESCs per well of a six-well plate at Day 7 of culture on UVPS (red) and on standard mouse embryonic feeder (mEF)-containing substrates (gray). UVPS was precoated with vitronectin. All error bars indicate 95%confidence intervals (n = 3). (Figure from reference [45] with permission).
In addition to printing monomer solutions and synthesizing polymeric materials on slides, the technology can be used to spot existing polymers and their mixtures onto microarray. Anderson and coworkers fabricated microarrays composed of a large number of biodegradable polymers and examined their effects on human mesenchymal stem cells, neural stem cells and primary articular chondrocytes.34 Recently, Hay and coworkers printed 380 presynthesized polyurethanes and polyacrylates onto agarose coated glass slides and used them to screen for substrates supporting hESC-derived hepatocytes (hESC-HE).46 Six polymers were found to support the attachment of hESC-HE. However, neither chemical structures nor physical properties of these materials were found to be correlated with biological responses. This highlights the importance of unbiased screening as a practical solution to identify substrates for stem cell applications. When coated with a clinically approved bioartificial liver matrix, the best candidate, a simple polyurethane, was found to promote hepatic phenotype and function in vitro. Notably, a broader range of dose-dependent drug (e.g., phenobarbital) inducible CYP3A metabolism was found in the hESC-HE cultured on the polyurethane when compared to freshly isolated primary human hepatocytes.
Peptide Microarray
The interactions between stem cells and the above microarrayed materials typically are mediated a layer of proteins adsorbed onto materials surface during the precoating step and/or from cell culture media. Instead of using proteins, peptides have been studied as an attractive alternative because they can be chemically synthesized with defined biological functions. To this end, Derda and coworkers developed a self-assembled monolayer microarray of peptides derived from laminin, an ECM protein known to promote pluripotence, to support short-term self-renewal of hESCs.47 The group further utilized phage display to identify novel cell-binding peptides for supporting hESC undifferentiated growth.48 Recently, the group extended the technology to identify heparin-binding peptides, which can interact with cell-surface glycosaminoglycans, to support long-term self-renewal of multiple hESC and hIPSC lines when used with a chemically defined medium.49 These studies have clearly demonstrated the power of peptides as synthetic signaling molecules to modulate stem cells.
Small-molecule microarrays
Instead of using peptides as signaling molecules, an alternative is to study small molecules that capture chemical aspects of the native extracellular space. To this end, Benoit and coworkers developed a microarray of hydrogels modified with small molecules to mimic functional groups of various tissue types to direct differentiation of human mesenchymal stem cells (hMSCs).38 (Fig 4) As one of the most studied adult stem cells, hMSCs are well known to possess the capacity to differentiate into cells of connective tissue lineages, which include cartilage, bone and fat. In this work, pedant carboxyl groups were hypothesized to resemble glycosaminoglycans in cartilage, phosphate groups were chosen for their role in bone mineralization, and tert-butyl groups were thought to mimic the lipid rich environment in adipose tissue. As the group hypothesized, hydrogels functionalized with these small molecules can induce differentiation of hMSCs down chondrogenic, osteogenic or adipogenic pathways without the use of soluble differentiation factors.
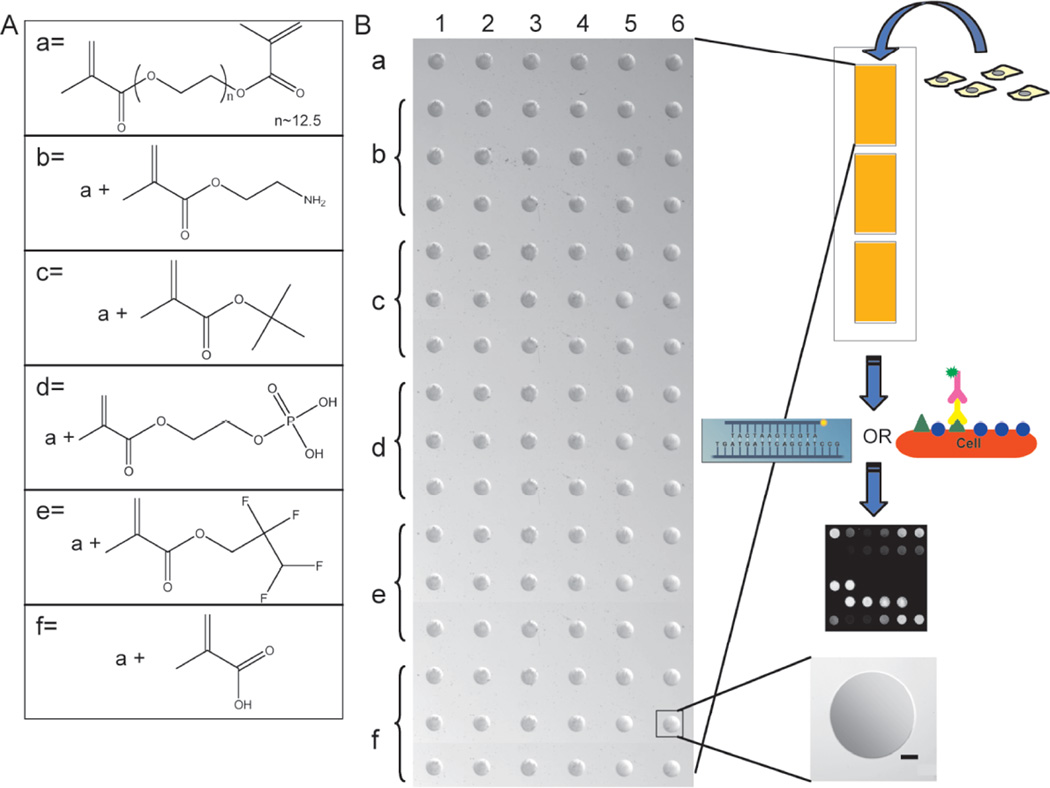
Figure 4.
Depiction of the chemical moiety array set-up for high-throughput evaluation of hMSC expression. (A) Chemical structures of PEG dimethacrylate (PEGDM) and the methacrylated moieties utilized for hydrogel spot formulation. (B) Microscope Image of chemical moiety arrays spotted with a Bio-Rad VersArray ChipWriter and utilized for hMSC culture and subsequent on-chip analysis by a Bio-Rad VersArray ChipReader to analyze protein expression via immunostaining, gene expression via in situ hybridization (left), or protein production by immunocytochemistry (right). There are 3 identical arrays per microscope slide with each array consisting of a 16x6 matrix for immunostaining of 6 different compositions at three different concentrations (0.5, 5, or 50 mM) or 6x6 for in situ hybridization of 6 different compositions at a concentration of 50 mM. Each spot is replicated six times and is approximately 600 µm (bar = 100 µm) in diameter and 10 µm thick, resulting in a volume of about 3 nL; each enables the culture of up to 300 hMSCs/spot for analyses. Chemical composition for individual spots was verified using X-ray photoelectron spectroscopy and physical properties (e.g., contact angle and equilibrium swelling ratio) were confirmed to be statistically similar. (Figure from reference [38] with permission).
Combinatorial ECM protein microarrays
In addition to synthetic materials, ECM proteins represent a source of materials to construct combinatorial library. They are important signaling molecules to regulate stem cell growth and differentiation in vivo. MacBeth and coworkers pioneered the development of protein microarray technology.50 Later, Flaim and coworkers fabricated ECM protein microarrays to study their effects on mouse embryonic stem (ES) cells and hepatocytes. The combinations of ECM proteins that affect hepatocyte function and ES cell differentiation were identified. By using factorial analysis methods, the effects of each ECM protein in the combinations were also revealed. Collagen IV and fibronectin were found to increase the level of intracellular albumin, a marker of liver-specific function, while collagen III and laminin led to decreased albumin levels. 51 Recently, the group extended the technology to study the combinatorial signaling of ECM proteins and growth factors.52 Like ECM proteins, growth factors are potent signaling molecules for stem cells. They are a major component of soluble factors in vivo with cell regulation functions. In this work, the authors fabricated microarrays composed of 20 mixtures of 5 ECM proteins (fibronectin, laminin, collagen I, collagen III, collagen IV), and arranged them in a multiwell format. The multiwell ECM microarrays were cultivated in 12 different growth factor mixtures, which allowed for 1200 simultaneous experiments on 240 unique signaling environments. This miniaturized assay format has allowed the authors to rapidly identify co-signaling between ECM protein-ECM proteins, ECM protein-growth factors, and growth factor-growth factors in a cost-effective manner.
Instead of screening the effects of growth factors in a soluble form, Soen and coworkers used a non-contact, piezoelectric arrayer to print premixed combinations of growth factors, ECM components, cell adhesion molecules, and morphogens onto microarrays. Subsequently, primary bipotent human neural precursor cells were cultivated on the array, and their proliferation and differentiation were examined.53 Recently, Brafman and coworkers further expanded the idea to develop arrayed cellular microenvironment (ACMEs).54 In addition to growth factors and ECM proteins, ACMEs also include glycans, the carbohydrate chains that constitute a large portion of the materials surrounding cells. The authors have used ACMEs to study effects of extracellular microenvironments on multiple hESC and hIPSC lines.
Most of the ECM protein microarrays discussed here are composed of protein spots (Diameter: 150~600 µm) significantly larger than a single cell. It is well known that cells can discern and react to features smaller than themselves. To this end, we have developed microscale direct writing (MDW) technology to generate complex ECM protein arrays at subcellular feature size with multiple components.55 The cell-compatible, multicomponent protein arrays with subcellular feature resolution were used to study muscle cell adhesion and spreading.
As in vivo signaling molecules, ECM proteins and growth factors are powerful molecules to control stem cells. However, they are usually associated with high cost, batch-to-batch variation and contaminants of animal origin. On the other hand, synthetic materials can be prepared at low cost and with high purity, but their biological activity is usually limited. Given the rapid development in organic synthesis and chemical biology, we are optimistic that potent synthetic materials can be developed to interact with stem cells in a highly specific manner.
Section 2: Assessing the effects of materials physical properties on stem cells
Hydrogel microwell arrays
In addition to chemical structures, it has been increasingly apparent that materials physical properties can play a critical role in controlling stem cell fate. For example, the elastic modulus of materials has been shown to direct differentiation of MSCs when cells are attached to substrates or encapsulated in matrices.24,56–58 One limiting factor for adult stem cell research is that almost all adult stem cells can be isolated only in a limited purity. This leads to heterogonous cell behavior and makes it difficult to understand and regulate adult stem cells.59 To address this challenge, Gilbert and coworkers have combined microcontact printing and click chemistry to develop ECM proteins functionalized hydrogel microwell arrays.59,60 The arrays permit the assessment of effects of chemical and physical cues on single cell behavior. By using a laminin modified PEG hydrogel microwell array with different elasticity, the authors have showed that hydrogel that resemble the elastic modulus of endogenous skeletal muscle tissue can promote self-renewal of muscle stem cells.59,60
Topographical biomaterials microarrays
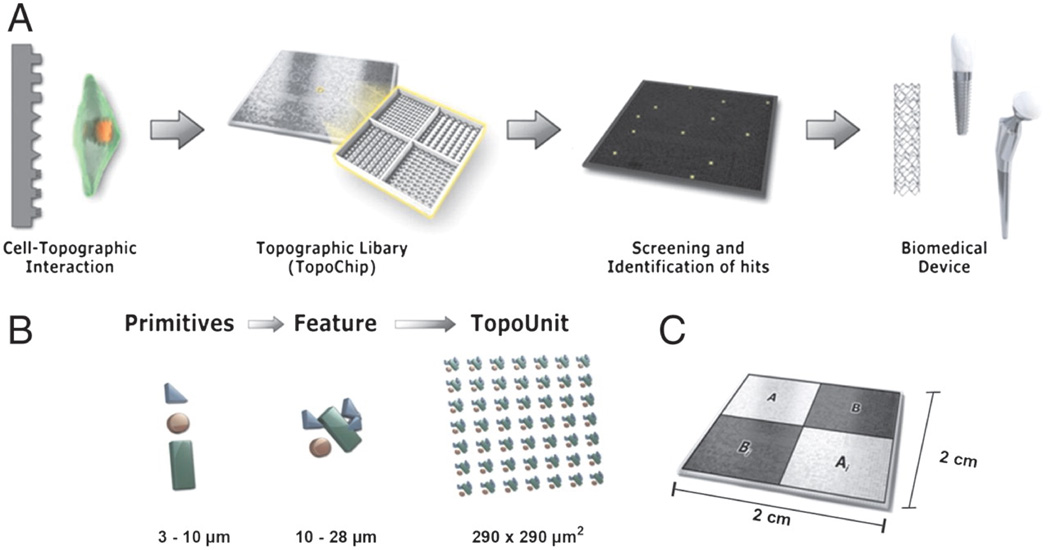
Stem cells can discern and react to a variety of materials physical properties. Like materials elasticity, surface topography is able to provide instructive signals to stem cells. Unadkat and coworkers combined mathematical algorithms and microfabrication to design and fabricate microarrays contains 2,176 random, unbiased topographies (TopoChip).61 (Fig 5) After seeding with hMSCs, the authors have identified surface topographies that are capable of inducing MSC proliferation or osteogenic differentiation.
Figure 5.
TopoChip design. (A) A schematic representation of a sequence of events that is proposed to be followed for high-throughput screening of biomedical materials starting from initial design to clinical application. (B) Design of the TopoChip is based on the use of primitives. Three types of primitives, namely circles, triangles, and lines were used to construct features. Repeated features constitute a TopoUnit, and two times 2,176 = 4,352 TopoUnits constitute a TopoChip (size ranges are indicated). In addition, four flat control surfaces are included. (C) TopoChip is divided into four quadrants. TopoUnits in quadrant A are repeated in quadrant Ai; similarly TopoUnits in quadrant B are repeated in quadrant Bi in order to exclude site specific or localized effects. (Figure from reference [61] with permission).
Recent advances in micro- and nano-fabrication allow for development of substrates with well-defined nanotopography. By using electron beam lithography (EBL), Dalby and coworkers have fabricated nanoarrayed polymethyl methacrylate (PMMA) with different symmetry and disorder as substrates to direct hMSCs.26 The authors reported the nanoscale disorder can differentiate hMSCs into osteogenic lineage (bone formation), and the efficiency was reported to be similar to that of osteogenic media. Further analysis showed these nanoscale surfaces can stimulate similar signaling cascades as osteogenic media. Later, the authors extended the technology to develop nanotopography for long-term self-renewal of hMSCs.25 It is noteworthy that unlike chemical structures, the effects of materials physical properties have been under-appreciated until recently. Given the high attention this topic received nowadays, we expect rapid growth in microarray-assisted, high-throughput investigations of effects of materials physical properties on stem cells.
Section 3: Simultaneously testing the effects of chemical and physical cues
Hydrogel microwell arrays with simultaneously varied chemical structures and physical properties
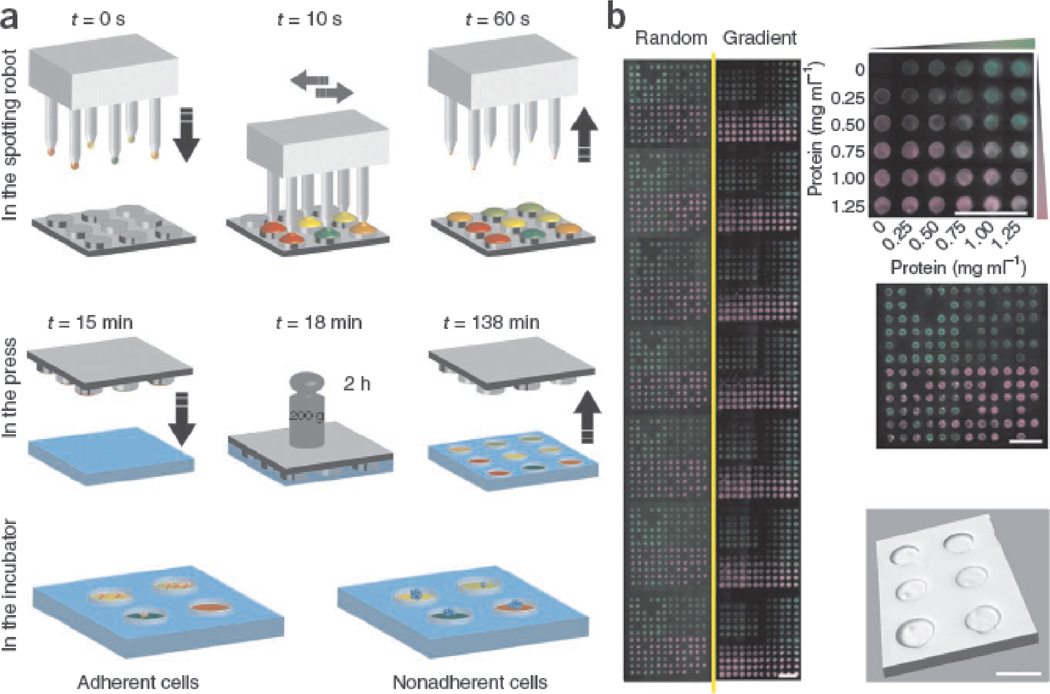
To harness the synergy of materials chemical structures and physical properties to control stem cell fate, Gobaa and coworkers have recently developed artificial niche microarrays with simultaneously varied biochemical structures (ECM proteins) and physical properties (stiffness). By combining robotic DNA spotter and microfabrication technology, the group has developed a hydrogel microarray in which each well was functionalized with different combinations of ECM proteins and having distinct stiffness.39 By integrating biochemical and physical cues, the arrays offered a unique opportunity to study cell-cell interaction, cell-substrate stiffness interaction of hMSCs, and self-renewal of nonadherent neural stem cells (NSCs). (Fig 6)
Figure 6.
Production of artificial niche microarrays. (a) First a DNA spotter with solid pins was used to spot, in multiple rounds, different protein solutions (represented by different colors) on micropillars of a microfabricated silicon stamp. Then the printed stamps were pressed against a thin, partially cross-linked layer of PEG hydrogel. Finally, the stamp was demolded from patterned hydrogel layer. t, time. The obtained artificial niche microarray can be seeded with either adherent or nonadherent stem cells. (b) Representative example of two full arrays (combined in mosaic fashion from individual images) spotted with two fluorescently labeled model proteins. Six concentrations of FITC-BSA (green) and six concentrations of rhodamine-BSA (magenta) were printed either in 12×12 random motifs (left) or as overlapping gradients (right), all in the context of a topographically patterned gel substrate. Scale bars, 2 mm. A three-dimensional reconstruction of confocal stacks showing microwells with a diameter of 450 µm (bottom right). Scale bar, 500 µm. (Figure from reference [39] with permission).
Design considerations for microarrayed materials synthesis and testing
Materials microarray technology is developed by combining (robotic) liquid dispensing technology, microfabrication, materials synthesis and characterization, and automated (fluorescence) microscopy. Microarrays in Section 1 were usually prepared by using (robotic) liquid dispensing systems to spot nanoliters of reagent (e.g., monomer/polymer/proteins) solutions onto 2D substrates. Suitable reagents and solvents for the synthesis usually possess the following properties: 1) high boiling points due to high surface area to volume ratio of reagent solution spotted onto substrates; 2) high solubility of reagents in solvents to ensure sufficient amount of materials can be spotted onto substrates; 3) for fabricating combinatorial polymer microarray synthesis, monomers should have similar reaction activities to ensure homogeneity of microarrayed materials; 4) for printing ECM protein microarrays, printing buffers and substrates must be carefully selected to prevent denaturation of proteins. On the other hand, the preparation of microarrays in Section 2 and 3 usually involved microfabrication. Although powerful, some microfabrication techniques may require sophisticated equipment and/or extensive manual manipulations, which can limit throughput of screening processes. In addition, immunohistochemistry has extensively been utilized to identify the desired cellular phenotype. Availability, specificity, and cost of suitable antibodies can be limiting factors for applications of the technology.
Perspective and conclusion
Over the past decade, significant progress has been made in the stem cell field. To date, it is feasible to maintain hESCs and hIPSCs in a xeno-free, chemically defined condition.21,42,62 In addition, therapeutically important cell types such as cardiomyocytes can be efficiently derived from hESCs and hIPSCs in a chemically defined condition.18 To accelerate the transition to clinical therapeutics, there is an unmet need to develop completely synthetic materials to facilitate efficient derivation, robust expansion, and homogenous differentiation of stem cells in a fully defined condition. This opens up opportunities for future development of materials microarray technology. The throughput of the microarray technology discussed here typically is in the range of hundreds or thousands. One direction would be to increase throughput by sequential use of a biological screening system and microarray technology. For example, Little and coworkers have used an unbiased bacterial peptide display library to identify peptides that bind to adult neural stem cells (NSC).63 Hydrogel surfaces modified with the best candidates were found to support NSC self-renewal and differentiation. This peptide display technology can be easily combined with combinatorial microarray technology. We think the “screening of screening” approach could dramatically increase throughput and accelerate discovery of next generation biomaterials with high specificity to direct stem cells.
Most applications of materials microarray technology have been focused on screening the interactions between stem cell and two-dimensional substrates. Because most stem cells are cultivated on two-dimensional surfaces in vitro, the format is appropriate for developing chemically defined culture substrates for production of medical grade stem cells. For regenerative medicine and tissue engineering applications, the paradigm in stem cell research is gradually shifting from cell production to technology development for cell delivery and integration of delivered (stem) cells with host tissues. These applications necessitate understanding and engineering of stem cell behaviors in three-dimensional, in vivo microenvironments. We believe development of innovative technology for high throughput study of stem cell behavior in microarrayed three-dimensional cultures would significantly move the field forward.28
As an emerging technology, microarrayed materials can have impacts in a wide range of applications beyond stem cell research. For instance, we recently utilized polymer microarrays to develop materials to resist bacteria attachment in a high throughput manner.64 Liberski and coworkers have applied the technology to study polymorphism of small molecules.65 With the current growth rate in lab automation, we expect rapid advancement in development of materials microarray technology and accelerated materials discovery for a fast-growing range of applications.
Acknowledgment
For financial support, we thank Clemson University for startup funding, National Institutes of Health (P20RR021949), and National Science Foundation (The South Carolina Project for Organ Biofabrication). We also thank Ms. Jenny Bourne for the critical review and suggestions.
References
- 1.Pittenger MF, et al. Science. 1999;284(5411):143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, et al. Cell. 2007;131(5):861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Cell. 2006;126(4):663. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JA. Science. 1998;282(5391):1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, et al. Science. 2007;318(5858):1917. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 6.Weissman IL, et al. Annual review of cell and developmental biology. 2001;17:387. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 7.Daley GQ. Cell stem cell. 2012;10(6):740. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissman I. JAMA : the journal of the American Medical Association. 2005;294(11):1359. [Google Scholar]
- 9.Saha K, Jaenisch R. Cell stem cell. 2009;5(6):584. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okita K, Yamanaka S. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366(1575):2198. doi: 10.1098/rstb.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melton DA. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366(1575):2307. doi: 10.1098/rstb.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scadden DT. Nature. 2006;441(7097):1075. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 13.Lutolf MP, Hubbell JA. Nature biotechnology. 2005;23(1):47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 14.Laflamme MA, et al. Nature biotechnology. 2007;25(9):1015. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 15.Ding S, Schultz PG. Nature biotechnology. 2004;22(7):833. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, et al. Nature. 2008;453(7193):338. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, et al. Nature chemical biology. 2009;5(4):258. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 18.Lian X, et al. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(27):E1848. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, et al. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(8):3317. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei Y, et al. Nature materials. 2010;9(9):768. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melkoumian Z, et al. Nature biotechnology. 2010;28(6):606. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 22.Anderson DG, et al. Nature biotechnology. 2004;22(7):863. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 23.Levengood SL, Murphy WL. Current stem cell research & therapy. 2010;5(3):261. doi: 10.2174/157488810791824557. [DOI] [PubMed] [Google Scholar]
- 24.Engler AJ, et al. Cell. 2006;126(4):677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 25.McMurray RJ, et al. Nature materials. 2011;10(8):637. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 26.Dalby MJ, et al. Nature materials. 2007;6(12):997. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 27.Mei Y, et al. Current opinion in chemical biology. 2007;11(4):388. doi: 10.1016/j.cbpa.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranga A, Lutolf MP. Current opinion in cell biology. 2012;24(2):236. doi: 10.1016/j.ceb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, et al. Combinatorial chemistry & high throughput screening. 2009;12(6):554. doi: 10.2174/138620709788681916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes PF, et al. Progress in polymer science. 2008;33(8):787. doi: 10.1016/j.progpolymsci.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akinc A, et al. Nature biotechnology. 2008;26(5):561. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon CG, Jr., Lin-Gibson S. Adv Mater. 2011;23(3):369. doi: 10.1002/adma.201001763. [DOI] [PubMed] [Google Scholar]
- 33.Mei Y, et al. Journal of biomedical materials research. Part A. 2006;79(4):974. doi: 10.1002/jbm.a.30883. [DOI] [PubMed] [Google Scholar]
- 34.Anderson DG, et al. Biomaterials. 2005;26(23):4892. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 35.Davies MC, et al. Journal of drug targeting. 2010;18(10):741. doi: 10.3109/1061186X.2010.521941. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, et al. Biomaterials. 2010;31(34):8827. doi: 10.1016/j.biomaterials.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urquhart AJ, et al. Analytical chemistry. 2008;80(1):135. doi: 10.1021/ac071560k. [DOI] [PubMed] [Google Scholar]
- 38.Benoit DS, et al. Nature materials. 2008;7(10):816. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gobaa S, et al. Nature methods. 2011;8(11):949. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 40.Mignone JL, et al. Circulation Journal. 2010;74(12):2517. doi: 10.1253/circj.cj-10-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laflamme MA, et al. Annual review of pathology. 2007;2:307. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 42.Villa-Diaz LG, et al. Nature biotechnology. 2010;28(6):581. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, et al. Nature methods. 2011;8(5):424. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludwig TE, et al. Nature biotechnology. 2006;24(2):185. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 45.Saha K, et al. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(46):18714. doi: 10.1073/pnas.1114854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hay DC, et al. Stem cell research. 2011;6(2):92. doi: 10.1016/j.scr.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Derda R, et al. ACS chemical biology. 2007;2(5):347. doi: 10.1021/cb700032u. [DOI] [PubMed] [Google Scholar]
- 48.Derda R, et al. Journal of the American Chemical Society. 2010;132(4):1289. doi: 10.1021/ja906089g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klim JR, et al. Nature methods. 2010;7(12):989. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacBeath G, Schreiber SL. Science. 2000;289(5485):1760. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 51.Flaim CJ, et al. Nature methods. 2005;2(2):119. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 52.Flaim CJ, et al. Stem cells and development. 2008;17(1):29. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 53.Soen Y, et al. Molecular systems biology. 2006;2:37. doi: 10.1038/msb4100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brafman DA, et al. Nature protocols. 2012;7(4):703. doi: 10.1038/nprot.2012.017. [DOI] [PubMed] [Google Scholar]
- 55.Mei Y, et al. Small. 2008;4(10):1600. doi: 10.1002/smll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huebsch N, et al. Nature materials. 2010;9(6):518. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaudhuri O, Mooney DJ. Nature materials. 2012;11(7):568. doi: 10.1038/nmat3366. [DOI] [PubMed] [Google Scholar]
- 58.Trappmann B, et al. Nature materials. 2012;11(7):642. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert PM, et al. Integrative biology : quantitative biosciences from nano to macro. 2012;4(4):360. doi: 10.1039/c2ib00148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilbert PM, et al. Science. 2010;329(5995):1078. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Unadkat HV, et al. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(40):16565. doi: 10.1073/pnas.1109861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irwin EF, et al. Biomaterials. 2011;32(29):6912. doi: 10.1016/j.biomaterials.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Little LE, et al. Biomaterials. 2011;32(6):1484. doi: 10.1016/j.biomaterials.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hook AL, et al. Nature biotechnology. 2012 doi: 10.1038/nbt.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liberski A, et al. Chem Commun (Camb) 2009;(3):334. doi: 10.1039/b816920a. [DOI] [PubMed] [Google Scholar]