Abstract
Over the past few years, significant progress has been made in cancer therapy. Indeed, the lifespan of cancer patients has significantly increased. Although patients live longer, cancer-related pain remains a daily problem affecting their quality of life, especially when metastases reach the bone. In patients coping with cancer-induced bone pain, morphine and NSAIDs, often used in combination with other medications, are the most commonly used drugs to alleviate pain. However, these drugs have dose-limiting side effects. Morphine and other routinely used opioids are mu opioid receptor (MOPR) agonists. The MOPR is responsible for most opioid-related adverse effects. In the present study, we revealed potent analgesic effects of an intrathecally-administered selective delta opioid receptor (DOPR) agonist, deltorphin II, in a recently developed rat bone cancer model. Indeed, we found that deltorphin II dose-dependently reversed mechanical allodynia 14 days post-surgery in this cancer pain model, which is based on the implantation of mammary MRMT-1 cells in the femur. This effect was DOPR-mediated as it was completely blocked by naltrindole, a selective DOPR antagonist. Using the complete Freund’s adjuvant model of inflammatory pain, we further demonstrated that deltorphin II was equipotent at alleviating inflammatory and cancer pain (i.e., similar ED50 values). Altogether, the present results show, for the first time, that activation of spinal DOPRs causes significant analgesia at doses sufficient to reduce inflammatory pain in a rat bone cancer pain model. Our results further suggest that DOPR represents a potential target for the development of novel analgesic therapies to be used in the treatment of cancer-related pain.
Keywords: Delta opioid receptor, bone cancer, MRMT-1 cells, allodynia, complete Freund’s adjuvant, pain
Introduction
According to the American Cancer Society, more than 194,000 US patients were diagnosed with breast cancer and 192,000 with prostate cancer in 2009 (Jemal et al., 2009). Advanced breast and prostate cancers often metastasize to the skeleton (Coleman, 1997, 2006). Indeed, bones are among the three most common sites of metastatic diseases (Schulman and Kohles, 2007). Unfortunately, pain is the major debilitating factor in bone metastasis-bearing patients and is responsible for a reduced quality of life (Coleman, 1997, Mercadante, 1997, Mantyh et al., 2002, Zeppetella, 2009).
When tumor cells invade bone, the growing tumors induce nerve compression, ischemia and the release of proinflammatory substances (Honore and Mantyh, 2000). During these changes within the bone, cancer patients commonly experience intermittent periods of severe pain against a background of tonic pain, and these intense pain episodes are further aggravated by the use of the affected limb (movement-evoked pain) (Mercadante, 1997, Urch, 2004). Although the “World Health Organization analgesic ladder” provides a structured approach to drug selection for the treatment of cancer pain, most medications effective for patients coping with bone cancer pain have dose-limiting side effects (Mercadante, 1997, de Wit et al., 2001, Meuser et al., 2001).
In cancer pain therapy, mu opioid receptor (MOPR) agonists are the most commonly used in a structured approach treatment (Mercadante and Arcuri, 1998, Ruiz-Garcia and Lopez-Briz, 2008). Morphine and nonsteroidal anti-inflammatory drugs (NSAIDs) are used in combination and have limited efficacy at reducing bone cancer pain (Portenoy and Lesage, 1999, Kirou-Mauro et al., 2009). In fact, although morphine is efficacious at alleviating inflammatory pain, higher doses are usually required to treat cancer-related bone pain (Luger et al., 2002, Wacnik et al., 2003). In some patients, sustained treatment and escalating doses of morphine may be accompanied by analgesic tolerance and/or opioid-induced hyperalgesia (Mercadante et al., 2003, Ossipov et al., 2005, Silverman, 2009) in addition to other common side effects, i.e., constipation, somnolence and sedation (Portenoy and Lesage, 1999).
Currently, advances in cancer therapy have significantly increased the lifespan of patients diagnosed with bone cancer metastasis (Halvorson et al., 2006). However, these patients still suffer from severe episodes of bone-related pain that greatly affect daily activities (Mantyh et al., 2002, Halvorson et al., 2006, Christo and Mazloomdoost, 2008). Therefore, further efforts are required to develop novel therapies to alleviate bone cancer-induced pain over extended periods of time without any adverse side effects.
Early studies have demonstrated that delta opioid receptor (DOPR) agonists produce potent analgesia when administered intrathecally (i.t.) to mice (Porreca et al., 1987). Over the years, the DOPR has emerged as a potential new target for the development of novel painkillers to treat pain arising from different etiologies. Interestingly, in humans coping with cancer pain, i.t.-administered D-Ala-D-Leu-enkephalin (DADLE), a DOPR-preferred agonist, has been shown to produce analgesia (Onofrio and Yaksh, 1983, Moulin et al., 1985, Krames et al., 1986).
Using a newly validated preclinical model that closely mimics the human conditions of metastatic breast cancer-induced bone pain (Dore-Savard et al., 2010), we examined the analgesic properties of i.t. deltorphin II, a DOPR-selective agonist, to treat cancer-induced bone pain. For comparison, the analgesic potency of i.t. deltorphin II in cancer pain was also determined in the well-characterized complete Freund’s adjuvant (CFA) model of chronic inflammatory pain.
Experimental Procedures
Cell Culture
Mammary rat metastasis tumor (MRMT-1) cells were provided by the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer (Tohoku University 4-1, Seiryo, Aoba-ku, Sendai, Japan). These cells originated from female Sprague-Dawley rats. Cells were cultured in RPMI 1640 medium (Gibco, Montreal, Quebec, Canada) supplemented with 10% fetal bovine serum (FBS, heat-inactivated) and 2% penicillin/streptavidin (Gibco, Montreal, Qc, Canada). Cells were detached using a gentle mechanical medium-flow and then prepared for implantation. Briefly, cells were pelleted by centrifugation (3 min at 200 X g), rinsed with 1 mL of Hank’s balanced salt solution without calcium, magnesium or phenol (HBSS; Gibco, Montreal, Qc, Canada) and then centrifuged again (3 min at 200 X g). The pellet was re-suspended in 1 mL of RPMI 1640, and cells were counted using a hematocytometer. For injection, cells were diluted to achieve a final concentration of 30,000 cells in 20 μL of RPMI 1640 medium and were maintained on ice prior to surgery. For the sham group, 20 μL of RPMI 1640 was injected into the femur.
Animals
Two adult male Sprague-Dawley rats (175–225 g; Charles River Laboratories, St-Constant, Quebec, Canada) were housed per cage and maintained on a 12 hour light/dark cycle with access to food and water ad libitum. All animal procedures were approved by the Ethical Committee for Animal Care and Experimentation of the Université de Sherbrooke (protocol #170-07) and carried out according to the regulations of the Canadian Council on Animal Care (CCAC) to refine and reduce the number of animals used and ensure humane endpoints. Behavioral studies were conducted between 6:00 and 11:00 (light cycle). Rats were acclimated for 4 days to the animal facility and 3 days to manipulations and devices prior to behavioral studies.
Surgery
Animals’ anesthesia was induced with 5% isoflurane (Abbott Laboratories, Montreal, Qc, Canada) and then maintained with 2.5% isoflurane with an O2 flow of 1 L/min during the surgery. Throughout the surgery, the eyes were protected with an ophthalmic liquid gel (Tear-gel, Novartis, Mississauga, ON). Next, animals were laid supine, and their left hind paw was shaved and disinfected with 70% v/v ethanol and 10% v/v povidone-iodine (Rougier Pharma, Mirabel, Qc, Canada). A minimal skin incision (8–10 mm) exposed the quadriceps femoris. To reach the femoral condyles, the vastus lateralis was incised (5–8 mm in length), and the patellar ligament was moved adjacent to the medial condyle to expose the patellofemoral groove area. Minimal damage was inflicted to the surrounding muscles and blood vessels. With this procedure, knee ligaments remained intact. Using a 25-gauge needle, a small and superficial cavity was bored between the medial and lateral condyle (approximately 1 mm in depth). A 25-gauge needle was inserted into the cavity at a 45° angle allowing it to reach the intramedullar canal of the femur. The needle was then substituted with a blunt-ended 25-gauge needle connected to a 50 μL Hamilton syringe containing 20 μL of the cell suspension (30,000 cells). The syringe was left in place for 1 min to allow the cell suspension to disperse into the bone marrow. The needle was then removed, and the cavity was sealed using dental amalgam (Prodigy A3, Kerr, Orange, CA) and polymerized with a curing light (QHL75, Sinclair Dental, Montreal, Canada). The site was thoroughly washed with sterile saline solution. The muscle and the connective tissue were closed using a discontinuous suture made with a synthetic absorbable suture (Monocryl, Ethicon, Sommerville, NJ). The skin was closed using a continuous suture made with a non-absorbable surgical suture (Prolene, Ethicon, Sommerville, NJ). Finally, the wound was washed with 3% v/v peroxide and sprayed with a bitter solution (Aventix animal health, Flamborough, ON). After the surgery, animals recovered from anesthesia within 3 minutes and could walk easily on their operated-limb immediately following surgery.
Imaging
Femurs were gently dissected to preserve their unique pathological condition. To preserve the architecture, rat femurs were immersed in 70 % ethanol for a minimum of 72 hours. Radiographs of dissected bones from sarcoma- and sham-operated animals 14 days after surgery were performed using a Faxitron MX-20 (Faxitron, Lincolnshire, IL). Pictures were taken at 26 kV with an exposure time of 10 s and at 2X magnification. Micro-computed tomography analyses were performed on the same samples with a 1072 MicroCT system (Skyscan, Kontich, Belgium). The parameters were as follows: source: 80 kV, 124 μA; zoom: 20X; pixel size: 14.06 μm; exposition time: 3.0 s; rotation: 0.9°.
Induction of Inflammation
Unilateral inflammation of the hind limb was induced by a single subcutaneous injection of 100 μL emulsified complete Freund’s adjuvant (CFA 200 μg/100 μL; Calbiochem, San Diego, CA, USA) into the plantar surface of the left hind paw of rats under brief isoflurane anesthesia (3%; 1 L/min). Behavioral testing was carried out 6 days after CFA injection. The level of edema was measured by water displacement with a Plethysmometer (IITC Life Science Inc., Woodland Hills, CA, USA).
Drugs
Deltorphin II (lot #077K14561; Sigma, St. Louis, MO, USA, batch no 5/20720; Tocris Bioscience, Ellisville, MO, USA, and lot #M08048T1; American Peptide, Sunnyvale, CA, USA) was dissolved in sterile saline solution (0.9% NaCl) to a concentration of 1 mg/mL and stored at −20°C in aliquots until use. For behavioral testing, deltorphin II was diluted in sterile saline and injected i.t. Briefly, deltorphin II or vehicle saline were gently administered (in 30 μL final volume over 2 s) into the L4-L5 intervertebral space (corresponding to the cauda equina) using a 26G ½-inch needle mounted on a Luer tip Hamilton syringe (VWR, Montreal, Qc, Canada) under light isoflurane anesthesia (3%; 1 L/min). Needle placement was confirmed by the observation of a light tail flick. Selectivity of deltorphin II for the DOPR was verified by blocking DOPR binding sites with the selective DOPR antagonist, naltrindole (72 μg in final volume 30 μL; batch no 10A/93089; Tocris Bioscience, Ellisville, MO, USA), injected 10 min prior to deltorphin II. The putative effect of the same dose of naltrindole alone was also evaluated.
Behavioral testing
von Frey hair test
Sensitivity to non-noxious mechanical stimuli was measured using the von Frey test. To detect the appearance of allodynia, bilateral hind paw withdrawal responses to von Frey hair stimulation of the plantar surface of the footpads were determined at postoperative days 3, 7, 11 and 14. Animals were acclimated to a transparent plastic box with a wire mesh floor (Stoelting, Wood Dale, IL, USA) for 30 min, during the 3 consecutive days prior to the first test (on day 3). A 15 min habituation period was allowed before all other testing days (day 7, 11 and 14). The mid-plantar surface of the left hind paw was stimulated using a series of 8 Touch Test® von Frey hairs with logarithmically incremental stiffness capable of exerting bending forces of 0.41, 0.70, 1.20, 2.0, 3.63, 5.50, 8.50 and 15.10 g (Stoelting). The force of stimulation that caused rapid withdrawal in 50% of trials was calculated using the up-and-down Dixon method (Dixon, 1980, 1991). Tests started with a 2.0 g filament and each filament was held for 10 s. An absence of response led to a stronger subsequent stimulus, while the next weaker hair was used following a paw withdrawal event. According to the Dixon method, threshold calculation requires 6 responses in the immediate vicinity of the 50% threshold. In cases where continuous positive or negative responses were obtained from the entire range of hair stimuli, values of 15.00 g and 0.25 g were assigned, respectively. The resulting pattern of positive and negative responses was tabulated using the convention X = withdrawal and O = no withdrawal, and the 50% response threshold was interpolated using the formula: 50% g threshold = (10[Xf+κδ]/10,000) where Xf = value (in log units) of the final von Frey hair used; κ = tabular value (Appendix from (Chaplan et al., 1994)) for the positive/negative responses; and δ = mean difference (in log units) between stimuli (here δ = 0.175).
Testing timeline
MRMT-1 cells were implanted into the femur, or CFA was injected into the left hind paw, as described above. Fourteen and 6 days after cancer-induction and CFA injection, respectively, the 50% paw withdrawal threshold (indicated as 0 min) was measured prior to i.t. injection of saline, deltorphin II or naltrindole. Thereafter, the 50% withdrawal threshold was measured every 15 min over a 60 min period. The maximum possible effects (MPE) of deltorphin II in the presence or absence of naltrindole in cancer animals and CFA-injected rats were calculated according to the following formula: %MPE = 100X[(test 50% paw withdrawal threshold)-(baseline 50% paw withdrawal threshold)]/[(cutoff value, i.e., 15 g)-(baseline 50% paw withdrawal threshold)]. From the latter calculation, 0% MPE represents no anti-allodynic effect of the drug while a 100% MPE corresponds to a complete relief of the mechanical allodynia or hypersensitivity, i.e., response withdrawal thresholds to von Frey hair equal to baseline values (prior to cancer cells or CFA injection).
Calculations and statistical analysis
Calculations were done using Excel 2007, graphs using SigmaPlot 11.0, and statistical analysis using GraphPad Prism 5.0. Data are expressed as the mean ± standard error of the mean (S.E.M.).
Results
General observations
Sarcoma and sham animals were frequently weighed throughout the duration of the experiments. On day 14, no difference in weight was observed between cancer-(331.2±8.9 g; n=13) and sham-operated rats (332.5±7.3 g; n=8). At that time, sarcoma rats frequently displayed limping and guarding and avoided bearing body weight on their sarcoma paw, suggesting the presence of cancer-induced continuous and movement-evoked pain. Such behaviors were not observed in sham-operated animals.
Bone cancer induced mechanical allodynia
As shown in Fig. 1A, implantation of 30,000 MRMT-1 cells into the femur of Sprague-Dawley rats resulted in the development of mechanical allodynia. Seven days following tumor cell injection, the ipsilateral paw of tumor-bearing rats showed decreased withdrawal threshold to von Frey hair stimuli compared to the contralateral paw (50% withdrawal threshold = 7.45±1.15 g and 13.37±0.80 g for ipsi- and contralateral hind paw, respectively; *** P<0.001, two-way ANOVA followed by Bonferroni’s multiple comparison test). Over the course of the experiment, the 50% paw withdrawal threshold of cancer-bearing rats progressively decreased reaching 5.51±0.95 g on day 11 and 4.29±0.62 g on day 14 post-surgery (*** P<0.001 compared to contralateral hind paw, two-way ANOVA followed by Bonferroni’s multiple comparison test). These data suggest that cancer-bearing rats have developed mechanical allodynia simultaneously with tumor progression (Fig. 1A). The 50% withdrawal threshold for the ipsilateral hind paw was not changed over time in sham-operated rats (Fig. 1A).
Figure 1. Mechanical allodynia and femur remodeling following implantation of MRMT-1 cells.
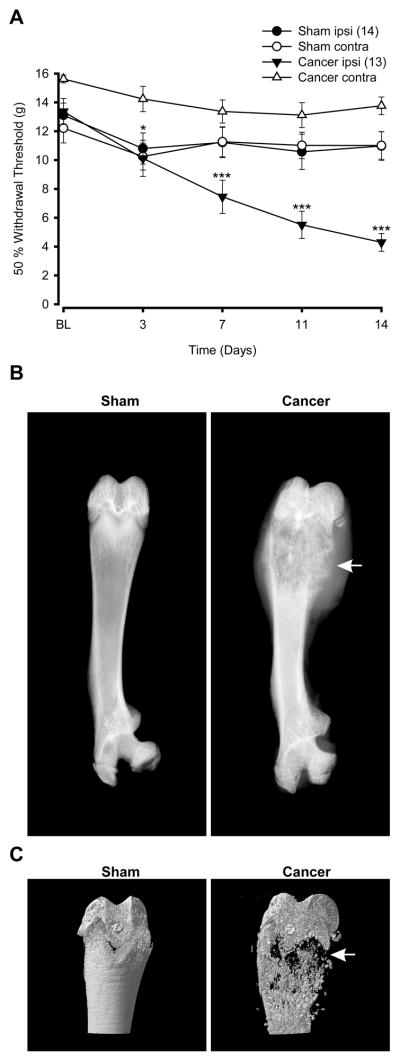
(A) Allodynia was evaluated using the manual von Frey hairs in cancer and sham-operated rats. In tumor-bearing rats, the 50% paw withdrawal threshold was significantly diminished by 7 days following tumor cell implantation (*** P<0.001 when compared to paw withdrawal threshold of the contralateral hind paw of cancer rats; two-way ANOVA followed by Bonferroni’s multiple comparison test). The contralateral hind paw of cancer-bearing rats remained similar to both hind paws of sham animals at day 7, 11 and 14 but was slightly decreased on day 3 (* P<0.05 when comparing cancer contralateral to sham contralateral hind paw; two-way ANOVA followed by Bonferroni’s multiple comparison test). Numbers in parentheses indicate the number of animals per group. (B) Faxitron radiographs of sham and cancer rats at 14 days post-surgery. In cancer-bearing femurs, cortical bone integrity is strongly compromised (white arrows) compared to sham-operated femurs. (C) Microcomputed tomodensitometry of sham-operated and cancer-bearing rat femurs. Fourteen days after surgery, sham femur showed an intact structure, whereas the bone integrity of a sarcoma femur is seriously compromised showing marked signs of potential fracture (white arrows).
Tumor progression induced bone loss and fragility
We have recently reported that proliferation of MRMT-1 cells into the femur induces structural bone remodeling and bone loss (Dore-Savard et al., 2010). In the present study, mechanical allodynia was accompanied by enhanced bone fragility 14 days post-surgery. Whole femur X-rays (Fig. 1B) and tomodensitometry of the distal femur (Fig. 1C) from tumor-bearing animals revealed structural changes when compared to sham animals. Indeed, in the cancer-bearing femur, severe cortical bone loss was observed at the distal level (white arrow; Fig. 1B and 1C).
DOPR activation reversed bone cancer-induced mechanical allodynia
To investigate the possible role of DOPRs in alleviating cancer-induced mechanical allodynia, increasing doses of deltorphin II were injected i.t. 14 days after MRMT-1 cell implantation. As shown in Fig. 2A, i.t. deltorphin II (3–30 μg) time- and dose-dependently increased the 50% paw withdrawal threshold. The peak response was observed 15 min after administration of 3 μg (5.33±0.93 g) and 10 μg (7.97±1.70 g) of deltorphin II. When a 30 μg bolus was administered, the maximal effect of deltorphin II was maintained from 15 to 30 min after the injection (13.29±1.60 g for 15 min and 12.93±1.36 g for 30 min). Responses returned to baseline values 45 minutes after injection for all doses (Fig. 2A). To determine whether the anti-allodynic effect of deltorphin II was DOPR-mediated, naltrindole was injected i.t. 10 min prior to deltorphin II. As illustrated in Fig. 2A, a 3:1 molar ratio of naltrindole (72 μg) completely abolished the analgesic effect of deltorphin II (30 μg). In the presence of naltrindole, deltorphin II failed to produce anti-allodynia (50% paw withdrawal threshold = 3.31±0.42 g at 15 min and 4.11±0.42 g at 30 min post deltorphin II; *** P<0.001 compared with deltorphin II alone). The %MPE of 30 μg deltorphin II in the presence or absence of 72 μg naltrindole are shown in Fig. 2B. In naltrindole-treated animals, deltorphin II was unable to exert analgesic effects at either 15 (%MPE = −7.3±6.6% compared to 87.7±12.0% for deltorphin II alone) or 30 min after administration (%MPE = −2.9±5.2% compared to 89.0±7.5% for deltorphin II alone). As shown in Fig. 2C, i.t. injection of naltrindole alone had no effect.
Figure 2. Anti-allodynic effects of i.t. deltorphin II in cancer rats.
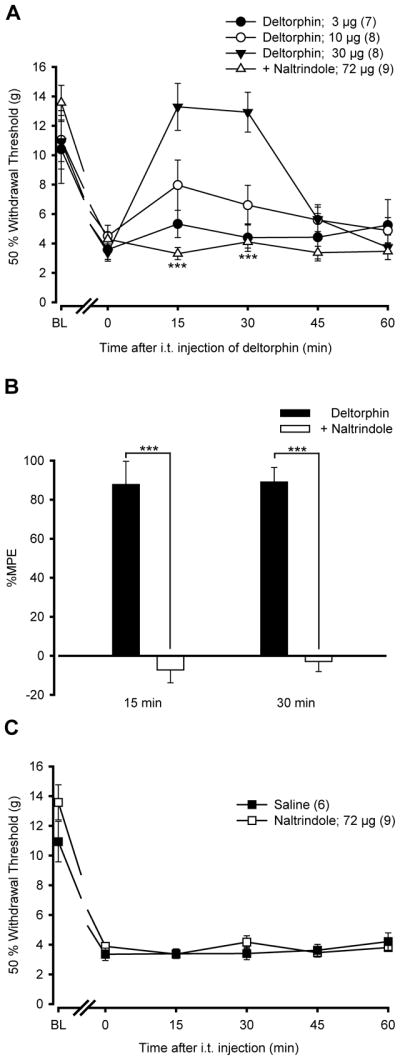
(A) Mechanical thresholds were measured using von Frey filaments before (BL) and 14 days after MRMT-1 cell implantation. The paw withdrawal threshold (in g) was measured every 15 min (from 15 to 60 min) following i.t. injection of deltorphin II. Time 0 indicates the latency to paw withdrawal before i.t. injection of deltorphin II. Intrathecally-administered deltorphin II induced a dose-dependent (3, 10, 30 μg) reduction in mechanical allodynia in the ipsilateral hind paw (FTreatment=6.50 with P=0.002, two-way ANOVA followed by Bonferroni post hoc test). The anti-allodynia of deltorphin II peaked 15 min after the injection. Intrathecally-administered naltrindole (NTI), 10 min before deltorphin II (Delt II) injection (a 3:1 molar ratio of NTI/Delt II = 72/30 μg), antagonized deltorphin II anti-allodynic effects at both 15 and 30 min post-injection (FTreatment=32.15 with *** P<0.001, two-way ANOVA followed by Bonferroni post hoc test). (B) %MPE ± S.E.M. were determined 15 and 30 min after injection of deltorphin II ± NTI. Percentage MPE of deltorphin II administered after NTI are significantly different from %MPE in deltorphin II-treated rats, indicating that the effect of deltorphin II was DOPR-mediated (*** P<0.001, two-tailed unpaired t-test). (C) As compared with saline, by itself i.t. NTI (72 μg) has no effect on the cancer-induced mechanical allodynia (FTreatment=0.38 with P>0.05, two-way ANOVA followed by Bonferroni post hoc test).
DOPR activation reduced CFA-induced tactile hypersensitivity
It has been shown that morphine is less efficacious at alleviating bone cancer pain than inflammatory pain (Luger et al., 2002, Wacnik et al., 2003). To compare the analgesic efficacy of deltorphin II in these pain models, we measured the anti-allodynia in the CFA model of inflammation. Injection of 100 μL of CFA emulsion into the plantar surface of the left hind paw rapidly induced the development of edema and swelling. We determined that 6 days after CFA injection, the level of mechanical allodynia was comparable to tumor-bearing animals at 14 days post-MRMT-1 cell implantation (see Fig. 1A). Six days after the CFA injection, the ipsilateral hind paw volume (i.e., edema) was 2.52±0.07 mL compared to 1.65±0.03 mL for the contralateral hind paw (*** P<0.0001 when compared to contralateral hind paw; Student’s two-tailed paired t-test).
As shown in Fig. 3A, i.t. deltorphin II dose-dependently (3–30 μg) decreased CFA-induced allodynia. While there was no difference observed following 3 μg of deltorphin II (50% paw withdrawal threshold = 2.98±0.40 g), the analgesic effect using 10 μg and 30 μg peaked 15 min after deltorphin II administration (50% paw withdrawal threshold = 8.70±1.71 g and 11.09±1.65 g, respectively). Paw withdrawal thresholds returned to baseline 45 min after deltorphin II injection. The anti-allodynic effect of 30 μg deltorphin II was DOPR-mediated because i.t. naltrindole (72 μg) injection 10 min prior to deltorphin II administration significantly reduced deltorphin II-mediated analgesia (Fig. 3A; 50% paw withdrawal threshold = 4.33±0.38 g at 15 min and 2.73±0.22 g at 30 min post deltorphin II; *** P<0.001 compared with deltorphin II 30 μg). In the presence of naltrindole, the %MPE of deltorphin II was 12.8±3.3% and -0.7±4.6% after 15 and 30 min, respectively, whereas it reached 60.5±16.7% and 41.5±17.7% for deltorphin II alone (Fig. 3B; * P<0.05 for naltrindole compared to deltorphin alone, two-tailed unpaired t-test). As shown in Fig. 3C, i.t. injection of naltrindole had no effect by itself.
Figure 3. Anti-allodynic effects of i.t. deltorphin II in CFA rats.
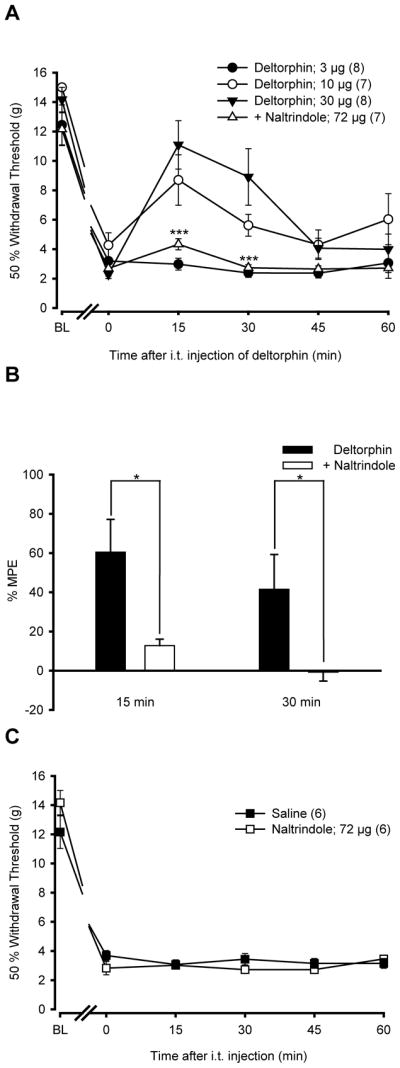
(A) Rats were injected with CFA in the plantar surface of the left hind paw. Six days after CFA injection, the 50% paw withdrawal threshold (in g) was measured using von Frey hairs every 15 min over a period of 60 min following i.t. injection of deltorphin II (Delt II; 3, 10, 30 μg) alone or following naltrindole (NTI; 72 μg) injection. BL indicates the baseline for 50% withdrawal threshold just before CFA injection, and time 0 indicates the paw withdrawal threshold immediately before deltorphin II injection. Intrathecally-administered deltorphin II induced a dose-dependent (3, 10, 30 μg) reduction of mechanical allodynia of the ipsilateral hind paw of CFA-treated rats (FTreatment=15.83 with P<0.0001, two-way ANOVA followed by Bonferroni post hoc test). The anti-allodynia induced by deltorphin II peaked 15 min after the injection. Intrathecally-administered naltrindole, 10 min prior to deltorphin II injection (a 3:1 molar ratio of NTI/Delt II = 72/30 μg), reversed the anti-allodynia induced by deltorphin II at 15 min post-injection (FTreatment=25.53 with P<0.0001, two-way ANOVA followed by Bonferroni post hoc test). (B) The %MPE ± S.E.M. was determined 15 and 30 min after injection of deltorphin II alone or in combination with NTI. The %MPE for deltorphin II injected after NTI was lower than the %MPE of deltorphin II-injected rats, indicating that the effect of deltorphin II was DOPR-mediated (* P<0.05 at 30 min, two-tailed unpaired t-test). (C) As compared with saline, by itself i.t. NTI (72 μg) has no effect on the CFA-induced mechanical allodynia (FTreatment=3.13 with P>0.05, two-way ANOVA followed by Bonferroni post hoc test).
Deltorphin II has equal potency at alleviating bone cancer pain and inflammatory pain
We determined the potency of deltorphin II to inhibit bone cancer pain and inflammatory pain. As shown in Fig. 4, doses of deltorphin II required to induce 50% of the maximal possible effect (ED50) at 15 min are 10.1 μg and 17.6 μg for cancer-bearing and CFA animals, respectively. Note that the %MPE used for this calculation were determined from the results shown in Fig. 2B and Fig. 3B. The relative potency of deltorphin II at alleviating bone cancer pain and inflammatory pain were comparable (P>0.05, two-way ANOVA followed by Bonferroni’s multiple comparison test).
Figure 4. Analgesic efficacy of deltorphin II in bone cancer pain and inflammatory pain.
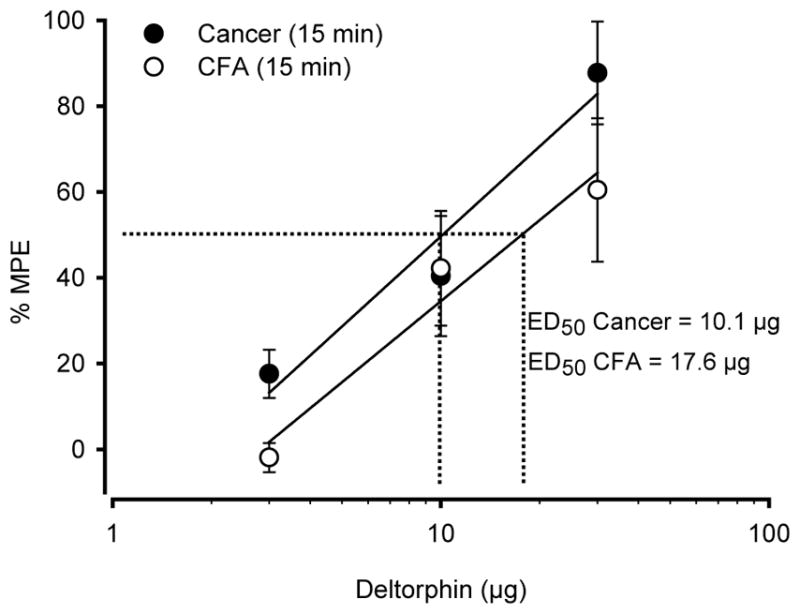
A graph of the effective dose of deltorphin II (ED50) inducing 50% relief of the mechanical allodynia and hypersensitivity was obtained from the data presented in Fig. 2B and 3B at the corresponding peak responses (i.e., 15 min after i.t. injection of deltorphin II). No difference was observed between cancer (ED50=10.1 μg) and CFA-treated rats (ED50=17.6 μg) (Statistical analysis was determined using two-way ANOVA followed by Bonferroni’s post test).
Discussion
In this study, we investigated the analgesic potency of deltorphin II in a rat bone cancer pain model 14 days after MRMT-1 cancer cell implantation in the femur. Our results demonstrated that i.t. deltorphin II, a selective DOPR agonist, alleviates bone cancer-induced mechanical allodynia. Interestingly, we also observed that i.t. deltorphin II is equally efficacious at reducing bone cancer pain and complete Freund’s adjuvant-induced inflammatory pain.
Bone cancer pain is among the most difficult types of pain to treat (Honore et al., 2000, Kirou-Mauro et al., 2009). Although morphine and other MOPR agonists are the most common treatment of cancer pain, they are ineffective in a large proportion of patients (Portenoy and Lesage, 1999, Kirou-Mauro et al., 2009). For this reason, it is common that increasing doses of morphine are required to adequately reduce bone cancer pain, a strategy often accompanied by unwanted side effects (Portenoy and Lesage, 1999, Mercadante et al., 2003, Ossipov et al., 2005, Silverman, 2009). More alarmingly, prolonged morphine treatment has been shown to accelerate bone degradation and fragility and sarcoma-induced bone pain (King et al., 2007).
Recently, DOPRs became an attractive target to develop new therapies for the treatment of chronic and severe pain. In fact, drugs selectively acting on DOPR could represent an interesting alternative to MOPR drugs for the treatment of diverse types of pain. We and others have reported that spinally-administered DOPR agonists have potent analgesic effects in preclinical animal models of inflammatory (Stewart and Hammond, 1994, Fraser et al., 2000, Hurley and Hammond, 2000, Cahill et al., 2003, Petrillo et al., 2003, Morinville et al., 2004b, Gendron et al., 2007a, Gendron et al., 2007b) and neuropathic pain (Mika et al., 2001, Petrillo et al., 2003, Holdridge and Cahill, 2007). Bone cancer-induced pain is, however, a distinct and more complex type of pain. Although bone cancer pain shares common characteristics with both neuropathic and inflammatory pain, Honore and colleagues have suggested that bone cancer pain generates a unique set of neurochemical changes in both dorsal root ganglion (DRG) and spinal cord (Honore et al., 2000).
The present study investigated the role of spinal DOPRs in the treatment of pain induced by bone cancer. Using a rat model of cancer-induced bone pain characterized in our laboratory (Dore-Savard et al., 2010), we first measured the onset of mechanical allodynia following MRMT-1 cell injection in the femur. We observed that mechanical allodynia rapidly and progressively developed from day 7 to day 14 following tumor cell implantation. At day 14, mechanical allodynia was shown to be associated with bone structural changes, degradation, and microfractures. Additionally, MRMT-1 cells increased osteoclast activity leading to progressive bone remodeling, as demonstrated previously (Dore-Savard et al., 2010). These observations are consistent with studies performed in other rat and mouse models of bone cancer pain (Medhurst et al., 2002, Donovan-Rodriguez et al., 2004, Brainin-Mattos et al., 2006, King et al., 2007).
The analgesic potency of i.t.-delivered deltorphin II, a selective DOPR agonist, was thus measured 14 days after tumor cell implantation. In tumor-bearing rats, i.t. deltorphin II produced significant dose-dependent anti-allodynic effects. This effect was DOPR-mediated because it was prevented by naltrindole, a DOPR-selective antagonist. In mouse models of tibial (Baamonde et al., 2005) and femoral osteosarcoma (Brainin-Mattos et al., 2006), peripheral (local or systemic) administration of DOPR agonists produced analgesia. This is however the first study demonstrating that i.t.-delivered DOPR agonist can adequately alleviate bone cancer-induced pain in rodents. Accordingly, in humans coping with cancer-induced pain, i.t. DADLE, a DOPR-preferred agonist, was also found to induce potent analgesia (Onofrio and Yaksh, 1983, Moulin et al., 1985, Krames et al., 1986). In most patients, DADLE was as potent as morphine in alleviating bone cancer pain (Moulin et al., 1985). More importantly, DADLE remained active in patients tolerant to systemic (Moulin et al., 1985) or i.t. opiates (Krames et al., 1986). In the present study, we only evaluated mechanical hypersensitivity. Additional studies will be necessary to determine whether spinally-injected DOPR agonists are effective at reducing movement-evoked pain and spontaneous pain, two modalities often present in patients with cancer pain.
Because morphine was previously shown to have reduced potency in alleviating bone cancer pain compared to inflammatory pain (Luger et al., 2002, Wacnik et al., 2003), we sought to determine the analgesic effects of i.t. deltorphin II in these two pain models. At comparable levels of mechanical allodynia for each model, we found that deltorphin II had a similar relative potency at alleviating bone cancer- and inflammation-induced pain. These observations contrast with morphine’s apparent four- to ten-fold lower potency against tumor-induced bone pain (Luger et al., 2002, Wacnik et al., 2003) and further support the utility of using DOPR selective agonists to treat this type of pain. The reduced efficiency of morphine in animals suffering from bone cancer pain could possibly be explained by the down-regulation of MOPR in a distinct population of DRG neurons (Yamamoto et al., 2008). Similarly, humans suffering from bone cancer pain generally need higher doses of morphine compared to individuals coping with inflammatory pain (Mercadante and Arcuri, 1998, Mercadante, 2010). Therefore, it is interesting that a spinal DOPR agonist produced similar analgesia in both pain models. Because morphine only has a narrow selectivity for MOPR and DOPR, it could be hypothesized that the high doses of morphine required to alleviating pain in bone cancer pain patients might also activate DOPR in addition to MOPR.
In a rodent CFA model, we have previously demonstrated that the increased DOPR agonist efficiency was accompanied by an up-regulation of DOPRs at the cell surface of DRG and spinal cord neurons (Cahill et al., 2003, Gendron et al., 2006, Gendron et al., 2007a). The same phenomenon was also observed in neuropathic pain models (Morinville et al., 2004a). Currently, it is not known if the levels of membrane-associated DOPRs in neurons are increased following injection of tumor cells in the femur. However, because the doses of deltorphin II used in this study had no effect in the contralateral paw, this suggests that DOPRs are activated poorly in the absence of pain and therefore, supports the hypothesis that its membrane expression is enhanced following tumor-induced bone pain. It was shown previously that DOPR deletion in mice induced exaggerated responses to noxious stimuli (Martin et al., 2003) and increased neuropathic and inflammatory pain in corresponding animal models (Nadal et al., 2006, Gaveriaux-Ruff et al., 2008). In the present study, acute pharmacological blockade of DOPR had no effect on the allodynia induced by bone cancer pain and by inflammation, suggesting that if these pain conditions changed the properties of DOPR, naltrindole administration had no hyperalgesic effect.
In addition to the analgesia induced by DOPR agonists (present study and (Baamonde et al., 2005, Brainin-Mattos et al., 2006), activation of DOPRs was shown to reduce cancer cell invasion and proliferation (Martin-Kleiner et al., 2003, Jaglowski et al., 2005, Debruyne et al., 2010, Kuniyasu et al., 2010). In the present study, only acute i.t. administration of deltorphin II was performed. Therefore, it is possible that sustained systemic infusion of a DOPR agonist could decrease MRMT-1 cell proliferation and invasion.
Conclusion
In conclusion, we demonstrated for the first time in a rat bone cancer pain model the analgesic effects of a spinally-administered selective DOPR agonist, deltorphin II. In particular, we showed the dose-dependent anti-allodynic effects of deltorphin II in sarcoma-induced bone cancer and inflammatory pain. In both models, we found similar analgesic potency of deltorphin II. Bone pain symptoms are generated by various mechanisms that remain unclear. A better understanding of the mechanisms underlying bone cancer pain and the regulation of DOPRs in chronic pain conditions will be important in the development of DOPR-targeted medications.
Acknowledgments
The authors are thankful to Karine Belleville who provided technical help and advice on the bone cancer model. This work was primarily supported by grant MOP-84538 from the Canadian Institutes of Health Research (CIHR) to LG, but also by grant MOP-106574 from the CIHR awarded to P.S and L.G and by a grant from the Cancer Research Society (CRS) awarded to PS. LG is the recipient of a Fonds de la Recherche en Santé du Québec (FRSQ) Junior 1 salary support, and PS is a CIHR Young Investigator.
References
- Baamonde A, Lastra A, Juarez L, Garcia V, Hidalgo A, Menendez L. Effects of the local administration of selective mu-, delta-and kappa-opioid receptor agonists on osteosarcoma-induced hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:213–219. doi: 10.1007/s00210-005-0013-6. [DOI] [PubMed] [Google Scholar]
- Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, Yaksh TL. Cancer-related bone pain is attenuated by a systemically available delta-opioid receptor agonist. Pain. 2006;122:174–181. doi: 10.1016/j.pain.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christo PJ, Mazloomdoost D. Cancer pain and analgesia. Ann N Y Acad Sci. 2008;1138:278–298. doi: 10.1196/annals.1414.033. [DOI] [PubMed] [Google Scholar]
- Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- de Wit R, van Dam F, Litjens MJ, Abu-Saad HH. Assessment of pain cognitions in cancer patients with chronic pain. J Pain Symptom Manage. 2001;22:911–924. doi: 10.1016/s0885-3924(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Leroy A, ODEW, Vakaet L, Mareel M, Bracke M. Direct effects of delta opioid receptor agonists on invasion-associated activities of HCT-8/E11 colon cancer cells. Anticancer Res. 2010;30:9–17. [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15:47–50. doi: 10.1016/s0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- Donovan-Rodriguez T, Dickenson AH, Urch CE. Superficial dorsal horn neuronal responses and the emergence of behavioural hyperalgesia in a rat model of cancer-induced bone pain. Neurosci Lett. 2004;360:29–32. doi: 10.1016/j.neulet.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Dore-Savard L, Otis V, Belleville K, Lemire M, Archambault M, Tremblay L, Beaudoin JF, Beaudet N, Lecomte R, Lepage M, Gendron L, Sarret P. Behavioral, medical imaging and histopathological features of a new rat model of bone cancer pain. PLoS One. 2010;5:e13774. doi: 10.1371/journal.pone.0013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GL, Pradhan AA, Clarke PB, Wahlestedt C. Supraspinal antinociceptive response to [D-Pen(2,5)]-enkephalin (DPDPE) is pharmacologically distinct from that to other delta-agonists in the rat. J Pharmacol Exp Ther. 2000;295:1135–1141. [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27:2558–2567. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O’Donnell D, Vincent JP, Devi LA, Cahill CM, Stroh T, Beaudet A. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007a;144:263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O’Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007b;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson KG, Sevcik MA, Ghilardi JR, Rosol TJ, Mantyh PW. Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin J Pain. 2006;22:587–600. doi: 10.1097/01.ajp.0000210902.67849.e6. [DOI] [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain. 2007;11:685–693. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Honore P, Mantyh PW. Bone cancer pain: from mechanism to model to therapy. Pain Med. 2000;1:303–309. doi: 10.1046/j.1526-4637.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglowski JR, Zagon IS, Stack BC, Jr, Verderame MF, Leure-duPree AE, Manning JD, McLaughlin PJ. Opioid growth factor enhances tumor growth inhibition and increases the survival of paclitaxel-treated mice with squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2005;56:97–104. doi: 10.1007/s00280-004-0929-4. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, Vanderah TW, Lai J, Porreca F. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain. 2007;132:154–168. doi: 10.1016/j.pain.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirou-Mauro AM, Hird A, Wong J, Sinclair E, Barnes EA, Tsao M, Danjoux C, Chow E. Has pain management in cancer patients with bone metastases improved? A seven-year review at an outpatient palliative radiotherapy clinic. J Pain Symptom Manage. 2009;37:77–84. doi: 10.1016/j.jpainsymman.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Krames ES, Wilkie DJ, Gershow J. Intrathecal D-Ala2-D-Leu5-enkephalin (DADL) restores analgesia in a patient analgetically tolerant to intrathecal morphine sulfate. Pain. 1986;24:205–209. doi: 10.1016/0304-3959(86)90043-6. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H, Luo Y, Fujii K, Sasahira T, Moriwaka Y, Tatsumoto N, Sasaki T, Yamashita Y, Ohmori H. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut. 2010;59:348–356. doi: 10.1136/gut.2009.178376. [DOI] [PubMed] [Google Scholar]
- Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, Rathbun M, Clohisy DR, Honore P, Yaksh TL, Mantyh PW. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain. 2002;99:397–406. doi: 10.1016/S0304-3959(02)00102-1. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2:201–209. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- Martin-Kleiner I, Gabrilovac J, Kusec R, Boranic M. Methionine enkephalin suppresses metabolic activity of a leukemic cell line (NALM-1) and enhances CD10 expression. Int Immunopharmacol. 2003;3:707–711. doi: 10.1016/S1567-5769(03)00058-4. [DOI] [PubMed] [Google Scholar]
- Martin M, Matifas A, Maldonado R, Kieffer BL. Acute antinociceptive responses in single and combinatorial opioid receptor knockout mice: distinct mu, delta and kappa tones. Eur J Neurosci. 2003;17:701–708. doi: 10.1046/j.1460-9568.2003.02482.x. [DOI] [PubMed] [Google Scholar]
- Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O’Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. Pain. 2002;96:129–140. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- Mercadante S. Intravenous morphine for management of cancer pain. Lancet Oncol. 2010;11:484–489. doi: 10.1016/S1470-2045(09)70350-X. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev. 1998;24:425–432. doi: 10.1016/s0305-7372(98)90005-6. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Ferrera P, Villari P, Arcuri E. Hyperalgesia: an emerging iatrogenic syndrome. J Pain Symptom Manage. 2003;26:769–775. doi: 10.1016/s0885-3924(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001;93:247–257. doi: 10.1016/S0304-3959(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Mika J, Przewlocki R, Przewlocka B. The role of delta-opioid receptor subtypes in neuropathic pain. Eur J Pharmacol. 2001;415:31–37. doi: 10.1016/s0014-2999(01)00814-7. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O’Donnell D, Clarke PB, Collier B, Henry JL, Vincent JP, Beaudet A. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004a;24:5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004b;109:266–273. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Moulin DE, Max MB, Kaiko RF, Inturrisi CE, Maggard J, Yaksh TL, Foley KM. The analgesic efficacy of intrathecal D-Ala2-D-Leu5-enkephalin in cancer patients with chronic pain. Pain. 1985;23:213–221. doi: 10.1016/0304-3959(85)90099-5. [DOI] [PubMed] [Google Scholar]
- Nadal X, Banos JE, Kieffer BL, Maldonado R. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur J Neurosci. 2006;23:830–834. doi: 10.1111/j.1460-9568.2006.04569.x. [DOI] [PubMed] [Google Scholar]
- Onofrio BM, Yaksh TL. Intrathecal delta-receptor ligand produces analgesia in man. Lancet. 1983;1:1386–1387. doi: 10.1016/s0140-6736(83)92170-0. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–324. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- Petrillo P, Angelici O, Bingham S, Ficalora G, Garnier M, Zaratin PF, Petrone G, Pozzi O, Sbacchi M, Stean TO, Upton N, Dondio GM, Scheideler MA. Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta, 12bbeta)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]iso quinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses. J Pharmacol Exp Ther. 2003;307:1079–1089. doi: 10.1124/jpet.103.055590. [DOI] [PubMed] [Google Scholar]
- Porreca F, Heyman JS, Mosberg HI, Omnaas JR, Vaught JL. Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. J Pharmacol Exp Ther. 1987;241:393–400. [PubMed] [Google Scholar]
- Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Garcia V, Lopez-Briz E. Morphine remains gold standard in breakthrough cancer pain. BMJ. 2008;337:a3104. doi: 10.1136/bmj.a3104. [DOI] [PubMed] [Google Scholar]
- Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–2342. doi: 10.1002/cncr.22678. [DOI] [PubMed] [Google Scholar]
- Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679–684. [PubMed] [Google Scholar]
- Stewart PE, Hammond DL. Activation of spinal delta-1 or delta-2 opioid receptors reduces carrageenan-induced hyperalgesia in the rat. J Pharmacol Exp Ther. 1994;268:701–708. [PubMed] [Google Scholar]
- Urch C. The pathophysiology of cancer-induced bone pain: current understanding. Palliat Med. 2004;18:267–274. doi: 10.1191/0269216304pm887ra. [DOI] [PubMed] [Google Scholar]
- Wacnik PW, Kehl LJ, Trempe TM, Ramnaraine ML, Beitz AJ, Wilcox GL. Tumor implantation in mouse humerus evokes movement-related hyperalgesia exceeding that evoked by intramuscular carrageenan. Pain. 2003;101:175–186. doi: 10.1016/s0304-3959(02)00312-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Kawamata T, Niiyama Y, Omote K, Namiki A. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience. 2008;151:843–853. doi: 10.1016/j.neuroscience.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Zeppetella G. Impact and management of breakthrough pain in cancer. Curr Opin Support Palliat Care. 2009;3:1–6. doi: 10.1097/SPC.0b013e3283260658. [DOI] [PubMed] [Google Scholar]


