Abstract
For many patients, chronic pain is often accompanied, and sometimes amplified, by co-morbidities such as anxiety and depression. Although it represents important challenges, the establishment of appropriate preclinical behavioral models contributes to drug development for treating chronic inflammatory pain and associated psychopathologies. In this study, we investigated whether rats experiencing persistent inflammatory pain induced by intraplantar injection of complete Freund’s adjuvant (CFA) developed anxiety-like behaviors, and whether clinically used analgesic and anxiolytic drugs were able to reverse CFA-induced anxiety-related phenotypes. These behaviors were evaluated over 28 days in both CFA- and saline-treated groups with a variety of behavioral tests. CFA-induced mechanical allodynia resulted in increased anxiety-like behaviors as evidenced by: 1) a significant decrease in percentage of time spent and number of entries in open arms of the elevated-plus maze (EPM), 2) a decrease in number of central squares visited in the open field (OF), and 3) a reduction in active social interactions in the social interaction test (SI). The number of entries in closed arms in the EPM and the distance travelled in the OF used as indicators of locomotor performance did not differ between treatments. Our results also reveal that in CFA-treated rats, acute administration of morphine (3 mg/kg, s.c.) abolished tactile allodynia and anxiety-like behaviors, whereas acute administration of diazepam (1 mg/kg, s.c) solely reversed anxiety-like behaviors. Therefore, pharmacological treatment of anxiety-like behaviors induced by chronic inflammatory pain can be objectively evaluated using multiple behavioral tests. Such a model could help identify/validate alternative potential targets that influence pain and cognitive dimensions of anxiety.
Keywords: complete Freund’s adjuvant, chronic pain, comorbidity, elevated-plus maze, social interaction test, open field test, light/dark box exploration test, actimetry
1. INTRODUCTION
In the United States, it is estimated that 9% of the adult population is suffering from moderate to severe chronic non-cancer pain1. For a large proportion of those patients, this situation is accompanied by sleep, anxiety and/or depressive disorders that contribute to quality of life deterioration [1, 2]. Accordingly, the prevalence of anxiety disorders among patients with chronic pain ranges from 20–40%, whereas it is between 7–18% in the general population [3, 4]. Clinically, patients living with chronic pain present 2–3 times more chances to develop mild psychiatric disorders [5]. Since genetic background, psychological state, cognitive variables and personality traits, among others, modulate factors of pain intensity [6], undermanaged pain can have significant impact on the development of anxiety [7]. Conversely, clinical studies also revealed that patients dealing with anxiety disorders are at higher risk of developing chronic pain [8].
Recent surveys report that arthritis is one of the most common chronic pain conditions in North America, affecting approximately 16% of the adult population in United States2 and Canada3. Not surprisingly, psychiatric disorders are highly associated with inflammatory arthritis (e.g. rheumatoid arthritis) [9, 10]. Furthermore, anxiety symptoms related to arthritis have also been shown to increase pain [11]. Thus, all indications point toward the mutual maintenance of chronic inflammatory pain and anxiety disorders [12, 13]. The actual management of chronic inflammatory pain requires a combination of several drugs (non-steroidal anti-inflammatory drugs [NSAID], disease-modifying antirheumatic drugs [DMARD], analgesics, anxiolytics and antidepressants) to achieve the desired level of pain relief and reduce sleep, anxiety and depression co-morbidities [14–17]. As a matter of fact, polytherapy carries its risk of drug interaction and side effects [14, 18, 19]. When inadequately considered, pharmacological interaction can result in suboptimal analgesia, supporting the idea that treatment of associated psychopathologies with array of drugs may decrease the success of arthritis management [10].
Although there is now compelling evidence demonstrating that chronic pain and anxiety are interrelated [12, 20], little is known about the underlying mechanisms involved in the co-occurrence of those pathophysiological phenomena. To date, very few preclinical studies have been conducted to investigate the co-morbidity between chronic pain and anxiety-like behaviors. For instance, only a few neuropathic pain studies have highlighted the development of anxiety-like behaviors in mice or rats using only a limited number of behavioral tests [21–28]. The aim of the present study was therefore to develop and validate a rat model of anxiety-like behaviors caused by chronic inflammatory pain, in line with the clinical reality. Analgesic and anxiolytic actions of morphine and diazepam were also evaluated in this behavioral paradigm.
2. MATERIAL and METHODS
2.1 Animals and housing conditions
Adult male Sprague-Dawley (SD) rats, weighing 175–225 g (Charles River laboratories, St-Constant, Canada), were individually housed on Aspen shavings in a quiet and controlled environment on a 12 h light/dark cycle with free access to lab chow and water. Animals were habituated to housing conditions for 5 days before any manipulation. All procedures were approved by the Animal Care Committee of the Université de Sherbrooke (protocol #148-07) and were in accordance with policies and directives of the Canadian Council on Animal Care (CCAC) and with the International Association for the Study of Pain (IASP) guidelines for pain research on animals.
2.2 Induction of inflammatory pain
Inflammatory pain was induced with an intraplantar injection of 100 μl of complete Freund’s adjuvant (CFA) in the plantar surface of the left hind paw of SD rats. The injected volume (injected as a 1:1 emulsion of oil and saline 0.9%) contained the equivalent of 200 μg of lyophilized bacterial membranes (Mycobacterium Butyricum). Control animals were injected with the same volume of saline 0.9%. CFA injections were performed under isoflurane anaesthesia (5%; Abbott Laboratories, Montreal, QC, Canada) mixed with medical air (2 L/min). We have shown previously that CFA injection in the hind paw of rats induced sustained mechanical [29] and thermal hyperalgesia [30].
2.3 Nociceptive behavioral testing
Mechanical hypersensitivity was a primary outcome measure. The day prior to testing, rats were acclimatized to the Plexiglas enclosures and the mesh floor for 60 min. On the testing day, rats were given 10 minutes to habituate to the enclosures. The same experimenter determined the baselines (BL) before (day 0) and after the CFA injection at days 7, 14, 21 and 28. Allodynia was determined using the Dynamic Plantar Aesthesiometer (DPA, Stoelting, USA). The single blunt filament (0.5 mm in diameter) is applied against the mid-plantar surface of the animal’s hind paw. The preset force increases linearly from 0 to 50 g over a 15 s period (3.33 g/s). The paw withdrawal threshold is automatically recorded by the device. Fifty grams were considered as the cut-off. Three successive stimuli were alternatively applied to both ipsilateral and contralateral hind paws at 150 s intervals. Mechanical hypersensitivity was assessed for all animals included in the study.
2.4 Anxiety-like behavioral tests
Separate animal groups were assayed in the different behavioral tests and all animals were acclimatized twice to the appropriate experimental conditions developed for each specific test (light intensity, white noise and testing room environment). A 30 min period the day prior testing, as well as a 30 min period immediately before the test (on the testing day, set between days 28 to 30) were sufficient for proper acclimatization. Depending on the test, lighting conditions were adjusted with a digital lux meter (DX-100, INS Enterprise Co., Taipei, Taiwan). Continuous white noise, generated by the image acquisition software ANY-maze (Stoelting, Wood Dale, IL, USA), was played during the acclimatization and testing periods. Testing platforms were cleaned with a humid cloth between animals and individual tests were separated by 3 min intervals.
2.4.1 Elevated-plus maze
The elevated-plus maze (EPM) is a cross-shaped platform constituted of four equally sized arms (50 cm × 10 cm) elevated 50 cm above ground. There are 2 closed arms (opposing each other), flanked by 40-cm opaque walls, while the remaining two arms are without walls (open arms). All arms communicate through a central zone (10 cm × 10 cm), allowing animals to move freely into each zone of the maze. Light intensities in the central zone, opened and closed arms were set to 16, 16 and 4 lx, respectively. For this test, animals were habituated to experimental conditions, but not to the maze. Their movements on the platform were monitored for a 5-min period with a camera. The animal was initially placed in the central zone facing an open arm. Percentage of time spent in open arms (time in open arms/[time in open arms + time in closed arms] × 100), as well as the number of entries in opened and closed arms were analyzed with the ANY-maze software. A zone entry was defined as the presence of 85% of the animal’s body in a specific zone. Using these parameters, we have confirmed that diazepam (1 mg/kg, s.c.) was able to inhibit anxiety-like behaviors, i.e. that diazepam increased the time spent in open arms and the number of entries in the open arms without affecting locomotor activity (not shown). Note that diazepam at 3 mg/kg was also tested but was found to induce profound dizziness therefore inducing important motoric effects on the EPM.
2.4.2 Open field test
The open field test (OF) consists of a clear Plexiglass enclosure (1 m × 1 m) placed in the center of a normally lit (400 lx) experimental room. Once again, animals were habituated to experimental conditions, but not to the open field. To initiate testing, rats were placed in one corner of the enclosure and their exploratory behaviors were recorded for 5 min across the arena virtually divided into 16 squares. Parameters measured were the number of entries and the time spent in the center of the arena (four virtual central squares), and the total distance traveled throughout the arena. An entry in a virtual square occurred when the animal’s midpoint crossed a frontier.
2.4.3 Social interaction test
The social interaction test (SI) was performed in a lid-free opaque Plexiglass enclosure (40 cm × 40 cm). Light intensity in the center of the interaction arena was set at 35 lx. This test allows measuring time spent in active interaction (sniffing, grooming, playing or chasing) by a focal (saline- or CFA-treated animal) with a social and naive animal (untreated animal used as interaction partner that has never been in presence of the focal animal before). Simple passive physical contacts between both animals were not considered as interactions. As opposed to other tests, animals performing this test were given a supplemental 30-min period (one extra day) to acclimate to experimental conditions. During the first two periods, each animal was consecutively introduced in the enclosure for a 1-min bout before being returned into its home cage. As for other behavioral tests, a standard acclimatization period was provided on the day of the test. Testing started when the focal and social animals were simultaneously placed in opposing corners of the enclosure. Active social interactions of the focal animal were monitored for 5 min and visually scored later by a blind observer. Social and focal animals were paired according to their body weight (i.e. ± 10% of body weight difference between focal and social animals).
2.4.4 Light/dark box exploration test
The light/dark box exploration test (L/D) is constituted of two adjacent 40 cm × 40 cm boxes communicating by an opening of 5 cm × 5 cm at the floor level. Light intensity in the center of the clear box was set at 100 lx, whereas it was maintained at 0 lx in the dark box. For this test, animals were also habituated to experimental conditions, but not to the boxes. Rats were placed in the center of the lit box and their behaviors were recorded for a 10-min period. The analyzed parameters were the percentage of time spent and number of entries in the lit box. An animal was considered to be in a box when all the paws had crossed the opening separating both boxes.
2.5 Ambulatory/locomotor activities
The MotorMonitor (Kinder Scientific Company, Poway, CA, USA) was used to assess ambulatory functions of both experimental groups (saline- vs CFA-injected animals). In the housing room, clear cages were placed into infrared beam-mounted racks for 24h at noon, on day 28. Parameters analyzed were basic movements, resting time and total distance travelled, all determined by the occurrence of X and Y beam breaks.
2.6 Drugs
The efficacy of acute morphine sulfate (ms) and diazepam (dzp) treatments on inflammatory pain- and anxiety-related behaviors was investigated in CFA-injected rats on day 28. Rats were injected subcutaneously (s.c.) with morphine sulfate (0.3–3 mg/kg, Sabex Inc., Boucherville, QC, Canada) or diazepam (1 and 3 mg/kg, Sandoz, Boucherville, QC, Canada) diluted in physiological saline (0.9% NaCl), 30 min (dzp) and 60 min (ms) before behavioral testing. CFA control rats received a s.c. injection of 0.9% saline.
2.7 Statistical analysis
All data are expressed as means ± standard error of the mean (SEM). Analyses were performed with GraphPad Prism 5.d software. Statistical analyses were performed using the parametric Student’s unpaired t-test, the one-way ANOVA followed by the Dunnett’s multiple comparison test, or the two-way ANOVA followed by a Bonferroni post-hoc test, as detailed in the legend section. A P-value < 0.05 was considered statistically significant.
3. RESULTS
3.1 Temporal development of mechanical hypersensitivity following CFA injection
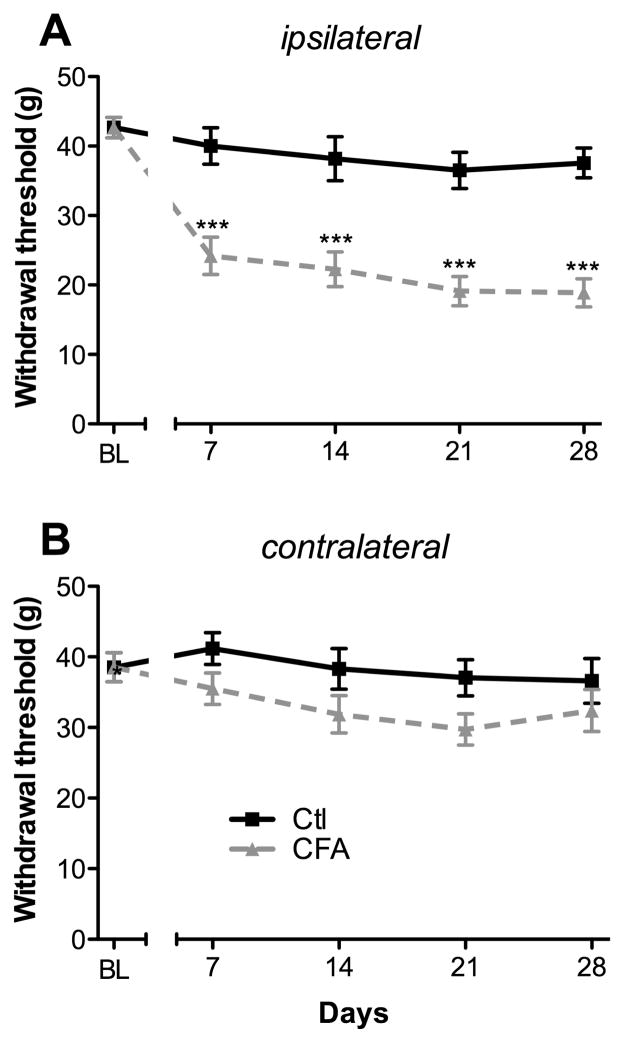
The time course of tactile allodynia was examined in CFA- and saline-injected rats during a 4-week period (Fig. 1). As measured with the von Frey device, baselines to punctuate mechanical stimuli were similar in both groups before CFA administration. Mechanical hypersensitivity was evidenced from day 7 by a significant decrease in withdrawal threshold of the ipsilateral hind paw of CFA-injected animals, compared to saline-treated rats (24.2 ± 2.7 g versus 40 ± 2.6 g, respectively; P < 0.001; Ftreatment = 89.81 and Ftime = 21.12; Fig. 1A). Mechanical allodynia was maintained for 28 days after CFA injection, representing a significant 40% to 50% decrease of the mechano-nociceptive threshold of the affected limb compared to control animals (Fig. 1A). At the same time, saline-injected rats did not develop hypersensitivity to innocuous von Frey filament stimulation (Fig. 1A). Over time, both CFA- and saline-injected rats contralateral paw was unaffected (Fig. 1B), as previously reported [31, 32]. Overall, CFA induced a persistent mechanical allodynia confined to the ipsilateral hind paw.
FIGURE 1. Time course of CFA-induced inflammatory pain behavior.
Changes in withdrawal thresholds to mechanical stimulation with the dynamic von Frey filament were assessed on both (A) ipsilateral and (B) contralateral hind paws of CFA and control groups, at day 0 (BL), and days 7, 14, 21, and 28 post-CFA injection. All data represent means ± S.E.M. (n = 12 for the control group and n = 11 for the CFA group). Statistical analyses were performed using a 2-way ANOVA followed by the Bonferroni post-hoc test. Asterisks denote a significant difference from saline-injected animals; ***P < 0.001.
3.2 Development of anxiety-like behaviors following onset of chronic inflammatory pain
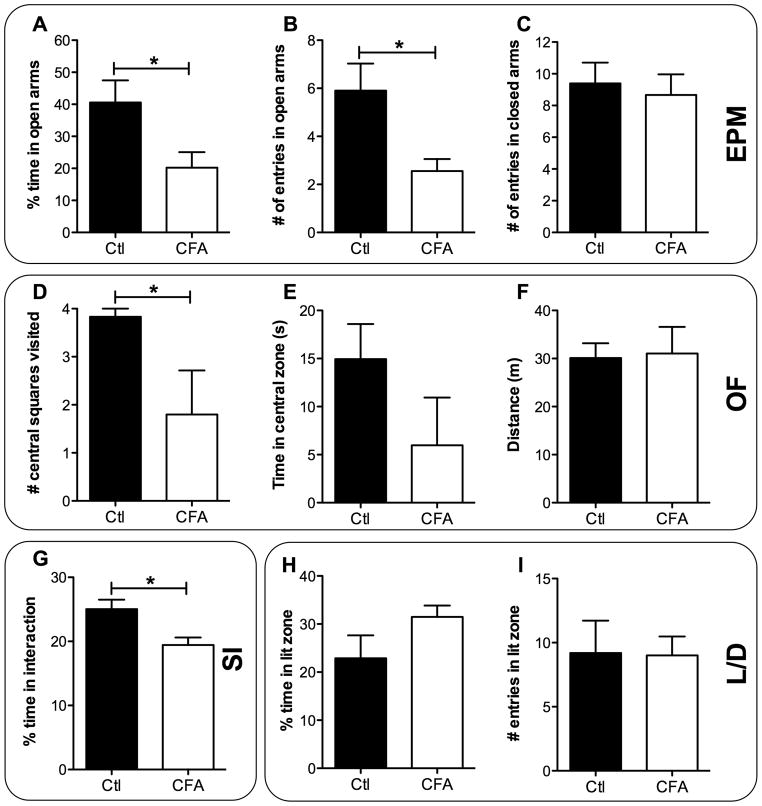
We next investigated whether rats experiencing chronic inflammatory pain developed anxiety-related behaviors. To this end, animals were confronted to different anxiety-like behavioral tests 4 weeks after pain induction. In the elevated-plus maze (EPM) test, rats suffering from chronic inflammatory pain displayed, on day 29 post-CFA, increased anxiety-like behaviors, when compared to controls (Fig. 2). Indeed, CFA-treated rats spent significantly less time in the open arms than rats receiving intraplantar saline (20.2 ± 4.8 % and 40.6 ± 6.9 %, respectively, P < 0.05; t = 2.358; Fig. 2A). Likewise, the number of entries into open arms was significantly decreased by the CFA administration (3.3 ± 1.3 vs 5.9 ± 1.1, P < 0.05; t = 2.601; Fig. 2B). The number of entries in closed arms was also measured to rule out the possible effect of CFA injection on mobility. As shown in Fig. 2C, no significant difference was observed between CFA- and saline-treated groups, suggesting that the development of this anxiety-like behavior was unrelated to a deficit in locomotor functions caused by CFA injection.
FIGURE 2. Development of anxiety-like behaviors caused by chronic inflammatory pain.
Anxiety-like behaviors were measured on CFA- and saline-treated (control) animals using the elevated-plus maze (EPM), open field (OF), social interaction (SI) and light/dark exploration (L/D) tests. (A) Percentage of time spent in open arms, (B) number of entries in open arms and (C) number of entries in closed arms measured in the EPM on day 29 (n = 9–10 in both CFA and control groups). (D) Number of virtual central squares visited (on a total of 4), (E) time in the central zone and (F) total distance travelled were measured in the OF test on day 30 (n = 5–6 in each group). (G) Percentage of time focal animals spent actively interacting with social animals measured in the SI test on day 28 (n = 9 in the control group and n = 25 in the CFA group). (H) Percentage of time spent and (I) number of entries in the lit zone measured in the L/D test on day 29 (n = 5 in both CFA and control groups). All data represent means ± S.E.M. Statistical analyses were performed using the parametric unpaired t-test. The asterisks denote significant differences from saline-injected animals; *P < 0.05.
In the open-field (OF) test, rats with chronic inflammatory pain exhibited a significant reduction in exploratory behavior, when compared to the control group (Fig. 2D and E). In fact, CFA-injected rats displayed a significant decrease in the number of central squares visited on day 30 post-CFA (1.8 ± 0.9 vs 3.8 ± 0.2, P < 0.05; t = 2.399; Fig. 2D). Moreover, a trend in decreased time spent in the central zone was measured (6.0 ± 5.0 s vs 14.9 ± 3.7 s, P = 0.08; t = 1.483; Fig. 2E). Interestingly, as observed in the EPM (data not shown), total distance travelled was similar for both groups (Fig. 2F). Further, no change in activity parameters was detected between groups on day 28 when animals were left in their housing environment. Indeed, when analyzing 6 hours of in-cage activity during the active period following lights extinction (starting at 6 p.m.), CFA animals showed similar basic and fine movements profiles as did control animals (data not shown).
The social interaction (SI) test was also used to assess anxiety-like behaviors in rodents, by using their natural tendency to interact with unfamiliar congener. Rats experiencing chronic inflammatory pain (focal animal), when forced to share a common enclosure with another rat (healthy/naive social animal) showed a difference in time spent interacting (Fig. 2G). Indeed, the percentage of time the focal animal spent in active interaction with the social one was significantly reduced on day 28 in the CFA-treated group (19.4 ± 1.2 % vs 25.0 ± 1.5 %, P < 0.05; t = 2.582; Fig. 2G).
Finally, we used the light/dark (L/D) test to measure the state anxiety, this test being based on a conflict between the aversion of rats to brightly illuminated areas and their spontaneous exploratory behavior in novel environments. Here, we found that chronic inflammatory pain was not sufficient to induce behavioral changes in parameters measured with the L/D test on day 29. No difference was observed in the percentage of time spent in the lit zone (Fig. 2H) and in the number of entries in the lit zone (Fig. 2I) between CFA- and saline-treated rats.
3.3 Effect of morphine and diazepam on CFA-induced allodynia
To characterize and validate this model of anxiety-like behaviors caused by chronic inflammatory pain, we next evaluated the effectiveness of analgesic and anxiolytic drugs in reversing mechanical hypersensitivity and anxiety-related behaviors. To isolate both “pain” and “anxiety” variables in behavioral tests, morphine and diazepam (used as standard analgesic and anxiolytic drugs, respectively) were acutely administered in CFA-injected rats.
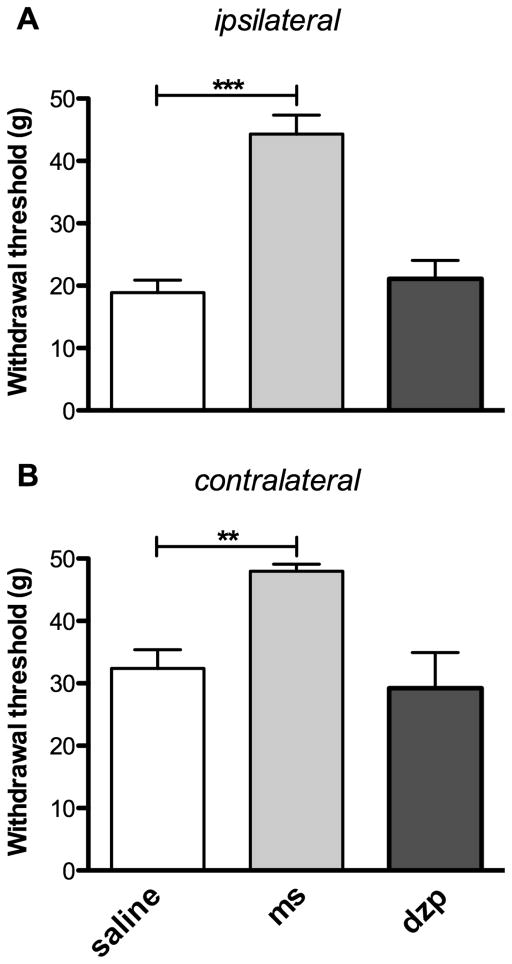
Four weeks after CFA injection, we first assessed the effects of subcutaneous (s.c.) administration of morphine (3 mg/kg) or diazepam (1 mg/kg) on “pain” levels. As expected, CFA-injected rats treated with morphine displayed a significant increase in the withdrawal threshold of their ipsilateral hind paw when compared to rats treated with s.c. saline (44.3 ± 3.0 g vs 18.9 ± 2.0 g respectively, P < 0.001; Fig. 3A). Morphine also exerted an antinociceptive effect on the contralateral side when compared to CFA-injected rats treated with saline (48.0 ± 1.2 g vs 32.4 ± 3.0 g respectively, P < 0.01; Fig. 3B). In contrast to morphine, no significant difference in withdrawal thresholds of ipsilateral or contralateral hind paws was observed when diazepam was administered to CFA-injected animals (Fig. 3A and 3B). These results thus demonstrate that a single s.c. injection of the anxiolytic drug diazepam does not reduce sensitivity to pain in CFA-inflamed rats, whereas morphine completely reverses tactile allodynia caused by chronic inflammatory pain.
FIGURE 3. Anti-allodynic action of morphine and diazepam in rats developing chronic inflammatory pain.
Changes in withdrawal threshold to mechanical stimulation with the dynamic von Frey filament were assessed on day 28 on both (A) ipsilateral and (B) contralateral hind paws of CFA animals after acute s.c. injections of saline 9%, morphine (3 mg/kg, ms) or diazepam (1 mg/kg, dzp). Morphine and diazepam were respectively injected 30 min and 60 min before testing. All data represent means ± S.E.M. (CFA-treated rats receiving morphine or diazepam, n = 5–6; and saline, n = 11). Statistical analyses were performed using a one-way ANOVA followed by a Dunnett’s multiple comparison test. The asterisks denote significant differences from saline-injected animals; ***P < 0.001 and **P < 0.01.
3.4 Effect of diazepam and morphine on anxiety-like behaviors induced by chronic pain
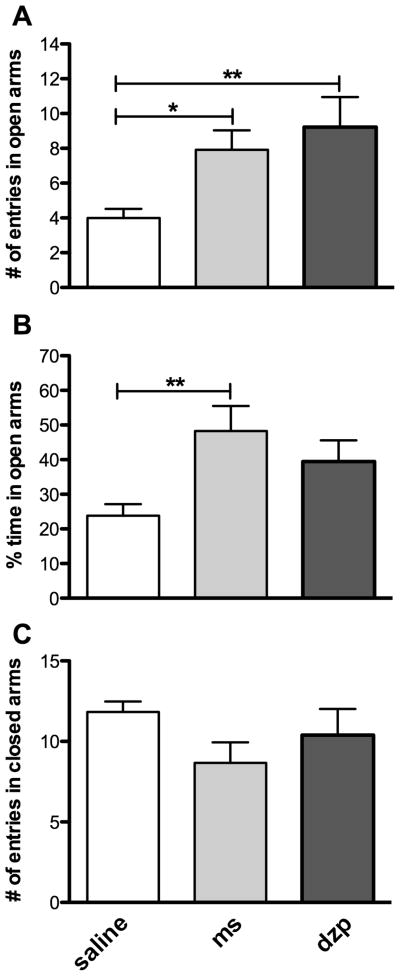
We next examined on day 28 the effects of s.c. acute administration of diazepam (1 mg/kg) and morphine (3 mg/kg) on “anxiety” levels in rats exposed to CFA. For this purpose, the EPM test was specifically used to evaluate the anxiolytic-like properties of those drugs. In this paradigm, CFA-injected rats treated with diazepam, 60 min prior to testing, displayed an increase in the number of entries in open arms (9.2 ± 1.7 vs 4.0 ± 0.5, P < 0.01; Fig. 4A) when compared to animals treated with s.c. saline. Although not significant, a trend toward an increase in the percentage of time spent in open arms (Fig. 4B) was also observed for rats treated with diazepam. Diazepam did not alter the locomotion, as observed by the absence of difference in the number of entries in closed arms between both groups (Fig. 4C). Similarly, a single injection of morphine administered 30 min before testing produced an anxiolytic-like effect that was evidenced by an increase in the number of entries in open arms (7.9 ± 1.1 vs 4.0 ± 0.5, P < 0.05; Fig. 4A) and in the percentage of time spent in open arms (48.3 ± 7.3 % vs 23.8 ± 3.3 %, P < 0.01; Fig. 4B). Similarly to diazepam, no significant effect on locomotion was observed with morphine on the EPM (Fig. 4C). Interestingly, a lower dose of morphine (0.3 mg/kg) was found to induce analgesia without having significant anxiolytic effects in the EPM (not shown). The latter observations suggest that the analgesic effect of morphine is reached at lower doses than its anxiolytic effect.
FIGURE 4. Anxiolytic effects of diazepam and morphine in rats experiencing chronic inflammatory pain-related anxiety-like behaviors.
(A) Number of entries in open arms, (B) percentage of time spent in open arms and (C) numbers of entries in closed arms measured with the elevated-plus maze on day 28 in CFA-treated animals receiving acute s.c. injections of either saline 0.9%, morphine (3 mg/kg, ms) or diazepam (1 mg/kg, dzp). Morphine and diazepam were respectively injected 30 min and 60 min before testing. All data represent means ± S.E.M. (n = 10–12 for saline-injected rats and rats treated with morphine or diazepam, respectively). Statistical analyses were performed using a one-way ANOVA followed by a Dunnett’s multiple comparison test. The asterisks denote significant differences from saline-injected animals; **P < 0.01 and *P < 0.05.
4. DISCUSSION
In the present study, we demonstrated that chronic inflammatory pain leads to the development of anxiety-like behaviors after 28 days. Indeed, in rats suffering from unilateral inflammation, anxiety-related phenotypes were revealed by a decrease in the percentage of time spent and in the number of entries in the open arms of the elevated-plus maze; a decrease in the number of central squares visited in the open field; and a decrease in social interactions. No anxiety-like behavior was, however, detected in the light/dark exploration test. Moreover, acute morphine significantly attenuated both mechanical hypersensitivity and anxiety-like behaviors, although only analgesia was observed at lower dose. On the other hand, systemic diazepam was able to reverse chronic inflammatory pain-induced anxiety-like behaviors, without exerting analgesic properties or locomotor side effects at the tested dose. Our results are therefore supportive of using animal models of chronic pain using controlled procedures to screen new therapies for treating pain and associated co-morbidities.
Chronic pain is, by itself, a debilitating phenomenon that affects approximately 20% of the population. Common co-morbidities such as sleep, anxiety and depressive disorders are reported in most cases [1]. Although development of analgesics is an active research area, R&D on concomitant mood disorders is often neglected. Considering that neurochemical mechanisms in the physiopathology of pain are in part shared with psychiatric co-morbidities, innovative treatments addressing both conditions remain to be discovered [33].
Over the past decade, a handful of groups have explored the changes in anxiety-like behaviors caused by chronic pain in rodents. Using pain models such as virally induced neuropathy [27], drug-induced toxic neuropathy [27, 28], nerve injury-induced neuropathy [21, 23, 25, 34, 35] or models mimicking arthritic pain [21, 36, 37], studies have shown that the development of pain influenced the onset of anxiety-like behaviors in rodents. The use of one or two behavioral tests in the experimental designs of these studies is, however, a salient feature to be addressed. In fact, because anxiety cannot be directly assessed in animals, most tests used in pre-clinical research (e.g. EPM, L/D and OF; with SI being one exception) rely on evaluating exploratory behaviors believed to be associated with anxiety. The motivation to explore being closely related to movement capabilities, obtaining an unbiased measure of emotionality can often be difficult. Interestingly, developed behavioral tests measure psychobiological parameters that are different in nature [38]. Consequently, utilization of a single or of a limited number of anxiety tests in rodents may underestimate the “mood disorder”, especially when the co-morbid condition results from persistent pain.
It has been well described that patients coping with inflammatory arthritis commonly develop anxiety [10]. In rodents, unilateral CFA injection mimics many pathological features of rheumatoid arthritis present in human patients [39]. Indeed, plantar injection of CFA stimulates a rapid, local inflammatory response causing peripheral and central sensitization. CFA-induced pain is usually intense and persists from weeks to months [21, 29, 40]. The physiologic features and duration of this model make it appropriate for the study of pain-induced psychiatric co-morbidities. In the present study, CFA-induced inflammatory pain was accompanied, in rats, by the development of anxiety-like behaviors 4 weeks after the induction of inflammation. Indeed, anxiety-related behaviors were observed in the EPM, the OF, and the SI tests. We, however, failed to observe any anxiety-like behaviors in the L/D exploration test. These disparities reinforce the hypothesis that, despite their similarities, each test seems to reflect different aspects of emotionality, emphasizing the necessity of using multiple tests to study pain co-morbidities.
Anxiety-like effects were also reported in mice after intraplantar injection of CFA [21]. Conversely to the present study, mice exhibited anxiety-like phenotypes in the L/D exploration tests, whereas the number of entries into open arms in the EPM was not changed [21]. Of course, one could argue that this may only be due to interspecies differences, since variability among strains has been reported. For instance, Hasnie et al. [34] found that male C57BL/6J mice subjected to partial sciatic nerve ligation do not exhibit anxiety-related behaviors in the EPM. In opposition, nerve-injured mice sharing the same genetic background were shown to develop anxiety-like phenotypes in the same behavioral paradigm by a different group [21]. Beyond species or genetic background, we might also explain some of the disparities across studies by a wide range of modulatory factors. Variations in environmental factors (ambient noise, temperature, bedding, handling, odors, housing density, etc.) or differences in experimental conditions (surgical procedures, time point measurement, single or repeated measures per subject) may indeed affect behavioral outcomes.
Morphine, a mu opioid receptor agonist, is well known for its high analgesic efficacy for a wide variety of pain conditions. As awaited, we observed a robust analgesic effect of morphine in the CFA model of chronic pain. Diazepam, used in the clinic as an anxiolytic as well as an anticonvulsant, belongs to the benzodiazepine’s family and binds to GABAA receptors [41]. Although it is generally accepted that diazepam holds anxiolytic-like properties, its pharmacological action to painful stimuli remains unclear. Indeed, several studies in laboratory animals [42–46] or in humans [47–49] revealed that acute systemic administration of diazepam results in significant pain inhibition. However, other studies reported that diazepam does not induce antinociceptive responses in acute and tonic pain paradigms and may even produce hyperalgesia [45, 50–52]. In our hands, this positive allosteric modulator (PAM) of GABAA receptors did not induce any analgesic nor significant sedative actions at the selected dose in the CFA-induced arthritis pain model; although sedation was observed when diazepam was used at higher doses. In neuropathic pain models, a similar absence of analgesic actions was observed with etizolam [21] and midazolam [26], two selective benzodiazepine receptor agonists. Interestingly, a recent study by Munro and collaborators suggests that subtype-selective GABAA PAMs (i.e. TPA023 and NS11394) have analgesic effects in injured animals while non-selective PAMs (i.e. diazepam) do not [53], further increasing the complexity of GABAA receptor pharmacology. From these studies, we might nevertheless conclude that the effective doses of diazepam required to attenuate pain-like behaviors were mostly associated with apparent sedation and ataxia [42, 45, 51].
As already mentioned, anxiety disorders are one of the primary indications for the prescription of benzodiazepines in humans [41]. Because diazepam has a proven efficacy to reduce anxiety in humans and anxiety-like behaviors in rats [54–56], it was used here to confirm that behavioral alterations induced by chronic pain could be reversed by a well-known anxiolytic. As demonstrated in the EPM, diazepam succeeded to reduce anxiety-like behaviors caused by chronic inflammatory pain. Interestingly, it was previously reported that induction of inflammatory nociception modifies the response to anxiolytic drugs [37]. These data reinforce the reciprocal relationship between pain and affective disorders such as anxiety. It also suggests that the structural reorganization of neural circuits observed under chronic pain conditions may change the expected responses observed in healthy subjects following treatment with classical anxiolytics and analgesics. In a clinical setting, this has to be taken into consideration when implementing anxiolytic therapy in chronic pain patients.
Many studies report opposite views about the anxiolytic potential of morphine. In fact, it was recently shown that morphine, in the Vogel conflict test, does not display anxiolytic effects [57]. When injected bilaterally in the rat dorsal hippocampus, morphine was rather shown to have anxiogenic effects detectable in the EPM test [58]. However, most publications seem to agree on the general anxiolytic status of morphine. In fact, morphine has already been shown to decrease fear- and anxiety-related behaviors in rhesus monkeys [59] and in humans [60]. Furthermore, injection of morphine in healthy rats either has no effect [26] or induces anxiolytic effects [61, 62] when tested in the EPM. Part of the explanation for this divergence in morphine effectiveness in relieving anxiety could involve the anxiety-like behavioral test employed, the dose used, the route of administration (systemic vs central vs local injection) and the injection protocol (acute vs sustained). Here, we demonstrated that morphine dose-dependently reduced anxiety-like behaviors in the EPM in the CFA model of chronic pain, an effect also observed by others in an animal model of neuropathic pain [26, 28]. Because morphine failed to decrease the number of closed-arm entries on the EPM, the anxiolytic-like effects of morphine cannot be attributed to motor impairment as a confounding factor.
In conclusion, we were able to develop a rat model of anxiety-like behaviors caused by chronic inflammatory pain. By opposition to other studies that had successfully developed a similar model in mice, or by inducing chronic neuropathic pain [21, 23–28], we managed to measure the onset of anxiety-like behaviors with three (out of four) different anxiety tests, namely the EPM, OF and SI. Such results cannot be attributed to impairment in locomotor functions induced by inflammatory pain since no significant difference in ambulatory behaviors was observed in CFA-injected animals. Moreover, in our model, we were able to demonstrate the anxiolytic effect of diazepam, as well as both analgesic and anxiolytic actions of morphine. This model could serve for preclinical screening of alternative bimodal therapies targeting chronic pain and its associated psychopathologies. From a patient’s perspective, improved pharmacological treatment decreasing the odds of drug-drug interactions and side effects would increase the quality of life of a large proportion of the population coping with chronic pain and anxiety disorders.
Acknowledgments
This work was supported by grants from the Quebec Pain Research Network funded by the “Fonds de Recherche Québec - Santé (FRQ-S)” and the “Centre de Recherche Clinique Etienne-Le Bel” to P.S. and L.G. A.P. was supported by the Sir Frederick Banting and Dr Charles Best Canada graduate student scholarship from the Canadian Institute for Health Research (CIHR) and by a graduate student scholarship from the Canadian Arthritis Network (CAN). H.B. was supported by a graduate scholarship from the FRQ-S. P.B. was supported by the Sir Frederick Banting and Dr Charles Best Canada graduate student scholarship from the CIHR. P.S. and L.G. are both the recipients of a FRQ-S Junior 2 salary support. The authors would also like to thank Karine Belleville, Karyn Kirby, Jean Lainé and Pascal Tétreault for their technical assistance, as well as Nathalie Carrier for her help with statistical analyses.
Footnotes
“Chronic Pain in America: Roadblocks to Relief,” a study conducted by Roper Starch Worldwide for American Academy of Pain Medicine, American Pain Society and Janssen Pharmaceutica, 1999.
Center for Disease Control and Prevention (2011), Prevalence of doctor-diagnosed arthritis and arthritis-attributable effects among Hispanic adults, by Hispanic subgroup--United States, 2002, 2003, 2006, and 2009. MMWR Morb Mortal Wkly Rep 60:167-171
Health Canada. Arthritis in Canada. An ongoing challenge. Ottawa : Health Canada, 2003
References
- 1.Meyer-Rosberg K, Kvarnstrom A, Kinnman E, Gordh T, Nordfors LO, Kristofferson A. Peripheral neuropathic pain--a multidimensional burden for patients. Eur J Pain. 2001;5:379–89. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- 2.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–84. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twillman RK. Mental disorders in chronic pain patients. J Pain Palliat Care Pharmacother. 2007;21:13–9. [PubMed] [Google Scholar]
- 5.Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, et al. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain. 2007;129:332–42. doi: 10.1016/j.pain.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol. 2002;70:678–90. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 7.Teh CF, Morone NE, Karp JF, Belnap BH, Zhu F, Weiner DK, et al. Pain interference impacts response to treatment for anxiety disorders. Depress Anxiety. 2009;26:222–8. doi: 10.1002/da.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sareen J, Cox BJ, Clara I, Asmundson GJ. The relationship between anxiety disorders and physical disorders in the U.S. National Comorbidity Survey. Depress Anxiety. 2005;21:193–202. doi: 10.1002/da.20072. [DOI] [PubMed] [Google Scholar]
- 9.Culpepper L. Generalized anxiety disorder and medical illness. J Clin Psychiatry. 2009;70 (Suppl 2):20–4. doi: 10.4088/jcp.s.7002.04. [DOI] [PubMed] [Google Scholar]
- 10.Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM. Psychological aspects of persistent pain: current state of the science. J Pain. 2004;5:195–211. doi: 10.1016/j.jpain.2004.02.576. [DOI] [PubMed] [Google Scholar]
- 11.Vaeroy H, Tanum L, Bruaset H, Morkrid L, Forre O. Symptoms of depression and anxiety in functionally disabled rheumatic pain patients. Nord J Psychiatry. 2005;59:109–13. doi: 10.1080/08039480510022945. [DOI] [PubMed] [Google Scholar]
- 12.Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001;21:857–77. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 13.Isik A, Koca SS, Ozturk A, Mermi O. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:872–8. doi: 10.1007/s10067-006-0407-y. [DOI] [PubMed] [Google Scholar]
- 14.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 15.Marvizon JC, Ma Y-Y, Charles AC, Walwyn W, Evans CJ. Pharmacology of the opioid system. In: Beaulieu P, Lussier D, Porreca F, Dickenson AH, editors. Pharmacology of pain. Seatle: IASP Press; 2010. pp. 87–110. [Google Scholar]
- 16.Devane CL, Chiao E, Franklin M, Kruep EJ. Anxiety disorders in the 21st century: status, challenges, opportunities, and comorbidity with depression. Am J Manag Care. 2005;11:S344–53. [PubMed] [Google Scholar]
- 17.Nicolson SE, Caplan JP, Williams DE, Stern TA. Comorbid pain, depression, and anxiety: multifaceted pathology allows for multifaceted treatment. Harv Rev Psychiatry. 2009;17:407–20. doi: 10.3109/10673220903463226. [DOI] [PubMed] [Google Scholar]
- 18.Megarbane B, Gueye P, Baud F. Interactions between benzodiazepines and opioids. Ann Med Interne (Paris) 2003;154(Spec No 2):S64–72. [PubMed] [Google Scholar]
- 19.Devulder J, Richarz U, Nataraja SH. Impact of long-term use of opioids on quality of life in patients with chronic, non-malignant pain. Curr Med Res Opin. 2005;21:1555–68. doi: 10.1185/030079905X65321. [DOI] [PubMed] [Google Scholar]
- 20.Asmundson GJ, Abrams MP, Collimore KC. Pain and anxiety disorders. In: Zvolensky MJ, Smits JAJ, editors. Health behaviors and physical ilness in anxiety and its disorders. New York: Springer; 2008. pp. 207–35. [Google Scholar]
- 21.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–50. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 22.Narita M, Kuzumaki N, Kaneko C, Hareyama N, Miyatake M, Shindo K, et al. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J Neurochem. 2006;97:1369–78. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- 23.Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, et al. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain. 2008;12:591–9. doi: 10.1016/j.ejpain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus-associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuzawa-Yanagida K, Narita M, Nakajima M, Kuzumaki N, Niikura K, Nozaki H, et al. Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology. 2008;33:1952–65. doi: 10.1038/sj.npp.1301590. [DOI] [PubMed] [Google Scholar]
- 26.Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139:349–57. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, et al. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130:2688–702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace VC, Segerdahl AR, Blackbeard J, Pheby T, Rice AS. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci Lett. 2008;448:153–6. doi: 10.1016/j.neulet.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otis V, Sarret P, Gendron L. Spinal activation of delta opioid receptors alleviates cancer-related bone pain. Neuroscience. 2011;183:221–9. doi: 10.1016/j.neuroscience.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaudry H, Proteau-Gagne A, Li S, Dory Y, Chavkin C, Gendron L. Differential noxious and motor tolerance of chronic delta opioid receptor agonists in rodents. Neuroscience. 2009;161:381–91. doi: 10.1016/j.neuroscience.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colpaert FC. Evidence that adjuvant arthritis in the rat is associated with chronic pain. Pain. 1987;28:201–22. doi: 10.1016/0304-3959(87)90117-5. [DOI] [PubMed] [Google Scholar]
- 32.Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, et al. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306:490–7. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- 33.Max MB, Stewart WF. The molecular epidemiology of pain: a new discipline for drug discovery. Nat Rev Drug Discov. 2008;7:647–58. doi: 10.1038/nrd2595. [DOI] [PubMed] [Google Scholar]
- 34.Hasnie FS, Wallace VC, Hefner K, Holmes A, Rice AS. Mechanical and cold hypersensitivity in nerve-injured C57BL/6J mice is not associated with fear-avoidance- and depression-related behaviour. Br J Anaesth. 2007;98:816–22. doi: 10.1093/bja/aem087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roeska K, Ceci A, Treede RD, Doods H. Effect of high trait anxiety on mechanical hypersensitivity in male rats. Neurosci Lett. 2009;464:160–4. doi: 10.1016/j.neulet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez-Velazquez G, Lopez-Munoz FJ, Fernandez-Guasti A. Parallel anxiolytic-like and antinociceptive actions of diazepam in the anterior basolateral amygdala and dorsal periaqueductal gray. Brain Res. 2010;1349:11–20. doi: 10.1016/j.brainres.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Guasti A, Reyes R, Martinez-Mota L, Lopez-Munoz FJ. Influence of inflammatory nociception on the anxiolytic-like effect of diazepam and buspirone in rats. Psychopharmacology (Berl) 2005;180:399–407. doi: 10.1007/s00213-005-2190-x. [DOI] [PubMed] [Google Scholar]
- 38.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–8. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Zhang WS, Xu H, Xu B, Sun S, Deng XM, Zhang YQ. Antihyperalgesic effect of systemic dexmedetomidine and gabapentin in a rat model of monoarthritis. Brain Res. 2009;1264:57–66. doi: 10.1016/j.brainres.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–60. [PubMed] [Google Scholar]
- 41.Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 42.Jourdan D, Ardid D, Bardin L, Bardin M, Neuzeret D, Lanphouthacoul L, et al. A new automated method of pain scoring in the formalin test in rats. Pain. 1997;71:265–70. doi: 10.1016/s0304-3959(97)03366-6. [DOI] [PubMed] [Google Scholar]
- 43.Munro G, Erichsen HK, Mirza NR. Pharmacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacology. 2007;53:609–18. doi: 10.1016/j.neuropharm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Pang CS, Tsang SF, Yang JC. Effects of melatonin, morphine and diazepam on formalin-induced nociception in mice. Life Sci. 2001;68:943–51. doi: 10.1016/s0024-3205(00)00996-6. [DOI] [PubMed] [Google Scholar]
- 45.Rosland JH, Hunskaar S, Hole K. The effect of diazepam on nociception in mice. Pharmacol Toxicol. 1987;61:111–5. doi: 10.1111/j.1600-0773.1987.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 46.Zambotti F, Zonta N, Tammiso R, Conci F, Hafner B, Zecca L, et al. Effects of diazepam on nociception in rats. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:84–9. doi: 10.1007/BF00167386. [DOI] [PubMed] [Google Scholar]
- 47.Singer E, Dionne R. A controlled evaluation of ibuprofen and diazepam for chronic orofacial muscle pain. J Orofac Pain. 1997;11:139–46. [PubMed] [Google Scholar]
- 48.Warms CA, Turner JA, Marshall HM, Cardenas DD. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. 2002;18:154–63. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Yang JC, Clark WC, Ngai SH, Berkowitz BA, Spector S. Analgesic action and pharmacokinetics of morphine and diazepam in man: an evaluation by sensory decision theory. Anesthesiology. 1979;51:495–502. doi: 10.1097/00000542-197912000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez-Velazquez G, Fernandez-Guasti A, Lopez-Munoz FJ. Influence of pharmacologically-induced experimental anxiety on nociception and antinociception in rats. Eur J Pharmacol. 2006;547:83–91. doi: 10.1016/j.ejphar.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 51.Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EO, Larsen JS, et al. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327:969–81. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- 52.Tatsuo MA, Salgado JV, Yokoro CM, Duarte ID, Francischi JN. Midazolam-induced hyperalgesia in rats: modulation via GABA(A) receptors at supraspinal level. Eur J Pharmacol. 1999;370:9–15. doi: 10.1016/s0014-2999(99)00096-5. [DOI] [PubMed] [Google Scholar]
- 53.Munro G, Erichsen HK, Rae MG, Mirza NR. A question of balance - Positive versus negative allosteric modulation of GABA(A) receptor subtypes as a driver of analgesic efficacy in rat models of inflammatory and neuropathic pain. Neuropharmacology. 2011;61:121–32. doi: 10.1016/j.neuropharm.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Berney P, Halperin D, Tango R, Daeniker-Dayer I, Schulz P. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41:39–47. [PubMed] [Google Scholar]
- 55.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 56.Rodgers RJ, Lee C, Shepherd JK. Effects of diazepam on behavioural and antinociceptive responses to the elevated plus-maze in male mice depend upon treatment regimen and prior maze experience. Psychopharmacology (Berl) 1992;106:102–10. doi: 10.1007/BF02253596. [DOI] [PubMed] [Google Scholar]
- 57.Basso AM, Gallagher KB, Mikusa JP, Rueter LE. Vogel conflict test: sex differences and pharmacological validation of the model. Behav Brain Res. 2011;218:174–83. doi: 10.1016/j.bbr.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 58.Solati J, Zarrindast MR, Salari AA. Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatry Clin Neurosci. 2010;64:634–41. doi: 10.1111/j.1440-1819.2010.02143.x. [DOI] [PubMed] [Google Scholar]
- 59.Winslow JT, Noble PL, Davis M. Modulation of fear-potentiated startle and vocalizations in juvenile rhesus monkeys by morphine, diazepam, and buspirone. Biol Psychiatry. 2007;61:389–95. doi: 10.1016/j.biopsych.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis. 2008;27:49–65. doi: 10.1080/10550880802122646. [DOI] [PubMed] [Google Scholar]
- 61.Rezayof A, Hosseini SS, Zarrindast MR. Effects of morphine on rat behaviour in the elevated plus maze: the role of central amygdala dopamine receptors. Behav Brain Res. 2009;202:171–8. doi: 10.1016/j.bbr.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 62.Zarrindast MR, Rostami P, Zarei M, Roohbakhsh A. Intracerebroventricular effects of histaminergic agents on morphine-induced anxiolysis in the elevated plus-maze in rats. Basic Clin Pharmacol Toxicol. 2005;97:276–81. doi: 10.1111/j.1742-7843.2005.pto_116.x. [DOI] [PubMed] [Google Scholar]