Abstract
Both neurotensin (NT) and opioid agonists have been shown to induce antinociception in rodents after central administration. Besides, previous studies have revealed the existence of functional interactions between NT and opioid systems in the regulation of pain processing. We recently demonstrated that NTS1 receptors play a key role in the mediation of the analgesic effects of NT in long-lasting pain. In the present study, we therefore investigated whether NTS1 gene deletion affected the antinociceptive action of mu opioid drugs. To this end, pain behavioral responses to formalin were determined following systemic administration of morphine in both male and female NTS1 knockout mice. Acute injection of morphine (2 or 5 mg/kg) produced strong antinociceptive effects in both male and female wild-type littermates, with no significant sex differences. On the other hand, morphine analgesia was considerably reduced in NTS1-deficient mice of both sexes compared to their respective controls, indicating that the NTS1 receptor actively participates in mu opioid alleviating pain. By examining specifically the flinching, licking and biting nociceptive behaviors, we also showed that the functional crosstalk between NTS1 and mu opioid receptors influences the supraspinally-mediated behaviors. Interestingly, sexual dimorphic action of morphine-induced pain inhibition was found in NTS1 null mice in the formalin test, suggesting that the endogenous NT system interacts differently with the opioid network in male and female mice. Altogether, these results demonstrated that NTS1 receptor activation operates downstream to the opioidergic transmission and that NTS1-selective agonists combined with morphine may act synergistically to reduce persistent pain.
Keywords: tonic pain, formalin, opioid, supraspinal, crosstalk
INTRODUCTION
Both neurotensin (NT) and opioid agonists have been shown to induce antinociception in rodents after intracerebral administration. It is now well established that the spinal nociceptive transmission is modulated by inhibitory and facilitatory systems that originate from supraspinal sites in the central nervous system (Porreca et al., 2002, Gebhart, 2004, Vanegas, 2004, Heinricher et al., 2009). In particular, the neuronal network connecting the midbrain periaqueductal gray (PAG) and the rostral ventromedial medulla (RVM) actively contributes to the analgesic actions of mu opioid agonists, including morphine as well as to those mediated by NT agonists (Dobner, 2006, Loyd and Murphy, 2009). In support, opioid- and NT-peptide immunoreactive nerve terminals, as well as high densities of opioid- and NT-receptors are reported in most descending antinociceptive circuits (Basbaum and Fields, 1984, Sarret and Beaudet, 2003). Therefore, from a R&D perspective, it is of particular interest to know whether the coexistence of NT and opioid systems in cerebral structures implicated in pain modulation leads to their functional interactions.
Originally, NT was found to exert a potent antinociceptive effect in a mu opioid-independent manner in a variety of analgesic screening tests, including tail-flick, hot-plate and writhing induced by acetic acid (Clineschmidt et al., 1979b, a, Clineschmidt et al., 1982). Indeed, the analgesic effects of NT, reported as being more potent than morphine at equimolar doses, were still effective in the presence of two structurally related opioid antagonists, naloxone or naltrexone (Nemeroff et al., 1979, Osbahr et al., 1981, Behbehani and Pert, 1984, Coquerel et al., 1986, al-Rodhan et al., 1991, Sarhan et al., 1997, Boules et al., 2009). In that respect, the neurotensinergic system plays a pivotal role in the non-opioid form of stress-induced analgesia (Seta et al., 2001, Gui et al., 2004, Lafrance et al., 2010). There is, however, existing data indicating that the analgesic actions of NT are dependent on the functional integrity of the brain opioid system (van Wimersma Greidanus et al., 1982, Yaksh et al., 1982, Furuta et al., 1984). Above all, mice rendered tolerant to morphine show an attenuated antinociceptive profile to central NT administration (Luttinger et al., 1983). Based on these studies, it would appear that NT-induced pain suppression is, at least in part, generated by distinct mechanisms from those of the opioid peptides.
Of obvious importance is whether opioid-induced antinociception is also independent of the endogenous NT system. In this regard, morphine and the mu opioid receptor-selective agonist DAMGO both induced a naloxone-reversible increase of the NT release in the medial PAG, suggesting that local NT release from either intrinsic neurons or afferent terminals within the PAG may be involved in opioid-induced analgesia (Stiller et al., 1997). Similarly, the antinociception induced by the mu-opioid receptor activation in the amygdala is partly dependent on the recruitment of NT receptors in the ventral PAG (Tershner and Helmstetter, 2000). However, it has also been demonstrated that NT signaling disruption, by the use of either NT antiserum or NT receptor antagonists reinforced the antinociceptive action of morphine, revealing this time an “anti-opioid” effect of NT (Urban and Smith, 1993, 1994, Smith et al., 1995, Smith et al., 1997). These opposing findings may nevertheless be explained by the bidirectional effect of NT: NT inducing pain-facilitatory or -inhibitory dose-related behavioral responses (Urban and Gebhart, 1997, Urban et al., 1999, Neubert et al., 2004). The fact that NT produces dose-dependent antinociceptive and anti-analgesic effects within the descending PAG-RVM pathway also suggests interaction with multiple pain modulatory systems, possibly via distinct NT receptor subtypes. Accordingly, the central role of NT in pain modulation is dependent on the activation of two seven-transmembrane domain G-protein-coupled receptors, designed NTS1 and NTS2 (Dobner, 2006, Sarret et al., 2007). Among the studies demonstrating the involvement of NT receptors in nociceptive processing, we recently demonstrated that NTS1 receptors play a key role in mediating the analgesic effects of NT in long-lasting pain (Roussy et al., 2008).
Based on the pivotal role of NT and opioid peptides in regulating pain transmission, the current study was undertaken to determine if alterations in NTS1 signaling would affect the antinociceptive action of systemically administered morphine. For this purpose, we evaluated the analgesia induced by morphine in mice deficient for the high affinity NTS1 receptor (NTS1-KO), by measuring the formalin-evoked nociceptive behaviors in a widely accepted model of persistent inflammatory pain (Tjolsen et al., 1992, Coderre et al., 1993, Sawynok, 2004). Since gender/sex-related differences in pain and analgesia are reported in human and rodents, both male and female mice were used to study the functional interaction between NT and opioid receptor systems (Kest et al., 2000, Mogil et al., 2000, Craft, 2003, Greenspan et al., 2007, Loyd and Murphy, 2009).
MATERIAL and METHODS
Animals, housing and habituation
NTS1-KO mice were backcrossed with C57BL/6 mice (Charles River, St-Constant, QC, Canada). NTS1-KO and wild-type littermate adult male and female mice (20–30 g) used throughout the experiments were generated by mating heterozygous mice. The absence of expression of the NTS1 gene in NTS1-deficient mice was confirmed by RT-PCR analyzes, as previously described (Maeno et al., 2004). Routine genotyping was conducted by PCR on purified tail DNA using two NTS1-specific primers (5′-GTT AAC ACC TTC ATG TCC TTC CTG-3′, 5′-TAC GTA AGA CGA GGA CTC CAT GGC G-3′) and two neo primers (5′-GGA TCG GCC ATT GAA CAA GAT GG-3′; 5′-CTT CAG CAA TAT CAC GGG TAG CC-3′). The expected sizes for the WT and KO alleles were 200 and 700 bp, respectively. Animals were kept on a 10h light/14h dark cycle and allowed ad libitum access to food and water. Animals were individually acclimatized to Plexiglas enclosures and handling for 3 consecutive days prior testing. Mice were randomly assigned to control and drug treatments. Furthermore, the behavioral observations were performed by two experimenters blinded to the genotypes in a quiet room, between 09:00 and 12:00 PM to reduce any variation related to circadian rhythm. Experiments were approved by the animal care committee at the Université de Sherbrooke in compliance with the policies and directives of the Canadian Council on Animal Care and guidelines from the International Association for the Study of Pain.
Behavioral studies
Intraperitoneal administration of mu-opioid receptor agonist before formalin injection
Mice were injected intraperitoneally with morphine (2 or 5 mg/kg) diluted in physiological saline (0.9% NaCl) 15 min before formalin administration. Control mice received physiological saline.
Formalin test
Antinociception was assessed using the formalin test as a model of tonic pain. For this purpose, mice were placed for a 60-min habituation period in the experimentation room. Thereafter, mice received a 20 μl intradermal injection of 2% formaldehyde solution (i.e. 5.4% formalin, Fisher Scientific, Montreal, QC, Canada) into the plantar surface of the right hind paw. Following this, mice were placed in clear plastic chambers (30 X 30 X 30 cm) positioned over a mirror angled at 45° in order to allow an unobstructed view of the paws. Their behaviors were then observed for the next 60 min. An intraplantar injection of formalin produced a biphasic nociceptive response, typical of this tonic pain model (Tjolsen et al., 1992, Coderre et al., 1993). The two distinct phases of spontaneous pain behaviors that occur in rodents are proposed to reflect a direct effect of formalin on sensory receptors (phase I) and a longer lasting pain due to inflammation and central sensitization (phase II). These two phases are separated by a period of quiescence, namely the interphase characterized by an active inhibition of the formalin-induced nociceptive behaviors (Sawynok, 2004).
Nocifensive behaviors were assessed using a weighted score method as described previously (Dubuisson and Dennis, 1977, Coderre et al., 1993). Following injection of formalin into the right hind paw, the experimenter measured the time spent in each of four behavioral categories: 0, the injected paw is comparable to the contralateral paw; 1, the injected paw has little or no weight placed on it; 2, the injected paw is elevated and is not in contact with any surface; 3, the injected paw is licked, bitten, or flinched. The behaviors believed to represent higher levels of pain intensity were given higher weighted scores. The weighted average pain intensity score ranging from 0 to 3 was then calculated by multiplying the time spent in each category by the category weight, summing these products and dividing by the total time in a given time interval. The pain score was thus calculated from the following formula (1T1 + 2T2 + 3T3)/180 where T1, T2 and T3 are the durations (in sec) spent in behavioral categories 1, 2 or 3 respectively during each 180 sec block. Phase I and II values were calculated between 0–9 min and 21–60 min, respectively. To statistically compare the magnitude of the morphine analgesia between wild-type and NTS1-KO mice, dose-response curves were generated for phase II by calculating the area under the curve (A.U.C.) for each dose and for each genotype. The A.U.C. of “pain score - time” above the weighted pain score of 1 were calculated by the trapezoidal rule using Prism 4.0. Alternatively, formalin-induced pain-related behaviors were quantified by monitoring the cumulative time spent in flinching/licking/biting during the inflammatory phase (phase II) (Tjolsen et al., 1992). This representation of the data allowed to determine whether the differences in pain responses between wild-type and NTS1-KO mice occurred at the supraspinal level.
Statistical analysis
Data are presented as means ± standard errors of the mean (S.E.M.). All calculations and statistical analysis were performed using Prism 4.0 and Instat 3.05 (Graph Pad Software, San Diego, CA, USA). Nociceptive scores over the 3 min time blocks were analyzed using a two-way analysis of variance for repeated measures, with comparisons between experimental groups and the control group at each time interval using Bonferroni’s post hoc test. A one-way ANOVA followed by Dunnett’s post hoc t-test was used to determine the significance of differences in the A.U.C. and in the time spent in flinching/licking/biting. A difference in responses between groups was considered significant with P values; * P < 0.05, ** P < 0.01 and *** P < 0.001.
RESULTS
Nociceptive sensitivity in NTS1-KO and wild-type mice of both sexes
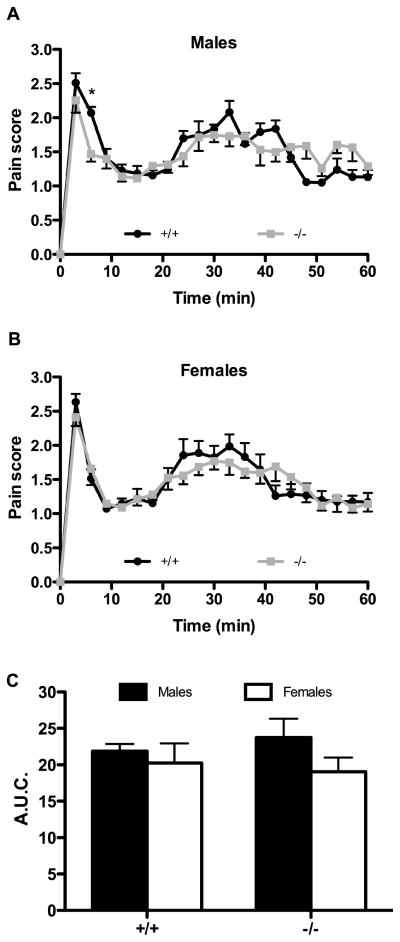
Since the introduction of the formalin test, different approaches have been used to assess formalin-induced pain-related behaviors (Porro and Cavazzuti, 1993, Sawynok, 2004). Using the weighted-scores method of behavioral pain rating (Dubuisson and Dennis, 1977), we found that subcutaneous injection of diluted formalin into the plantar surface of the right hind paw of saline pre-treated mice induced in both NTS1-deficient mice and wild-type littermates a typical biphasic pain response corresponding to the acute (phase I) and inflammatory (phase II) phases (Fig. 1). No effect of NTS1 deletion on baseline pain sensitivity was observed in the tonic pain test. Indeed, the nociceptive behavioral scores of NTS1 mice were indistinguishable from those of wild-type animals, in either male (Fig. 1A) or female groups (Fig. 1B). Beyond the genotype, there were also no sex differences in response to the noxious chemical stimulation of the hind paw with formalin (Fig. 1C).
Figure 1. Nociceptive processing in NTS1 knockout mice.
Nocifensive responses to intraplantar formalin in mice lacking NTS1 and their wild-type littermates, in both male (A) and female (B) mice. Formalin-induced pain responses are expressed in 3 min intervals with a weighted pain score. Both genotypes present similar biphasic nociceptive behavioral profiles following saline intraperitoneal injection (* P < 0.05 compared to wild-type mice; two-way ANOVA followed by Bonferonni’s post hoc t-test). (C) The cumulative pain response represented as area under curve (A.U.C.) is determined over the period of 21 to 60 minutes (phase II). In accordance with results presented in panels A and B, no difference is observed between wild-type and NTS1-KO mice during phase II. The A.U.C. also reveals no sex difference in the inflammatory phase. Each symbol represents the mean ± SEM of determinations made in eight animals.
Effect of NTS1 gene deletion on morphine-mediated analgesia in male mice
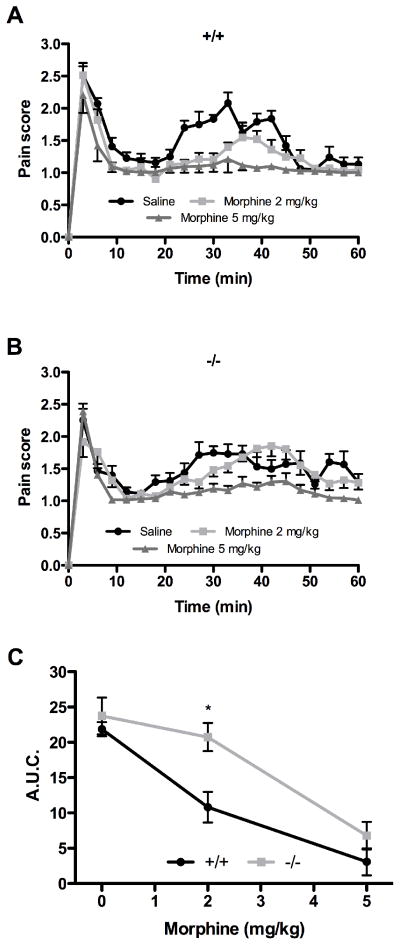
In both genotypes, morphine had no effect on pain score during phase I (Fig. 2A and B). In wild-type mice, morphine dose-dependently inhibited the nociceptive behaviors induced by formalin injection in phase II (Fig. 2A). This effect was statistically significant at both doses of morphine used (2 and 5 mg/kg). As opposed to their wild-type littermates, male NTS1-KO mice were less responsive to the analgesic action of morphine, the dose of 2 mg/kg losing its effectiveness in phase II (Fig. 2B). Figure 2C shows area under the curve (A.U.C.) analyses for the effects of morphine on formalin-evoked nociceptive behaviors in both genotypes. Compared to wild-type, NTS1-KO male mice displayed reduced sensitivity to morphine, this effect being statistically significant at 2 mg/kg (10.8 ± 2.2 versus 20.8 ± 2.0).
Figure 2. Dose and time-dependency of morphine antinociceptive effects in NTS1 null male mice.
Effect of morphine on nocifensive responses induced by intraplantar injection of formalin in wild-type (A) and NTS1-deficient (B) male mice. (C) A.U.C. of phase II shows a significant difference between genotypes at 2 mg/kg of morphine (* P < 0.05 compared to wild-type mice; two-way ANOVA followed by Bonferonni’s post hoc t-test). Note that the 0 mg/kg morphine data point represents male mice receiving the vehicle through intraperitoneal administration. Each point represents the mean ± SEM of determinations made in eight animals.
Effect of NTS1 gene deficiency on morphine-induced analgesia in female mice
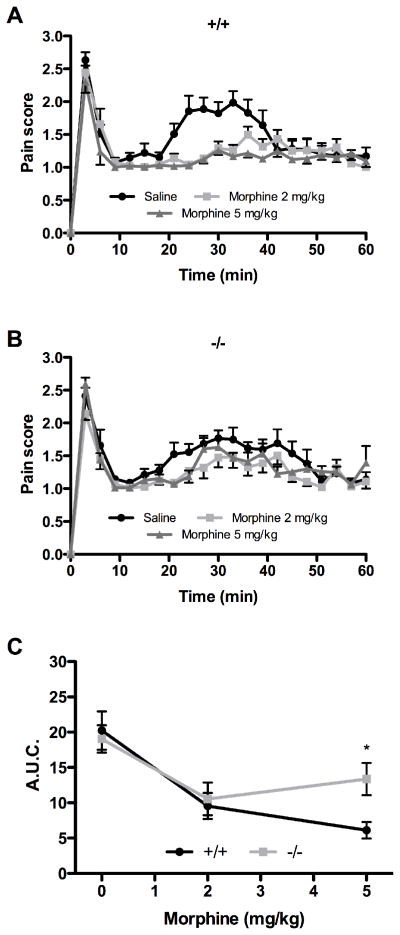
As in male mice, morphine administration provoked no effect on formalin-induced pain behaviors during phase I in both female genotypes (Fig. 3A and B). In wild-type female mice, both doses of morphine tested reversed the formalin-induced nociceptive behavioral responses manifested during the inflammatory phase (Fig. 3A). By contrast, the inhibition by morphine of the persistent noxious chemical stimulation was considerably altered in NTS1-KO female mice (Fig. 3B). Specifically, NTS1 gene deletion significantly reduced the analgesic effect induced by a subcutaneous dose of 5 mg/kg morphine, as demonstrated by the A.U.C. comparative analysis of both genotypes during phase II (6.1 ± 1.2 versus 13.4 ± 2.3) (Fig. 3C).
Figure 3. Effect of morphine on formalin-induced pain behaviors in NTS1-mutant female mice.
The nociceptive behaviors elicited by 5% formalin are quantified following intraperitoneal injection of different doses of morphine in both control littermates (A) and NTS1-KO (B) female mice. (C) A.U.C. obtained for the inflammatory phase demonstrates a significant difference between the two female genotypes at 5 mg/kg of morphine (* P < 0.05 compared to wild-type mice; two-way ANOVA followed by Bonferonni’s post hoc t-test). Note that the 0 mg/kg morphine data point represents female mice treated with intraperitoneal saline. Data represent the mean ± SEM of determinations made in eight animals.
Sex differences in morphine-induced analgesia in NTS1-KO mice involve supraspinally-mediated pain behaviors
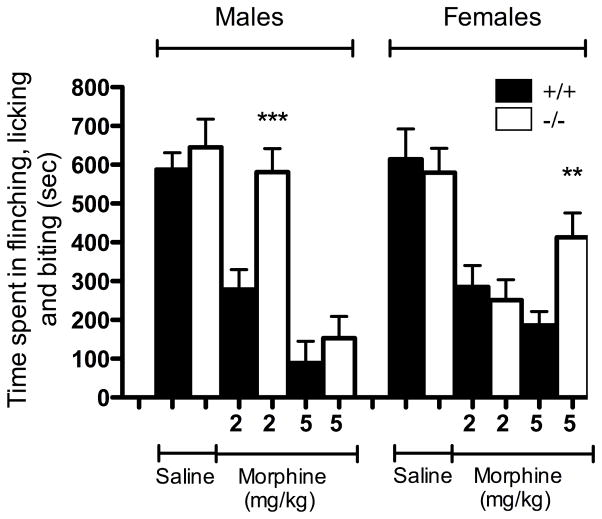
Pain intensity elicited by formalin administration may also be rated by monitoring the total time spent in different behavioral endpoints. Regarding these stereotypical nociceptive reactions, it was notably demonstrated that the persistence of flinching, licking and biting behavioral manifestations reflected the activity of supraspinal nociceptive circuits (Porro et al., 2003). By focusing on the inflammatory phase, we then evaluated whether these supraspinal-integrated behaviors were affected to the same extent in NTS1-KO and wild-type mice following morphine injection. As demonstrated using the weighted-scores method, we did not observe changes in baseline pain-related behaviors following formalin administration between NTS1-KO mice and wild-type littermates in either male (645 ± 73 sec in NTS1-KO compared to 587 ± 44 sec in WT) or female (580 ± 63 sec in NTS1-KO compared to 615 ± 78 sec in WT) (Fig. 4). In wild-type littermates, morphine delivery markedly decreased in a dose-dependent manner the cumulative time spent in flinching, licking and biting, reaching 85% and 75% of inhibition at the highest dose tested in male and female mice, respectively (Fig. 4). Interestingly, the analgesic effects of morphine were similar in wild-type male and female mice in the tonic pain test. In contrast to the effects observed in WT, targeted disruption of the NTS1 gene modified the action profile of morphine in reversing the spontaneous aversive behaviors. Indeed, male NTS1-KO mice were less sensitive to the pain relieving action of morphine (2 mg/kg) than their wild-type counterparts (581 ± 61 sec in NTS1-KO compared to 278 ± 52 sec in WT) (Fig. 4). No difference was however found between NTS1-KO and WT male mice treated with 5 mg/kg of morphine. Similarly, the duration of the nociceptive response was significantly increased in NTS1-KO female mice compared to wild-type animals, following 5 mg/kg of morphine (413 ± 63 sec in NTS1-KO compared to 186 ± 36 sec in WT). At the dose of 2 mg/kg of morphine, there was no difference between genotypes in female.
Figure 4. Effect of NTS1 receptor deficiency on morphine-induced decrease in stereotypic behavior reactions to formalin.
The cumulative nociceptive response time of flinching, licking and biting the injected paw is measured during the second phase (21–60 min) of the formalin test in both male and female mice. The duration of the nociceptive response is determined following morphine administration in NTS1-KO mice (white column) and their respective wild-type littermates (black column). In both sexes, the analgesic effect of morphine is reduced in a dose-dependent manner in NTS1-KO mice compared to controls. The vertical bars denote SEM. ** P < 0.01 and *** P < 0.001 when morphine-treated NTS1-KO mice are compared to morphine-treated wild-type littermates (ANOVA followed by Dunnett’s post hoc t-test). Results come from data obtained with animals used in figure 2 and 3 and are presented as the average ± SEM of eight animals.
DISCUSSION
In the present study, we used NTS1-deficient mice to evaluate the contribution of the NTS1 signaling pathways in modulating morphine-induced analgesia. Our data revealed for the first time that NTS1 gene disruption results in a significant decrease of morphine-induced analgesia in the formalin test, both in male and female mice. Our results also provided evidence that this interaction between opioid and neurotensin systems mainly influences supraspinally-mediated behaviors.
In the past few years, several studies have attempted to determine the respective role of NTS1 and NTS2 receptor subtypes in the pain modulating effects of the NT peptide (Dobner, 2006). There is now compelling evidence demonstrating that the high-affinity NTS1 subtype is involved in antinociception (Wustrow et al., 1995, Smith et al., 1997, Urban and Gebhart, 1997, Buhler et al., 2005, Sarret et al., 2005, Buhler et al., 2008, Roussy et al., 2008). Thus, microinjection of the selective NTS1 agonist PD149163 into the rostral ventromedial medulla produced increases in nociceptive thresholds in the tail-flick tests (Buhler et al., 2005, Buhler et al., 2008). Accordingly, injection of anti-NTS1 peptide nucleic acids into the periaqueductal gray reversed NT-induced analgesic responses in acute pain tests (Tyler et al., 1998). Studies in NTS1 knockout mouse derived from different genetic background also pointed to the NTS1 subtype as a NT antinociceptor. Indeed, NTS1-deficient mice failed to exhibit analgesic responses to thermal stimuli following central injection of NT or systemic delivery of brain penetrating NT analogs (NT-1, NT-2 and NT69L) (Pettibone et al., 2002, Mechanic et al., 2009).
In the present study, we first examined using formalin as a chemical noxious stimulus whether deletion of NTS1 affected baseline pain sensitivity. Our results demonstrate for the first time in a persistent pain model that the spontaneous nociceptive behaviors, analyzed either by the weighted-scores method or rated by monitoring the total time spent in flinching/licking/biting were equivalent in NTS1-KO male mice and their wild-type littermates. These results are consistent with previous findings showing similar hot-plate reaction times or tail-flick response latencies between NTS1-deficient and wild-type mice (Pettibone et al., 2002, Maeno et al., 2004, Mechanic et al., 2009). Interestingly, we recently demonstrated that baseline pain thresholds of NTS2-KO mice were also not different from wild-type mice after the application of thermal or chemical stimuli (Lafrance et al., 2010). This lack of difference between wild-type and NTS1/NTS2-KO mice may thus suggest that the neurotensin system does not mediate tonic effects on pain transmission, but comes into action under specific conditions to inhibit pain. As in male, we also found under tonic chemical pain stimulation that female NTS1 null mice failed to exhibit altered nociceptive behaviors compared to female littermates. Although experimental and clinical data reveal the presence of a basal sexual dimorphism in pain perception, no sex difference was observed here in both genotypes in the persistent pain model of formalin. With respect to sex/gender-related differences in nociception, females usually show a lower basal nociceptive threshold than males in the majority of experimental modalities (Berkley, 1997, Fillingim, 2000, Mogil et al., 2000). However, the reliability and direction of sex differences appear to depend on multiple factors, from genes and reproductive hormones to nociceptive assays and environmental considerations. Indeed, whereas some studies reported that females are more susceptible to the development of persistent pain following tissue injury, others have shown that female and male exhibit identical sensitivity to formalin (Aloisi et al., 1994, Kim et al., 1999, Khasar et al., 2001, Gaumond et al., 2002, Perissin et al., 2003, You et al., 2006, Leo et al., 2008).
Previous studies have established that NT-induced analgesia operates independently of the opioidergic transmission. Accordingly, it was pharmacologically demonstrated that the antinociceptive response induced by NT or NT receptor agonists could not be reversed by the opioid antagonists naloxone and naltrexone (Nemeroff et al., 1979, Osbahr et al., 1981, Behbehani and Pert, 1984, Coquerel et al., 1986, al-Rodhan et al., 1991, Wustrow et al., 1995, Sarhan et al., 1997, Bredeloux et al., 2006). Similarly, rats treated with antisense peptide nucleic acids targeting the mu opioid receptor maintained a normal antinociceptive response to NT (McMahon et al., 2001). Nevertheless, several lines of evidence reveal the existence of complex interactions between NT and opioid systems. First of all, microinjection of a mu opioid receptor agonist in the amygdala produced antinociception via NT release within the ventral PAG (Tershner and Helmstetter, 2000). Furthermore, perfusion of morphine or DAMGO in the PAG significantly increased in a naloxone-reversible manner the extracellular level of NT-like immunoreactivity (Stiller et al., 1997). In addition, morphine addiction also enhanced NT concentration in the thalamus of rats, thus suggesting that opioidergic inhibition does influence the analgesic response induced by activation of the neurotensinergic system (Morley et al., 1980). Along this line of thinking, it was demonstrated in the hot-plate test that tolerance to morphine attenuates the analgesic effects of NT (Luttinger et al., 1983, Bredeloux et al., 2006). Altogether, these results indicate that opioids interact at some level with NT neuronal circuits. There is, however, some uncertainty over the NT receptor subtype interfering with the endogenous opioid system.
The present study was thus conducted to evaluate the implication of NTS1 receptors in opioid-induced analgesia. Since we recently demonstrated that the activation of NTS1 receptors by selective NTS1 agonists reduced the nociceptive behaviors induced by the chemical irritant formalin (Roussy et al., 2008), we used the same experimental model to examine the functional interaction of opioid and NT systems. Specifically, we investigated whether morphine analgesia was affected by NTS1 gene silencing. Our results demonstrated that both male and female NTS1-deficient mice were less responsive to morphine than wild-type littermates during the persistent inflammatory phase of the formalin test. This suggests that NTS1 receptor activation is required for the full expression of the antinociceptive effects of morphine. Other approaches for characterizing potential opiate-NT interactions have, however, provided apparent opposite findings. Indeed, using acute thermal nociceptive tests, Richelson and coworkers found no difference in morphine-induced analgesia in rats pretreated with antisense peptide nucleic acids targeting NTS1 receptors (Tyler et al., 1999, McMahon et al., 2001). Different hypotheses may be raised to explain these inconsistencies. Since the antisense peptide nucleic acid strategy is reducing brain NTS1 receptor binding sites by only 35–40%, it could be argued that this might not be sufficient to significantly alter morphine-induced analgesia. Alternatively, such discrepancies regarding modulation of morphine analgesia could be related to the type of pain test used. Indeed, it has been demonstrated that anatomically or biochemically distinct neural pathways may be involved in the suppression of phasic (i.e. hot-plate and tail-flick) and tonic (i.e. formalin test) pain (Dennis and Melzack, 1979, Wall, 1984a, b, Ryan et al., 1985). Noteworthy, morphine analgesia is mediated by different neural systems in the tail-flick and formalin pain tests (Abbott and Melzack, 1982, Abbott et al., 1982, Morgan et al., 2006). Our results based on a genetic approach are also in apparent contradiction with previous pharmacological findings. Indeed, administration of the NT receptor antagonists, [D-Trp11] neurotensin or SR48692 into the RVM has been shown to potentiate the development of morphine analgesia (Urban and Smith, 1993, 1994, Smith et al., 1997). The fact that NT produces dose-dependent antinociceptive and anti-analgesic effects by acting on distinct classes of RVM neurons (ON-cells and OFF-cells) expressing different NT receptor types (NTS1 and NTS2) may explain these contrasting results (Urban and Gebhart, 1997, Urban et al., 1999, Neubert et al., 2004). The lack of potent selective NT receptor antagonists without NTS2 partial agonist properties and the potential compensatory mechanisms resulting from the generation of NTS1 null mice are however limiting all hypotheses raised to this day (Ryding et al., 2001, Kitabgi, 2002, Gama Sosa et al., 2010).
The pain-related behavioral responses, observed following formalin administration have been found to be integrated at all levels of the neuroaxis, from the spinal cord, via the brainstem to higher cerebral structures (Millan, 1999). Especially, the flinching/licking/biting behavioral manifestations have been proposed to be under supraspinal influences (Sawynok and Reid, 2001, Ceccarelli et al., 2003, You et al., 2006, Roussy et al., 2009, Lafrance et al., 2010). In an attempt to further characterize the implication of NTS1 in mediating morphine-induced analgesia, we analyzed the phenotypic changes in stereotypical nocifensive behaviors observed following formalin administration. We found by monitoring the total time spent in flinching/licking/biting that NTS1-KO mice exhibited altered responses to morphine. Hence, these results suggest that the functional interaction between opioid and NT systems affects the action of morphine on supraspinally-mediated nociceptive behaviors. These data are in accordance with recent findings demonstrating that NTS1 receptor activation modulates supraspinal nociceptive networks associated to formalin-induced tissue injury (Roussy et al., 2008, Roussy et al., 2009).
It is increasingly clear that gender is a critical determinant of responsiveness to opioid analgesics (Kest et al., 2000, Craft, 2003, Dahan et al., 2008). Here, we also used the formalin model of persistent pain to evaluate sex differences in mu opioid receptor-induced analgesia in wild-type and NTS1-KO mice. We found that the ability of morphine to block nociception was equivalent in female and male wild-type mice. Accordingly, morphine was reported to be equally potent and effective in males and females in reducing capsaicin-induced thermal hyperalgesic responses (Barrett et al., 2003). These findings contrast sharply with a wealth of data in acute somatic pain, in which morphine produces both longer-lasting and greater antinociception in male than in female rodents (Mogil et al., 2000, Loyd and Murphy, 2009). These results thus suggest that distinct mechanisms may underlie male and female sensitivity to opioid analgesia in phasic and tonic pain models (Morgan et al., 2006). We also determined whether NTS1 gene deficiency resulted in the sexually dimorphic actions of morphine. Interestingly, we noticed sex differences in opioid-induced supraspinal pain inhibition in NTS1-KO mice, morphine reducing formalin-induced nociceptive responses at 2 mg/kg and 5 mg/kg in females and males, respectively. This difference in the analgesic actions of morphine between NTS1-KO and wild-type mice of both sexes may be due to a lesser level of interaction between mu-opioid and neurotensin systems (Cicero et al., 1996). However, based on the experimental design of the present study we cannot conclude whether the reduction in morphine analgesia in NTS1-KO mice takes place at the supraspinal level rather than at the spinal level.
CONCLUSION
Mu opioid agonists, including morphine, are actually the mainstay of chronic pain management. Unfortunately, these powerful analgesic drugs induce multiple side effects and complications such as nausea, constipation, analgesic tolerance and physical dependence (Benyamin et al., 2008). Thus, these limitations have raised the necessity to develop new pain-relieving therapies. The data presented in this study lend further support to the hypothesis that complex interactions between NT and opioid systems partake in the regulation of supraspinal nociceptive processing and provide in vivo evidence for the implication of NTS1 receptors in morphine-induced analgesia in a context of persistent pain. Furthermore, as opposed to recent findings demonstrating that NTS2 receptor activation operates upstream to the opioidergic transmission (Bredeloux et al., 2006), we found here that NTS1 receptors act downstream of mu receptor stimulation. With this perspective, we could therefore suggest that reliable and effective pain relief could be reached by combining mu opioid agonists with NT analogs. Accordingly, synergistic effects of NT analogs with morphine or fentanyl have already been reported (Herman and Stachura, 1993, Boules et al., 2009). Future studies are still required to determine the temporal aspects of the antinociceptive synergistic interaction between NT and opioid systems.
Acknowledgments
This work is supported by grants from the Canadian Institutes of Health Research (CIHR, MOP-74618) and the Natural Sciences and Engineering Research Council of Canada (NSERC, 327122-06) awarded to P.S. G.R. is supported by the Sir Frederick Banting Dr. Charles Best Canada Graduate Scholarships, H.B. is recipient of Alexander Graham Bell NSERC and FRSQ fellowships, and M.L. holds a scholarship from NSERC and FRSQ. P.S. is a CIHR new investigator and director of the Sherbrooke’s Neurocience Center. L.G. holds a FRSQ Junior 1 salary support. P.S. and L.G. are members of the FRSQ-funded Centre de Recherche Clinique Étienne Lebel.
References
- Abbott FV, Melzack R. Brainstem lesions dissociate neural mechanisms of morphine analgesia in different kinds of pain. Brain Res. 1982;251:149–155. doi: 10.1016/0006-8993(82)91282-3. [DOI] [PubMed] [Google Scholar]
- Abbott FV, Melzack R, Samuel C. Morphine analgesia in tail-flick and formalin pain tests is mediated by different neural systems. Exp Neurol. 1982;75:644–651. doi: 10.1016/0014-4886(82)90031-0. [DOI] [PubMed] [Google Scholar]
- al-Rodhan NR, Richelson E, Gilbert JA, McCormick DJ, Kanba KS, Pfenning MA, Nelson A, Larson EW, Yaksh TL. Structure-antinociceptive activity of neurotensin and some novel analogues in the periaqueductal gray region of the brainstem. Brain Res. 1991;557:227–235. doi: 10.1016/0006-8993(91)90139-m. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Albonetti ME, Carli G. Sex differences in the behavioural response to persistent pain in rats. Neurosci Lett. 1994;179:79–82. doi: 10.1016/0304-3940(94)90939-3. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and mu-opioid-induced antihyperalgesia in male and female Fischer 344 rats. J Pharmacol Exp Ther. 2003;307:237–245. doi: 10.1124/jpet.103.054478. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Pert A. A mechanism for the analgesic effect of neurotensin as revealed by behavioral and electrophysiological techniques. Brain Res. 1984;324:35–42. doi: 10.1016/0006-8993(84)90619-x. [DOI] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–120. [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Boules M, Shaw A, Liang Y, Barbut D, Richelson E. NT69L, a novel analgesic, shows synergy with morphine. Brain Res. 2009;1294:22–28. doi: 10.1016/j.brainres.2009.07.086. [DOI] [PubMed] [Google Scholar]
- Bredeloux P, Costentin J, Dubuc I. Interactions between NTS2 neurotensin and opioid receptors on two nociceptive responses assessed on the hot plate test in mice. Behav Brain Res. 2006;175:399–407. doi: 10.1016/j.bbr.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Buhler AV, Choi J, Proudfit HK, Gebhart GF. Neurotensin activation of the NTR1 on spinally-projecting serotonergic neurons in the rostral ventromedial medulla is antinociceptive. Pain. 2005;114:285–294. doi: 10.1016/j.pain.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Buhler AV, Proudfit HK, Gebhart GF. Neurotensin-produced antinociception in the rostral ventromedial medulla is partially mediated by spinal cord norepinephrine. Pain. 2008;135:280–290. doi: 10.1016/j.pain.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli I, Fiorenzani P, Massafra C, Aloisi AM. Long-term ovariectomy changes formalin-induced licking in female rats: the role of estrogens. Reprod Biol Endocrinol. 2003;1:24. doi: 10.1186/1477-7827-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- Clineschmidt BV, Martin GE, Veber DF. Antinocisponsive effects of neurotensin and neurotensin-related peptides. Ann N Y Acad Sci. 1982;400:283–306. doi: 10.1111/j.1749-6632.1982.tb31576.x. [DOI] [PubMed] [Google Scholar]
- Clineschmidt BV, McGuffin JC, Bunting PB. Neurotensin: antinocisponsive action in rodents. Eur J Pharmacol. 1979a;54:129–139. doi: 10.1016/0014-2999(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Clineschmidt BV, McGuffin JC, Bunting PB. Neurotensin: antinocisponsive action in rodents. Eur J Pharmacol. 1979b;54:129–139. doi: 10.1016/0014-2999(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R. The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain. 1993;54:43–50. doi: 10.1016/0304-3959(93)90098-A. [DOI] [PubMed] [Google Scholar]
- Coquerel A, Dubuc I, Menard JF, Kitabgi P, Costentin J. Naloxone-insensitive potentiation of neurotensin hypothermic effect by the enkephalinase inhibitor thiorphan. Brain Res. 1986;398:386–389. doi: 10.1016/0006-8993(86)91501-5. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- Dennis SG, Melzack R. Comparison of phasic and tonic pain in animals. Advances in Pain Research and Therapy. 1979;3:747–760. [Google Scholar]
- Dobner PR. Neurotensin and pain modulation. Peptides. 2006;27:2405–2414. doi: 10.1016/j.peptides.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- Furuta S, Kisara K, Sakurada S, Sakurada T, Sasaki Y, Suzuki K. Structure-antinociceptive activity studies with neurotensin. Br J Pharmacol. 1984;83:43–48. doi: 10.1111/j.1476-5381.1984.tb10117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama Sosa MA, De Gasperi R, Elder GA. Animal transgenesis: an overview. Brain Struct Funct. 2010;214:91–109. doi: 10.1007/s00429-009-0230-8. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 (Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui X, Carraway RE, Dobner PR. Endogenous neurotensin facilitates visceral nociception and is required for stress-induced antinociception in mice and rats. Neuroscience. 2004;126:1023–1032. doi: 10.1016/j.neuroscience.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman ZS, Stachura Z. Pharmacological interaction between neuropeptides and pethidine or fentanyl in rat spinal cord. Pol J Pharmacol. 1993;45:481–492. [PubMed] [Google Scholar]
- Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–547. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Isenberg WM, Miao FJ, Gear RW, Green PG, Levine JD. Gender and gonadal hormone effects on vagal modulation of tonic nociception. J Pain. 2001;2:91–100. doi: 10.1054/jpai.2000.19295. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Calejesan AA, Li P, Wei F, Zhuo M. Sex differences in late behavioral response to subcutaneous formalin injection in mice. Brain Res. 1999;829:185–189. doi: 10.1016/s0006-8993(99)01353-0. [DOI] [PubMed] [Google Scholar]
- Kitabgi P. Targeting neurotensin receptors with agonists and antagonists for therapeutic purposes. Curr Opin Drug Discov Devel. 2002;5:764–776. [PubMed] [Google Scholar]
- Lafrance M, Roussy G, Belleville K, Maeno H, Beaudet N, Wada K, Sarret P. Involvement of NTS2 receptors in stress-induced analgesia. Neuroscience. 2010;166:639–652. doi: 10.1016/j.neuroscience.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Leo S, Straetemans R, D’Hooge R, Meert T. Differences in nociceptive behavioral performance between C57BL/6J, 129S6/SvEv, B6 129 F1 and NMRI mice. Behav Brain Res. 2008;190:233–242. doi: 10.1016/j.bbr.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural Plast. 2009;2009:462879. doi: 10.1155/2009/462879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttinger D, Burgess SK, Nemeroff CB, Prange AJ., Jr The effects of chronic morphine treatment on neurotensin-induced antinociception. Psychopharmacology (Berl) 1983;81:10–13. doi: 10.1007/BF00439265. [DOI] [PubMed] [Google Scholar]
- Maeno H, Yamada K, Santo-Yamada Y, Aoki K, Sun YJ, Sato E, Fukushima T, Ogura H, Araki T, Kamichi S, Kimura I, Yamano M, Maeno-Hikichi Y, Watase K, Aoki S, Kiyama H, Wada E, Wada K. Comparison of mice deficient in the high- or low-affinity neurotensin receptors, Ntsr1 or Ntsr2, reveals a novel function for Ntsr2 in thermal nociception. Brain Res. 2004;998:122–129. doi: 10.1016/j.brainres.2003.11.039. [DOI] [PubMed] [Google Scholar]
- McMahon BM, Stewart JA, Jackson J, Fauq A, McCormick DJ, Richelson E. Intraperitoneal injection of antisense peptide nucleic acids targeted to the mu receptor decreases response to morphine and receptor protein levels in rat brain. Brain Res. 2001;904:345–349. doi: 10.1016/s0006-8993(01)02511-2. [DOI] [PubMed] [Google Scholar]
- Mechanic JA, Sutton JE, Berson AE, Wu X, Kwan J, Schreiber R, Pang Z, Button DC. Involvement of the neurotensin receptor 1 in the behavioral effects of two neurotensin agonists, NT-2 and NT69L: lack of hypothermic, antinociceptive and antipsychotic actions in receptor knockout mice. Eur Neuropsychopharmacol. 2009;19:466–475. doi: 10.1016/j.euroneuro.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Morley JE, Yamada T, Walsh JH, Lamers CB, Wong H, Shulkes A, Damassa DA, Gordon J, Carlson HE, Hershman JM. Morphine addiction and withdrawal alters brain peptide concentrations. Life Sci. 1980;26:2239–2244. doi: 10.1016/0024-3205(80)90208-8. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Osbahr AJ, 3rd, Manberg PJ, Ervin GN, Prange AJ., Jr Alterations in nociception and body temperature after intracisternal administration of neurotensin, beta-endorphin, other endogenous peptides, and morphine. Proc Natl Acad Sci U S A. 1979;76:5368–5371. doi: 10.1073/pnas.76.10.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Osbahr AJ, 3rd, Nemeroff CB, Luttinger D, Mason GA, Prange AJ., Jr Neurotensin-induced antinociception in mice: antagonism by thyrotropin-releasing hormone. J Pharmacol Exp Ther. 1981;217:645–651. [PubMed] [Google Scholar]
- Perissin L, Facchin P, Porro CA. Tonic pain response in mice: effects of sex, season and time of day. Life Sci. 2003;72:897–907. doi: 10.1016/s0024-3205(02)02344-5. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Hess JF, Hey PJ, Jacobson MA, Leviten M, Lis EV, Mallorga PJ, Pascarella DM, Snyder MA, Williams JB, Zeng Z. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. J Pharmacol Exp Ther. 2002;300:305–313. doi: 10.1124/jpet.300.1.305. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M. Spatial and temporal aspects of spinal cord and brainstem activation in the formalin pain model. Prog Neurobiol. 1993;41:565–607. doi: 10.1016/0301-0082(93)90044-s. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M, Lui F, Giuliani D, Pellegrini M, Baraldi P. Independent time courses of supraspinal nociceptive activity and spinally mediated behavior during tonic pain. Pain. 2003;104:291–301. doi: 10.1016/s0304-3959(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Roussy G, Dansereau MA, Baudisson S, Ezzoubaa F, Belleville K, Beaudet N, Martinez J, Richelson E, Sarret P. Evidence for a role of NTS2 receptors in the modulation of tonic pain sensitivity. Mol Pain. 2009;5:38. doi: 10.1186/1744-8069-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussy G, Dansereau MA, Dore-Savard L, Belleville K, Beaudet N, Richelson E, Sarret P. Spinal NTS1 receptors regulate nociceptive signaling in a rat formalin tonic pain model. J Neurochem. 2008;105:1100–1114. doi: 10.1111/j.1471-4159.2007.05205.x. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Watkins LR, Mayer DJ, Maier SF. Spinal pain suppression mechanisms may differ for phasic and tonic pain. Brain Res. 1985;334:172–175. doi: 10.1016/0006-8993(85)90582-7. [DOI] [PubMed] [Google Scholar]
- Ryding AD, Sharp MG, Mullins JJ. Conditional transgenic technologies. J Endocrinol. 2001;171:1–14. doi: 10.1677/joe.0.1710001. [DOI] [PubMed] [Google Scholar]
- Sarhan S, Hitchcock JM, Grauffel CA, Wettstein JG. Comparative antipsychotic profiles of neurotensin and a related systemically active peptide agonist. Peptides. 1997;18:1223–1227. doi: 10.1016/s0196-9781(97)00145-9. [DOI] [PubMed] [Google Scholar]
- Sarret P, Beaudet A. Handbook of chemical neuroanatomy Vol. 20. Peptide Receptors, Part II. Amsterdam: Elsevier; 2003. Neurotensin receptors in the central nervous system; pp. 323–400. [Google Scholar]
- Sarret P, Beaudet N, Roussy G. NTS2 receptor: slamming the brakes on pain. Med Sci (Paris) 2007;23:11–12. doi: 10.1051/medsci/2007233s11. [DOI] [PubMed] [Google Scholar]
- Sarret P, Esdaile MJ, Perron A, Martinez J, Stroh T, Beaudet A. Potent spinal analgesia elicited through stimulation of NTS2 neurotensin receptors. J Neurosci. 2005;25:8188–8196. doi: 10.1523/JNEUROSCI.0810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J. The Formalin Test : Characteristics and Usefulness of the Model. Reviews in Analgesia. 2004;7:145–163. [Google Scholar]
- Sawynok J, Reid A. Antinociception by tricyclic antidepressants in the rat formalin test: differential effects on different behaviours following systemic and spinal administration. Pain. 2001;93:51–59. doi: 10.1016/S0304-3959(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Seta KA, Jansen HT, Kreitel KD, Lehman M, Behbehani MM. Cold water swim stress increases the expression of neurotensin mRNA in the lateral hypothalamus and medial preoptic regions of the rat brain. Brain Res Mol Brain Res. 2001;86:145–152. doi: 10.1016/s0169-328x(00)00279-5. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Hawranko AA, Monroe PJ, Gully D, Urban MO, Craig CR, Smith JP, Smith DL. Dose-dependent pain-facilitatory and -inhibitory actions of neurotensin are revealed by SR 48692, a nonpeptide neurotensin antagonist: influence on the antinociceptive effect of morphine. J Pharmacol Exp Ther. 1997;282:899–908. [PubMed] [Google Scholar]
- Smith DJ, Smith DL, Monroe PJ, Urban MO, Gully D. A non-peptide neurotensin antagonist (SR48692) potentiates the antinociceptive action of morphine in rats. Analgesia. 1995;1(4–6):750–753. [Google Scholar]
- Stiller CO, Gustafsson H, Fried K, Brodin E. Opioid-induced release of neurotensin in the periaqueductal gray matter of freely moving rats. Brain Res. 1997;774:149–158. doi: 10.1016/s0006-8993(97)81698-8. [DOI] [PubMed] [Google Scholar]
- Tershner SA, Helmstetter FJ. Antinociception produced by mu opioid receptor activation in the amygdala is partly dependent on activation of mu opioid and neurotensin receptors in the ventral periaqueductal gray. Brain Res. 2000;865:17–26. doi: 10.1016/s0006-8993(00)02179-x. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Tyler BM, Jansen K, McCormick DJ, Douglas CL, Boules M, Stewart JA, Zhao L, Lacy B, Cusack B, Fauq A, Richelson E. Peptide nucleic acids targeted to the neurotensin receptor and administered i.p. cross the blood-brain barrier and specifically reduce gene expression. Proc Natl Acad Sci U S A. 1999;96:7053–7058. doi: 10.1073/pnas.96.12.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler BM, McCormick DJ, Hoshall CV, Douglas CL, Jansen K, Lacy BW, Cusack B, Richelson E. Specific gene blockade shows that peptide nucleic acids readily enter neuronal cells in vivo. FEBS Lett. 1998;421:280–284. doi: 10.1016/s0014-5793(97)01575-5. [DOI] [PubMed] [Google Scholar]
- Urban MO, Coutinho SV, Gebhart GF. Biphasic modulation of visceral nociception by neurotensin in rat rostral ventromedial medulla. J Pharmacol Exp Ther. 1999;290:207–213. [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Characterization of biphasic modulation of spinal nociceptive transmission by neurotensin in the rat rostral ventromedial medulla. J Neurophysiol. 1997;78:1550–1562. doi: 10.1152/jn.1997.78.3.1550. [DOI] [PubMed] [Google Scholar]
- Urban MO, Smith DJ. Role of neurotensin in the nucleus raphe magnus in opioid-induced antinociception from the periaqueductal gray. J Pharmacol Exp Ther. 1993;265:580–586. [PubMed] [Google Scholar]
- Urban MO, Smith DJ. Localization of the antinociceptive and antianalgesic effects of neurotensin within the rostral ventromedial medulla. Neurosci Lett. 1994;174:21–25. doi: 10.1016/0304-3940(94)90109-0. [DOI] [PubMed] [Google Scholar]
- van Wimersma Greidanus TB, van Praag MC, Kalmann R, Rinkel GJ, Croiset G, Hoeke EC, van Egmond MA, Fekete M. Behavioral effects of neurotensin. Ann N Y Acad Sci. 1982;400:319–329. doi: 10.1111/j.1749-6632.1982.tb31578.x. [DOI] [PubMed] [Google Scholar]
- Vanegas H. To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lett. 2004;361:225–228. doi: 10.1016/j.neulet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Wall PD. Mechanisms of Acute and Chronic Pain. Advances in Pain Research and Therapy. 1984a;6:95–104. [Google Scholar]
- Wall PD. Neurophysiology of Acute and Chronic Pain. Advances in Pain Research and Therapy. 1984b;7:13–25. [Google Scholar]
- Wustrow DJ, Davis MD, Akunne HC, Corbin AE, Wiley JN, Wise LD, Heffner TG. Reduced amide bond neurotensin 8–13 mimetics with potent in vivo activity. Bioorg Med Chem Lett. 1995;5:997–1002. [Google Scholar]
- Yaksh TL, Schmauss C, Micevych PE, Abay EO, Go VL. Pharmacological studies on the application, disposition, and release of neurotensin in the spinal cord. Ann N Y Acad Sci. 1982;400:228–243. doi: 10.1111/j.1749-6632.1982.tb31572.x. [DOI] [PubMed] [Google Scholar]
- You HJ, Cao DY, Yuan B, Arendt-Nielsen L. Sex differences in the responses of spinal wide-dynamic range neurons to subcutaneous formalin and in the effects of different frequencies of conditioning electrical stimulation. Neuroscience. 2006;138:1299–1307. doi: 10.1016/j.neuroscience.2005.11.060. [DOI] [PubMed] [Google Scholar]