Abstract
We have isolated a new Drosophila mutant, satori (sat), the males of which do not court or copulate with female flies. The sat mutation comaps with fruitless (fru) at 91B and does not rescue the bisexual phenotype of fru, indicating that sat is allelic to fru (fru(sat)). The fru(sat) adult males lack a male-specific muscle, the muscle of Lawrence, as do adult males with other fru alleles. Molecular cloning and analyses of the genomic and complementary DNAs indicated that transcription of the fru locus yields several different transcripts. The sequence of fru cDNA clones revealed a long open reading frame that potentially encodes a putative transcription regulator with a BTB domain and two zinc finger motifs. In the 5' noncoding region, three putative transformer binding sites were identified in the female transcript but not in male transcripts. The fru gene is expressed in a population of brain cells, including those in the antennal lobe, that have been suggested to be involved in determination of male sexual orientation. We suggest that fru functions downstream of tra in the sex-determination cascade in some neural cells and that inappropriate sexual development of these cells in the fru mutants results in altered sexual orientation of the fly.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burtis K. C., Baker B. S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989 Mar 24;56(6):997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Castrillon D. H., Gönczy P., Alexander S., Rawson R., Eberhart C. G., Viswanathan S., DiNardo S., Wasserman S. A. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993 Oct;135(2):489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie D. A., Bate M. Innervation is essential for the development and differentiation of a sex-specific adult muscle in Drosophila melanogaster. Development. 1995 Aug;121(8):2549–2557. doi: 10.1242/dev.121.8.2549. [DOI] [PubMed] [Google Scholar]
- Dhordain P., Albagli O., Ansieau S., Koken M. H., Deweindt C., Quief S., Lantoine D., Leutz A., Kerckaert J. P., Leprince D. The BTB/POZ domain targets the LAZ3/BCL6 oncoprotein to nuclear dots and mediates homomerisation in vivo. Oncogene. 1995 Dec 21;11(12):2689–2697. [PubMed] [Google Scholar]
- Ferveur J. F., Störtkuhl K. F., Stocker R. F., Greenspan R. J. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995 Feb 10;267(5199):902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- Gailey D. A., Hall J. C. Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics. 1989 Apr;121(4):773–785. doi: 10.1093/genetics/121.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailey D. A., Taylor B. J., Hall J. C. Elements of the fruitless locus regulate development of the muscle of Lawrence, a male-specific structure in the abdomen of Drosophila melanogaster adults. Development. 1991 Nov;113(3):879–890. doi: 10.1242/dev.113.3.879. [DOI] [PubMed] [Google Scholar]
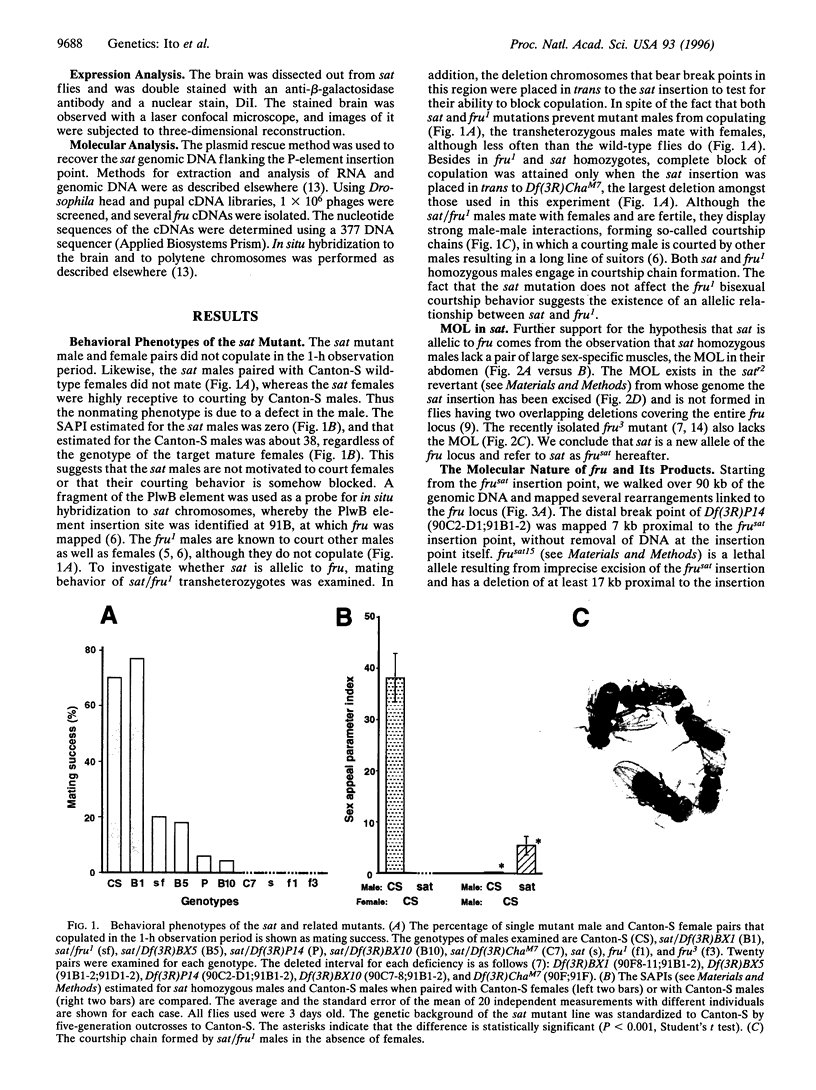
- Hall J. C. The mating of a fly. Science. 1994 Jun 17;264(5166):1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Hu S., Fambrough D., Atashi J. R., Goodman C. S., Crews S. T. The Drosophila abrupt gene encodes a BTB-zinc finger regulatory protein that controls the specificity of neuromuscular connections. Genes Dev. 1995 Dec 1;9(23):2936–2948. doi: 10.1101/gad.9.23.2936. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell. 1986 May 23;45(4):505–513. doi: 10.1016/0092-8674(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Nihonmatsu I., Kondo S., Ueda R., Togashi S., Hirata K., Ikegami Y., Yamamoto D. canoe encodes a novel protein containing a GLGF/DHR motif and functions with Notch and scabrous in common developmental pathways in Drosophila. Genes Dev. 1995 Mar 1;9(5):612–625. doi: 10.1101/gad.9.5.612. [DOI] [PubMed] [Google Scholar]
- O'Dell K. M., Armstrong J. D., Yang M. Y., Kaiser K. Functional dissection of the Drosophila mushroom bodies by selective feminization of genetically defined subcompartments. Neuron. 1995 Jul;15(1):55–61. doi: 10.1016/0896-6273(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Ryner L. C., Swain A. Sex in the '90s. Cell. 1995 May 19;81(4):483–493. doi: 10.1016/0092-8674(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Stocker R. F., Lienhard M. C., Borst A., Fischbach K. F. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990 Oct;262(1):9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- Taylor B. J. Differentiation of a male-specific muscle in Drosophila melanogaster does not require the sex-determining genes doublesex or intersex. Genetics. 1992 Sep;132(1):179–191. doi: 10.1093/genetics/132.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. J., Knittel L. M. Sex-specific differentiation of a male-specific abdominal muscle, the Muscle of Lawrence, is abnormal in hydroxyurea-treated and in fruitless male flies. Development. 1995 Sep;121(9):3079–3088. doi: 10.1242/dev.121.9.3079. [DOI] [PubMed] [Google Scholar]
- Taylor B. J., Villella A., Ryner L. C., Baker B. S., Hall J. C. Behavioral and neurobiological implications of sex-determining factors in Drosophila. Dev Genet. 1994;15(3):275–296. doi: 10.1002/dvg.1020150309. [DOI] [PubMed] [Google Scholar]
- Yokokura T., Ueda R., Yamamoto D. Phenotypic and molecular characterization of croaker, a new mating behavior mutant of Drosophila melanogaster. Jpn J Genet. 1995 Feb;70(1):103–117. doi: 10.1266/jjg.70.103. [DOI] [PubMed] [Google Scholar]
- Zhang S. D., Odenwald W. F. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5525–5529. doi: 10.1073/pnas.92.12.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollman S., Godt D., Privé G. G., Couderc J. L., Laski F. A. The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10717–10721. doi: 10.1073/pnas.91.22.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]