Abstract
Clinical and forensic toxicology laboratories are inundated with thousands of samples requiring lengthy chromatographic separations prior to mass spectrometry. Here, we employ differential mobility spectrometry (DMS) interfaced to nano-electrospray ionization-mass spectrometry to provide a rapid ion filtration technique for the separation of ions in gas phase media prior to mass spectral analysis on a DMS-integrated AB SCIEX API 3000 triple-quadrupole mass spectrometer. DMS is efficient at the rapid separation of ions under ambient conditions and provides many advantages when used as an ion filtration technique in tandem with mass spectrometry (MS) and MS/MS. Our studies evaluated DMS-MS/MS as a rapid, quantitative platform for the analysis of drug metabolites isolated from urine samples. In targeted applications, five metabolites of common drugs of abuse were effectively and rapidly separated using isopropanol and ethyl acetate as transport gas modifiers, eliminating the gas chromatography or liquid chromatography-based separations commonly employed in clinical and forensic toxicology laboratories. Calibration curves were prepared for the selected drug metabolites utilizing deuterated internal standards for quantitative purposes. The feasibility of separating and quantitating drug metabolites in a rapid fashion was evaluated by compensation voltage stepping followed by multiple reaction monitoring (MRM) detection. Rapid profiling of clinical and forensic toxicology samples could help to address an urgent need within the scientific community by developing high-throughput analytical methodologies, which could reduce significant case backlogs present within these laboratories.
Keywords: Differential mobility spectrometry - mass spectrometry, Rapid separation, Rapid quantitation, Toxicology, Drug metabolites, DMS, FAIMS, DMS-MS
Introduction
Analytical toxicology laboratories are charged with the separation and characterization of drug metabolites and poisons from a wide array of biological fluids and tissues. Gas and liquid chromatography in combination with mass spectrometry are well- established methods for the analysis of drugs and their metabolites in the pharmaceutical industry as well as in forensic and clinical toxicology laboratories. However, despite their proven effectiveness in addressing the analytical needs of most laboratories, chromatographic separation methods are often lengthy and time consuming. In particular, gas chromatographic methods usually require chemical derivatization steps for polar, non-volatile or thermally labile analytes, which may introduce further delays in the processing of samples as well as the possibility for generation of artifacts in the analysis. Use of faster separations via UPLC columns has helped to ameliorate some of these problems but the issue of high throughput analysis remains particularly acute in the case of clinical and forensic toxicology laboratories inundated with thousands of samples requiring lengthy chromatographic separations prior to characterization by mass spectrometry. Such laboratories often have lengthy turnaround times from the time samples are submitted for analysis until the results are reported to clinicians, investigators and attorneys. As a consequence, a search for alternative analytical approaches with rapid separation and quantitation capabilities remains highly desirable.
Ion mobility spectrometry (IMS), whose origins date back to the 1960’s and early 1970’s [1] has re-emerged over the past decade as a viable new approach to the orthogonal use of separation methods in combination with MS. Ion mobility separations can be accomplished in the millisecond time scale which makes the technique attractive for high throughput applications when interfaced to a mass spectrometric detector. In conventional ion mobility, ions travel through a drift tube under the influence of a weak DC electric field and their separation depends on their respective mobilities, which are in turn a function of the m/z value and the shape and size of the ions. In the 1990’s additional types of ion mobility based separation methods, which function at ambient conditions, emerged. These included methods dependent on the change of ion mobility with field such as field-asymmetric ion mobility spectrometry/differential ion mobility spectrometry (FAIMS/DMS) [1], as well as techniques that depend on the low field ion mobility such as traveling wave IMS (TWIMS) [2], differential mobility analysis – mass spectrometry (DMA-MS) [3], and aspiration ion mobility spectrometry (AIMS) [4]. We have focused on methods which depend on the change of mobility coefficient with field because of their useful dependence on chemical affinities rather than geometric cross-section, and their high resolution for small molecular ion targets [5], [6], [7]. DMS/FAIMS instrumentation can be classified as a spatial type separation; because these systems operate continuously, different ion species have distinct trajectories under the same electric field conditions. This mode is an advantage when used as an ion pre-filter for mass spectrometry because continuous operation provides efficient coupling, as opposed to conventional IMS, which suffers from a low duty cycle (injection time/drift time) and ion losses between cycles. We have employed the planar DMS design used in commercial AB SCIEX SelexION instrumentation [8] that has been found by Shvartsburg et al. [9] to provide higher selectivity and lower losses than the cylindrical configuration. In addition, planar designs enable the use of transport-gas modifiers – small organic molecules, also referred to as dopants – which enhance differential mobility separations by using reversible clustering to increase the difference between high and low field mobility [10], [11], [6], [12].
The rapid separation provided by differential mobility spectrometry, in combination with mass spectrometry, can be exploited in analytical applications. Recent work from our own laboratory has demonstrated the utility of DMS in tandem with an ion trap mass spectrometer for the rapid analysis of forensic drug (cocaine) samples adulterated with caffeine, benzocaine, theophylline, xylazine, levamisole, thiamine, diltiazem, lidocaine, diphenhydramine, tetramisole, acetaminophen, procaine, creatine, and/or creatinine [6, 13]. A primary goal of the research presented here was to illustrate the feasibility of implementing high throughput DMS-MS/MS methodologies into forensic toxicology laboratories in an effort to reduce analysis times for targeted metabolite quantitation.
In an effort to evaluate the analytical capabilities of DMS-MS/MS technology as a rapid separation and quantitation platform, DMS-MS/MS methods were developed by evaluating the conditions necessary for the effective separation of several drug metabolites from spiked urine samples. The following three considerations were evaluated during method optimization: 1) a determination of appropriate separation voltages (SV, CV), 2) the nature of the transport gas modifier and its concentration and 3) the temperature of the curtain gas.
Prior to the separation, characterization and quantitation of metabolites of interest within these samples, the analytes needed to first be isolated from biological samples. Effective strategies for the isolation of the targeted metabolites were essential before useful qualitative and quantitative DMS-MS/MS methodologies could be employed.
Materials and methods
The five metabolites investigated: norfentanyl, nordiazepam, benzoylecgonine, 6-acetylmorphine and morphine glucuronide were obtained as 1 mg/ml standards in MeOH (Cerilliant, Round Rock, TX). The internal standards: norfentanyl-D5, nordiazepam-D5, benzoylecgonine-D3, 6-acetylmorphine-D3 and morphine glucuronide-D3 were obtained as 100 μg/ml solutions in MeOH (Cerilliant, Round Rock, TX). A synthetic urine standard was also obtained from Cerilliant and served as a negative control for the preparation of the spiked samples. SPE was performed prior to the direct infusion of samples for analysis by ESI-DMS-MS. SPE cartridges were obtained from United Chemical Technologies (UCT, Bristol, PA) and consisted of 130 mg Clean Screen Xcel I columns. These columns have been designed to reduce the number of steps necessary as well as the amount of solvents consumed in comparison to traditional SPE procedures. In an effort to reduce the sample preparation time, amount of solvent consumed as well as their demonstrated high affinity for a wide range of basic drugs and metabolites, these columns were selected. The mobile phase for infusion consisted of 70 % MeOH/30 % H2O/0.1 % HCOOH v/v/v. LC/MS grade methanol and water (Optima) were obtained from Fisher Scientific and formic acid was obtained from Acros Organics. Optima ethyl acetate (Fisher Scientific) and Isopropanol (Fisher Scientific) were utilized as transport gas modifiers in the experiments conducted.
Experimental conditions: sample preparations and analysis by DMS-MS/MS
The primary metabolites of fentanyl, diazepam, cocaine, heroin and morphine were spiked into urine samples at a concentration of 5 ng/μL each, isolated by SPE and subjected to rapid separation and quantitation by DMS-MS/MS. Urine samples were spiked (in triplicate) with the primary metabolites of the five, aforementioned drugs of abuse and their corresponding deuterated analogs. The drug metabolites evaluated by DMS-MS/MS (norfentanyl, nordiazepam, benzoylecgonine, 6-acetylmorphine and morphine glucuronide) are represented in Fig. 1.
Fig. 1.
Five metabolites investigated by DMS-MS/MS for the development of rapid quantitative methods
The following procedure highlights the isolation of a selected drug metabolite (benzoylecgonine) from urine samples: Benzoylecgonine was spiked into urine samples within the concentration range of 0.1 to 10 ng/μL based on NIDA physiological values. To 1 mL of spiked urine, 1 mL of 0.1 M phosphate buffer (pH=6.0 +/− 0.5) was added. Benzoylecgnonine-d3 was utilized as an internal standard for quantitation at a concentration of 1 ng/μL and was normalized for all of the spiked samples. Samples were loaded directly onto the Xcel I SPE cartridges without any preconditioning of the stationary phase. Samples were pulled through at a rate of approximately 1 mL/min and the bed was dried for 2 min at approximately 100 psi. Next, 2 mL of 98:2 MeOH/acetic acid was used to wash the column and pulled through at a rate of approximately 1 mL/min. The bed was dried for 5 min at approximately 100 psi. Samples were eluted using 2 mL of 78:20:2 CH2Cl2/IPA/NH4OH. Samples were evaporated to dryness and reconstituted in 50 uL of 70:30:0.1 MeOH/H20/formic acid for quantitative analysis by nESI-DMS-MS/MS.
Samples were infused at 300 nL/min and ionized by nano-ESI at 3 kV into the orifice of the DMS. The compensation voltage (CV) range (typically −100 to +10 V) was scanned while the separation voltage was fixed. Once the CV peak positions for the target analytes were determined, fixed CV values were chosen for selected ion transmission into the triple quadrupole for structural analysis and quantitation by MRM. The planar DMS design utilized consists of a 1 mm filter gap, 10 mm in width and 15 mm in length, with a gas flow of 600 sccm through the DMS into the orifice of the AB SCIEX API 3000 triple quadrupole MS. In the system, the DMS ion filter is situated between the curtain plate and the orifice plate. The firmware and software are fully integrated and was developed by AB SCIEX research in Toronto, Ontario in cooperation with Sionex Corp. Effective separation of the targeted metabolites from background chemical noise was achieved in less than 30 s including time for scanning compensation voltage and signal acquisition.
Results and discussion
Development of DMS separation conditions for selected metabolites: BE and 6-AM
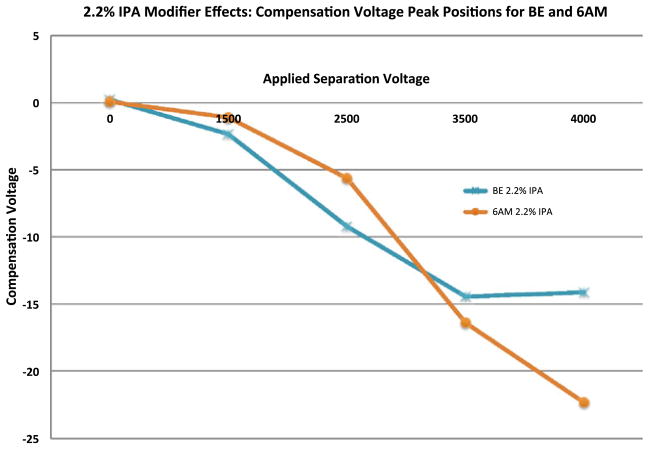
In an effort to evaluate appropriate analytical conditions for the rapid analysis and quantitation of two selected metabolites: benzoylecgonine (BE) and 6-acetylmorphine (6 AM), the optimal CV values for their selective transmission were determined by scanning the compensation voltage over the range of −40 to +5 volts. The separation voltage was fixed at the following voltages: 0, 1.5 kV, 2.5 kV, 3.5 kV and 4 kV, measured as a peak-to-peak amplitude of the two-harmonic waveform. The data for these experiments are represented in Fig. 2. The waveform shape and optimization is discussed in detail elsewhere and is not presented here [14].
Fig. 2.
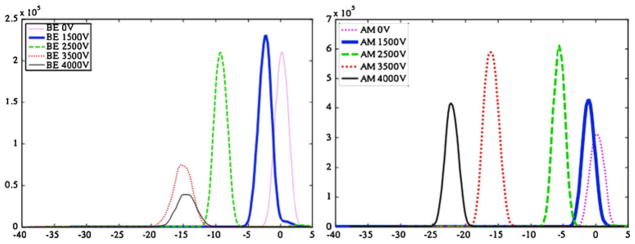
CV peak position plots for BE (left) and 6-AM (right) as a function of five applied separation voltages (SV) in the presence of 2.2 % IPA modifier introduced into the curtain gas
Isopropanol and ethyl acetate were investigated as transport gas modifiers during the separation process to increase differential mobility and ion selectivity. The modifiers were introduced into the heated curtain gas via infusion by capillary delivery at known delivery rates corresponding to desired concentrations. Calculations were performed according to the following equation to determine the percent modifier, f (mod), based upon a known curtain gas flow rate:
With the following numerical values: T (Kelvin), P (1 atm), Ftot (cm3/min), Fliq (μL/min), D (liquid density in g/cm3), M (molecular weight in g/mol) the modifier ratio is given by:
For example, performing the above calculation with the previously defined units for isopropanol: T=397, D=0.785, Fliq=20, M =60, Ftot =1,000, the isopropanol modifier concentration can be determined: fmod=0.0108 (or approximately 1 %).
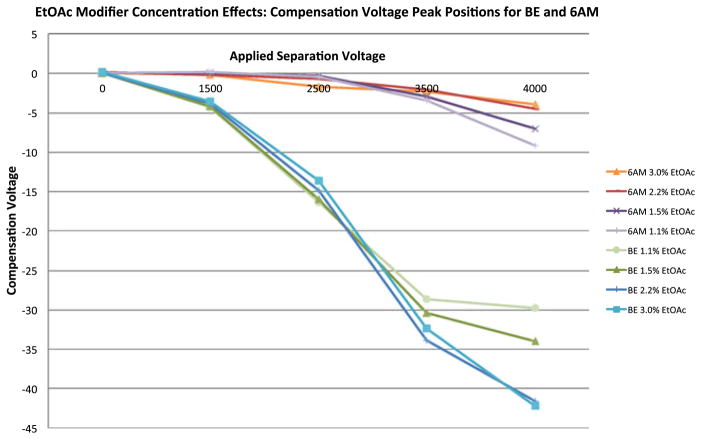
Ethyl acetate (Fig. 4) was evaluated in a concentration dependent fashion as a transport gas modifier. DMS-MS/MS method development for BE and 6 AM was accomplished by examining ethyl acetate at 1.1, 1.5, 2.2 and 3.0 % of the total curtain gas composition.
Fig. 4.
Peak position plot demonstrating the effects of EtOAc modifier concentration on the separation of BE and 6 AM at a curtain gas temperature of 170 °C. Separation voltage (SV) is represented on the x-axis (top) and compensation voltage (CV) is represented on the y-axis
The apex of the CV peak position was determined and plotted against separation voltage in an effort to evaluate trends as a function of CV peak position, separation voltage, and effective modifier concentration. Various plots (illustrated by Figs. 3 and 4) were generated to examine CV peak position effects based on the properties and concentrations of the isopropanol and ethyl acetate modifiers.
Fig. 3.
Compensation voltage peak position plot for BE and 6-AM as a function of five applied separation voltages in the presence of 2.2 % IPA modifier introduced into the curtain gas
Targeted isolation of additional metabolites from a biological matrix
Biological samples submitted for toxicological analysis often contain multiple metabolites from drugs of abuse due to the prevalence of both prescription and non-prescription drugs within society. As a result, in an effort to increase the complexity of the samples for the purposes of this study, three additional metabolites were investigated in addition to the data presented for BE and 6-AM. Nordiazepam, norfentanyl and morphine glucuronide, metabolites of diazepam, fentanyl and morphine, respectively, were spiked into urine at a concentration of 5 ng/μL prior to isolation by SPE and analysis by DMS-MS/MS. While structurally dissimilar, these metabolites were investigated and separation methods were developed due to their prevalence in clinical and forensic samples as metabolites of common drugs of abuse.
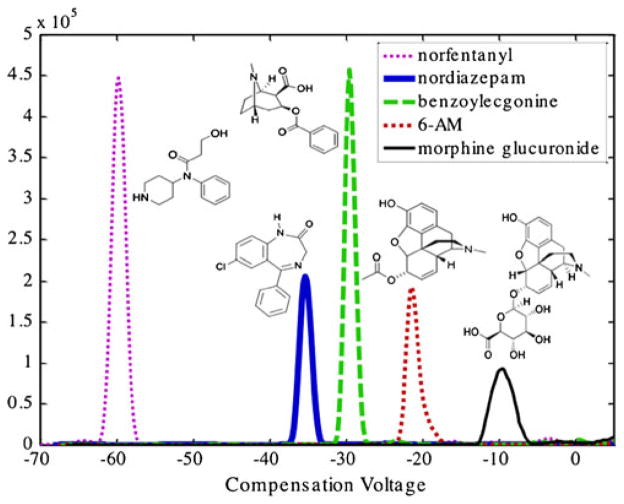
The DMS data presented in Fig. 5 show the baseline resolution of the five metabolites investigated during this study. This separation was accomplished by scanning the compensation voltage from −100 to +10 V while holding the separation voltage constant at 4,000 V. Isopropanol was introduced into the curtain gas via capillary infusion and was utilized as a transport gas modifier due to its demonstrated baseline resolution of the five metabolites of interest. It should be noted in the absence of isopropanol modifier, baseline separation of the five metabolites was not achieved (data not shown) suggesting that the choice of modifier is an important consideration in DMS-based separations.
Fig. 5.
DMS-MS/MS separation of five metabolites of common drugs of abuse with IPA as a modifier
Once the five species were resolved utilizing isopropanol as a modifier, MRM experiments were then performed to demonstrate the quantitative capabilities of the DMS-triple quadrupole platform following rapid separation.
Targeted quantitation of benzoylecgonine by DMS-MS/MS
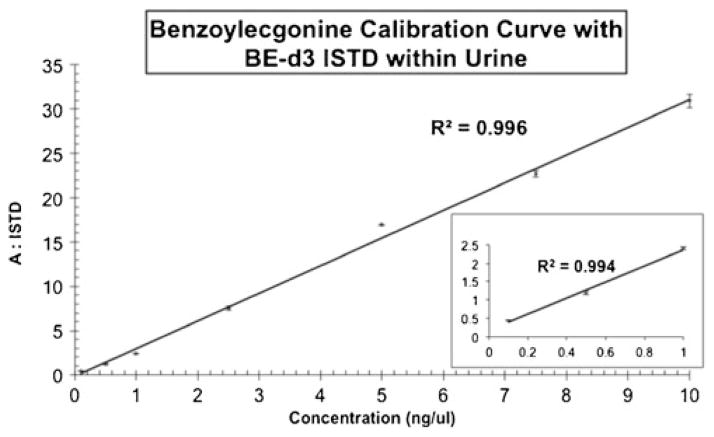
A seven point calibration curve was prepared by spiking 0.1 to 10 ng/μL of benzoylecgonine into twenty-one urine samples. Benzoylecgonine-d3 was utilized as an internal standard and was spiked at a concentration of 1 ng/μL into the samples prior to SPE isolation and was normalized for all of the spiked samples. The four additional metabolites were present at a concentration of 5 ng/μL within the urine samples. Seven samples were analyzed in triplicate in ~1.5 h as compared to ~10.5 h by traditional GC/MS based methods. Figure 6 demonstrates that the linear dynamic range of this quantitative DMS-MS/MS method for the targeted analyte (BE) was 100 pg to 10 ng for the experiments performed. The curve shows good linearity (R2=0.996) over the physiological range of interest based on SAMHSA quantitation guidelines.
Fig. 6.
Seven point calibration curve for BE with BE-d3 as an internal standard. The linear dynamic range of the quantitative DMS-MS/MS method was 100 pg to 10 ng
Conclusions
A mixture of five common metabolites of drugs of abuse was rapidly and effectively separated from urine samples by DMS prior to MS/MS analysis. Derivatization of these metabolites for traditional GC/MS analysis would typically involve silylation reactions to decrease the polarity, increase the volatility and/or decrease thermally labile drug metabolites such as BE and 6-AM. With DMS-MS methods, the analysis throughput is limited only by the delivery of samples to the DMS-MS/MS by infusion or by flow injection, and can be accelerated by the use of an auto-injector. Derivatization is not necessary when micro/nano flow rates on the order of 500 nL/min or less are used in ESI-DMS-MS/MS, saving both time and reagents. In addition to concerns related to the derivatization of compounds prior to GC/MS, the time-savings afforded by employing DMS and eliminating the necessity for chromatographic separations (GC or LC) prior to mass spectrometry provides an analytical improvement in sample throughput.
DMS-MS/MS separation and quantitation methods were developed for the primary metabolites of five common drugs of abuse, namely fentanyl, diazepam, cocaine, heroin and morphine. The DMS method development strategies described here are analogous to the development of a temperature gradient in a GC separation or mobile phase composition in an LC-based separation. A consideration of the appropriate SV and CV conditions, the nature and concentration of the modifier and the temperature of the curtain gas are essential method development considerations in DMS-based separations and could be extended to investigate the ability of DMS-MS/MS methods to separate and quantitate additional metabolites of interest. When used in MRM mode with a triple quadrupole mass spectrometer, DMS separation can lead to the development of methods for a wide range of metabolites and can serve as a viable analytical platform within forensic and clinical toxicology laboratories for the rapid quantitation of drug metabolites from urine samples in a high-throughput fashion. Based upon the data presented here, implementing DMS-MS/MS could significantly improve case backlogs in toxicology laboratories and provide investigative information in time-sensitive cases. Rapid profiling of drug metabolites from toxicology samples addresses an urgent need within the criminal justice and clinical chemistry communities to decrease the turnaround from the time of submission to the reporting of quantitative, analytical results.
Acknowledgments
The authors would like to sincerely thank Dr. Brad Schneider for his continued collaboration and for his contributions to the development of the field of differential mobility spectrometry –mass spectrometry. The authors would also like to acknowledge Dr. Jim Glick for countless hours of discussion and valuable contributions to this work. This manuscript is contribution #1021 from the Barnett Institute of Chemical and Biological Analysis. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R01CA69390.
Contributor Information
Adam B. Hall, Email: adamhall@bu.edu, Biomedical Forensic Sciences Program, Boston University School of Medicine, 72 East Concord Street, Boston, MA 02118, USA. Department of Chemistry and Chemical Biology and the Barnett Institute of Chemical and Biological Analysis, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA. Biomedical Forensic Sciences, Boston University School of Medicine, 72 East Concord Street, L-1004, Boston, MA 02118, USA
Stephen L. Coy, Department of Chemistry and Chemical Biology and the Barnett Institute of Chemical and Biological Analysis, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
Erkinjon Nazarov, Draper Laboratory - Bioengineering Center at USF, 3802 Spectrum Blvd, Suite 201, Tampa, FL 33612-9220, USA.
Paul Vouros, Department of Chemistry and Chemical Biology and the Barnett Institute of Chemical and Biological Analysis, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA.
References
- 1.Shvartsburg AA. Differential ion mobility spectrometry: nonlinear ion transport and fundamentals of FAIMS. CRC Press; Boca Raton: 2009. [Google Scholar]
- 2.Shvartsburg AA, Smith RD. Fundamentals of traveling wave Ion mobility spectrometry. Anal Chem. 2008;80(24):9689–9699. doi: 10.1021/ac8016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Mora JF. The differential mobility analyzer (DMA): adding a true mobility dimension to a preexisting API-MS. In: Wilkins CL, Sarah T, editors. Ion mobility spectrometry - mass spectrometry theory and applications. CRC Press; Boca Raton: 2010. [Google Scholar]
- 4.Adamov A, Viidanoja J, Karpanoja E, Paakkanen H, Ketola RA, Kostiainen R, Sysoev A, Kotiaho T. Interfacing an aspiration ion mobility spectrometer to a triple quadrupole mass spectrometer. Rev Sci Instrum. 2007;78(4):044101–044105. doi: 10.1063/1.2723742. [DOI] [PubMed] [Google Scholar]
- 5.Schneider B, Covey T, Coy S, Krylov E, Nazarov E. Planar differential mobility spectrometer as a pre-filter for atmospheric pressure ionization mass spectrometry. Int J Mass Spectrom. 2010;298 (1–3):45–54. doi: 10.1016/j.ijms.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall AB, Coy SL, Nazarov EG, Vouros P. Rapid separation and characterization of cocaine and cocaine cutting agents by differential mobility spectrometry-mass spectrometry. J Forensic Sci. 2012;57(3):750–756. doi: 10.1111/j.1556-4029.2011.02033.x. [DOI] [PubMed] [Google Scholar]
- 7.Coy SL, Krylov EV, Schneider BB, Covey TR, Brenner DJ, Tyburski JB, Patterson AD, Krausz KW, Fornace AJ, Nazarov EG. Detection of radiation-exposure biomarkers by differential mobility prefiltered mass spectrometry (DMS-MS) Int J Mass Spectrom. 2010;291(3):108–117. doi: 10.1016/j.ijms.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed 2012.04.24]; http://www.absciex.com/products/ion-mobility-spectrometry/ab-sciex-selexion-technology.
- 9.Shvartsburg AA, Li FM, Tang KQ, Smith RD. High-resolution field asymmetric waveform ion mobility spectrometry using new planar geometry analyzers. Anal Chem. 2006;78(11):3706–3714. doi: 10.1021/ac052020v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider BB, Covey TR, Coy SL, Krylov EV, Nazarov EG. Chemical effects in the separation process of a differential mobility/mass spectrometer system. Anal Chem. 2010;82 (5):1867–1880. doi: 10.1021/ac902571u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider BB, Covey TR, Coy SL, Krylov EV, Nazarov EG. Control of chemical effects in the separation process of a differential mobility mass spectrometer system. Eur J Mass Spectrom. 2010;16(1):57–71. doi: 10.1255/ejms.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blagojevic V, Chramow A, Schneider BB, Covey TR, Bohme DK. Differential mobility spectrometry of isomeric protonated dipeptides: modifier and field effects on ion mobility and stability. Anal Chem. 2011;83(9):3470–3476. doi: 10.1021/ac200100s. [DOI] [PubMed] [Google Scholar]
- 13.Hall A, Coy S, Kafle A, Glick J, Nazarov E, Vouros P. Extending the dynamic range of the ion trap by differential mobility filtration. J Am Soc Mass Spectrom. 2012 doi: 10.1007/s13361-013-0655-4. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krylov EV, Coy SL, Vandermey J, Schneider BB, Covey TR, Nazarov EG. Selection and generation of waveforms for differential mobility spectrometry. Rev Sci Instrum. 2010;81(2):024101–024111. doi: 10.1063/1.3284507. [DOI] [PMC free article] [PubMed] [Google Scholar]