Abstract
Regeneration of myocardium through regenerative therapy and tissue engineering is appearing as a prospective treatment modality for patients with end-stage heart failure. Focusing on this area, this review highlights the new developments and challenges in the regeneration of myocardial tissue. The role of various cell sources, calcium ion and cytokine on the functional performance of regenerative therapy is discussed. The evolution of tissue engineering and the role of tissue matrix/scaffold, cell adhesion and vascularisation on tissue engineering of cardiac tissue implant are also discussed.
Keywords: end-stage cardiac failure, new developments and challenges, regenerative therapy, tissue engineering
Introduction
The health care cost for millions of people who suffer tissue loss or end-stage organ failure increases every year. Every day thousands of people of all ages are admitted to hospitals because of the malfunction of some vital organ. According to a recent survey, the US occupies the first place as cardiovascular disease diagnostics trade.1 Europe occupies the second place and is followed by Japan. The US National Health expenditures have steeply grown from 2004 to 2009. According to the American Heart Association, cardiovascular disease (CVD) and death rates are expected to rise in China by nearly 73% by 2030.1 In India also, myocardial infarction (MI) is the main cause of death. According to a projection by the WHO and the ICMR, India will be the heart attack capital in 2020.
Treatment of patients for end-stage cardiac failure and post-ischemic complications of cardiac tissue is a challenge. Cardiac transplantation has been attempted for the treatment of end-stage cardiac failure. Because of a dearth of transplantable heart, many of these people will die. Cardiac patients with class III NYHA (New York Heart Association) heart failure caused by dilated cardiomyopathy or ischemic heart disease who are deteriorating despite maximal medical therapy, but still have some cardiac reserve, have a fair chance of survival. This review highlights the new developments and challenges in the regeneration of myocardial tissue.
Progress and Challenges in Stem Cell Regenerative Therapy
In recent years, stem cell technology is gaining pace in the management and regenerative therapy of heart failure.2 Research on cell-based regenerative therapy has focused on identifying an ideal cellular source. The optimal cell source to create an engineered myocardial patch should be easy to harvest, proliferate under controlled conditions, nonimmunogenic and should have the ability to differentiate into mature, functional cardiomyocyte. In principle, the natural electrophysiological, structural and mechanical properties of cardiomyocytes make them the ideal donor cell type. However, in practice cardiomyocytes are difficult to obtain and are sensitive to ischemic insults. Its in vitro maintenance is formidable and is allogenic.
On considering potential cells, options include embryonic stem cells and multipotent postnatal cells. Embryonic stem cells can be harvested from the inner cell mass of the blastocyst. These are well known for their pluripotency and unlimited capacity for self-renewal.3 The pluripotency of embryonic stem cells makes them an attractive cellular source. But the susceptibility of these cells for teratoma formation warns for understanding the mechanisms to control the tumorigenic properties.4 Furthermore, the political and the ethical issues dealing with the use of embryonic stem cells pose substantial challenges.5,6 Many researchers succeeded in the isolation of postnatal multipotent stem cells from various tissue sources which is displayed in Table 1.
Table 1. Various cell sources used in regenerative therapy.
| Cell sources |
|---|
| Endothelial progenitor cells7 |
| Bone marrow8,9 |
| Adipose tissue10 |
| Placenta and umbilical cord11 |
| Human amniotic fluid12 |
| Dental pulp13 |
| Skeletal muscle14 |
| Fetal cardiomyocytes15,16 |
| Skeletal myoblasts17,18 |
| Mesenchymal stem cells19 |
| Smooth muscle cells20 |
| Crude bone marrow21 |
| Fibroblasts22,23 |
| Cloned cells24 |
The most widely used cell types for cardiac cell therapy in human patients are skeletal muscle-derived progenitors or myoblasts, and crude bone marrow mononuclear cells.7 Such types of stem cells are easily available, autologous and in vitro expandable. But the demerit is their inability for differentiation into cardiac or endothelial cells. In contrast, bone marrow-derived stem cells need some responses from the target organ due to plasticity and the possibility of using the patient’s own cells.
The bone marrow-derived stem cells (BNMCs), isolated from bone marrow aspirates require no need of culture expansion and can be directly injected through coronary artery or can be engineered on a suitable scaffold. The presence of BMN stem cell progenitors in peripheral circulation made the situation more favorable to opt for these cells for cardiac tissue engineering. These stem cells are able to differentiate into endothelial cells, smooth muscle cells and cardiomyocytes. Consideration of specific surface markers like CD34+, CD133+, etc., is very crucial in dealing with these cells. Many authors reported successful differentiation of transplanted BMNCs to functional cardiomyocytes in both pre-clinical and clinical arenas.8 Still only a few recent clinical studies advocate the simple reinjection of unfractionated bone marrow cells in patients with acute MI. But such studies have been performed at the early stage after the ischemic insult. However, their relevance to chronically infarcted myocardium remains to be studied in detail.
The existence of resident stem cells in the myocardium is a clear-cut indication of natural regeneration capacity. Beltrami et al. reported the presence of Lin(-) c-kit(POS) cells in the myocardium which own the basic features of the stem cells like self renewal, multipotency and proliferation capacity. These cells were proved to be effective in the angiogenesis and the formation of differentiated myocardium at the ischemic injury site.10 Another group of researchers demonstrated the Sca-1-positive (Sca-1+) cells from adult murine hearts have stem cell like characteristics. These cells expressed cardiac-specific transcription factors, contractile proteins, showed sarcomeric arrangements and were able to beat spontaneously.25 But their mechanism of activation and signaling cascade for differentiation and their application in cardiac tissue engineering remains to be elucidated.
Forte G. et al.26 has cultured human cardiac progenitors obtained from the auricles of patients as scaffoldless engineered tissues fabricated using temperature-responsive surfaces. After engineered tissues were leant on visceral pericardium, a number of cells migrated into the murine myocardium and in the vascular walls, where they integrated in the respective textures. The study demonstrated the suitability of engineered tissues to deliver stem cells to the myocardium. Branco et al. reported small preclinical studies and suggested that such delivery of BMCs reach to the infarct site.27
Yu Suk Choi et al.28 have attempted to differentiate human adipose-derived stem cells in rat models using a tissue engineering chamber. They assigned an in vivo bioreactor system by implanting an arteriovenous loop by interposing a femoral vein graft between the femoral artery and the femoral vein at the groin region of nude rats. The loop was connected to a polycarbonate chamber, which in turn was anchored to the inguinal ligament. Onto this tissue engineering chamber they co-cultured human adipose-derived stem cells and rat cardiomyocytes which led to the effective differentiation of human adipose-derived stem cells to functional cardiomyocytes.28 Placenta and umbilical cord form one of the excellent sources of multi potent stem cells. The presence of comparatively longer telomeric DNA and higher telomerase activity of these cells is favorable as a precursor for cardiac tissue regeneration. They possess sufficient passage rate and express several markers of mesenchymal stem cells (MSCs).29
Attempts were made to use mesenchymal stem cells isolated from dental pulp. Administration of dental pulp stem cells (DPSC) in the infarcted area of rat heart aided in the repair and remodeling of the myocardium by enhancing neo-angiogenesis and by the suppression of apoptotic pathways. But the cardiac tissue engineering strategies using the DPSC are yet to emerge as a more promising route.11 Forced expression of genes like Gata4, Mef2c, Tbx5, Oct4 and Sox2 differentiate cardiac or dermal fibroblasts into cardiomyocytes like cells. Such ‘induced cardiomyocytes’ are able to express cardiac biomarkers and proteins and exhibit contractile properties. Induced-pluripotent fibroblasts are also applicable for cardiac tissue engineering.12 Research teams led by Yamanaka and Thompson also opened yet another avenue for cellular-based regenerative therapies through their demonstration of induced pluripotency in postnatal somatic cells.30 They demonstrated that the ectopic expression of a selected group of genes can dedifferentiate some of the postnatal human somatic cells to exhibit many of the hallmarks of human embryonic stem cells. These findings opened new opportunities for the potential reprogramming of postnatal somatic cells to a pluripotent state. The skin fibroblasts can be harvested autogenously and tailored to specific cell types that may be needed for tissue/organ regeneration, including heart. These studies remain only in principle and many studies are needed before the consideration of this approach in the clinical arena. Nevertheless, these results would direct the explorations for the generation of patient-tissue and disease-specific stem cells presumably without much immunological rejection. Bui QT et al.31 have reviewed potential therapeutic effects of myocardial stem cell and progenitor cell therapy delivered by various delivery modes for the treatment of infarcted myocardium and challenges in myocardial delivery and optimization of stem cells.
Role of calcium ions in regenerative therapy
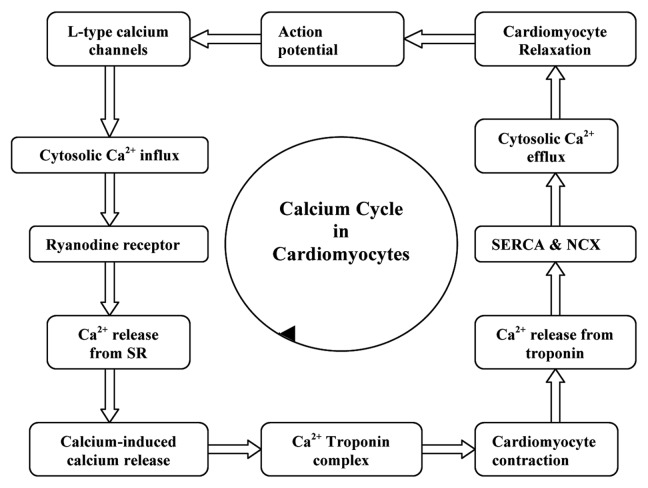
Cardiomyocyte contractility is determined by the amount of calcium released into the cytosol and the responsiveness of the contractile apparatus to calcium. Following an action potential, voltage-dependent L-type calcium channels are opened to flux the Ca2+ into the cytosol. This induces the release of calcium stored inside the sarcoplasmic reticulum (SR) via the ryanodine receptor by a process termed the calcium-induced calcium release mechanism.32 The molecular interaction between the contractile proteins, actin and myosin, imparts the contraction of cardiomyocytes which is driven by ATP hydrolysis which in turn is triggered by the increase in cytosolic calcium. Calcium binds to the troponin complex, inducing cardiomyocyte contraction by the formation of cross bridges between the actin and myosin myofilaments. The detachment of calcium from the troponin complex results in relaxation of cardiomyocytes; this calcium is effluxed from the cytosol via the sarcolemmal Na+-Ca2+ exchanger (NCX) and back into the sarcoplasmic reticulum via the sarcoplasmic reticulum Ca2+-ATPase (SERCA). The influence of calcium on the functioning of cardiomyocyte is narrated in Figure 1. The activity of SERCA is regulated by an associated protein, phospholamban. It is also reported that although the patients with heart failure have normal concentrations of SERCA II and phospholamban, the myocardial calcium uptake and calcium ATPase activity within sarcoplasmic reticulum are reduced.33,34 This results in a weak contractile force. In short, the sarcoplasmic reticulum is unable to release enough calcium during systole as a result of insufficient calcium uptake during the previous diastole.35 If the decreased sarcoplasmic reticulum calcium uptake persists, it will result in the accumulation of calcium ions in the cytosol of the cardiomyocytes.
Figure 1. Influence of calcium on the functioning of cardiomyocyte.
The Na+/Ca2+ exchanger (NCX) is a transmembrane protein antiport system, expressed in the membrane of almost every cell types. In cardiomyocytes it functions in the homeostasis of intracellular Ca2+ by the electrogenic exchange of Na+ and Ca2+ across the plasma membrane with a stoichiometry of 3 Na+ per each Ca2+. NCX operates on two modes. In the forward mode (Ca2+ extrusion mode) it will induce an inward current by the extrusion of 1 Ca2+ and the influx of 3 Na+. In the reverse mode (Ca2+ influx mode) it will generate an outward current by the extrusion of 3 Na+ and the influx of 1 Ca2+.36 The elevation of intracellular Ca2+ concentration and the deviation from normal NCX function during myocardial ischemia and reperfusion can be significantly correlated with the function and viability of cardiomyocytes.37 Oxidative stress and the activation of the Na+/Ca2+ exchanger in the reverse mode (due to the accumulation of H+ formed as a result of anaerobic glycolysis), would also produce intracellular Ca2+ overload.38 The degree of oxidative stress and the magnitude of intracellular Ca2+ overload in cardiomyocytes seem to be dependent upon the duration of ischemia. In fact, both oxidative stress and intracellular Ca2+ overload are considered to be the major mechanisms for the development of ischemic injury. The reperfusion appears to exacerbate the impact of these pathological processes. The pathological effects induced by intracellular Ca2+ overload are mediated by Ca2+-induced activation of membrane phospholipases and proteases and the associated molecular biosignaling pathways. The increases in intracellular Ca2+ are also associated with increases in L-type Ca2+ channel activity39 and a decrease in sarcoplasmic reticulum Ca2+ ATPase function.40 A recent report revealed that deficiency in nitric oxide synthase (NOS) leads to spontaneous Ca2+ fluctuations due to deficient nitrosylation of ryanodine receptors.41 Moreover, the intracellular Ca2+ overload also influences the opening of mitochondrial KATP channels and mitochondrial permeability transition pores, which activate apoptotic pathways.42
The therapeutic management of calcium overload in the post-infarcted cardiac tissue is mainly accomplished with the administration of calcium-channel blockers (CCBs) individually or in combination with other drugs like β-blockers. CCBs inhibit the L-type calcium channels of cardiomyocytes and cardiac nodal tissue (sinoatrial and atrioventricular nodes). In cardiac nodal tissue, L-type calcium channels mediate the pacemaker currents. By blocking calcium release into the cell, CCBs decrease the contraction (negative inotropy), heart rate (negative chronotropy) and conduction velocity within the heart (negative dromotropy). CCBs are used in treating hypertension, angina and arrhythmias. CCBs are of mainly three types. The first class of CCBs is phenylalkylamines (e.g., verapamil) which mainly target the heart to reduce the response to extra load. The second class, the dihydropyridines (amlodipine and nifedipine), mainly enlarge the arteries to lower the blood pressure. The third class, the benzothiazepines (diltiazem) target both the heart and the arteries. The mechanisms of action of these drugs are different and so these are administrated depending on the condition and severity of the infarction. The literatures on the tissue engineering approaches relating the post-MI calcium management are limited.
Cytokine-mediated regenerative therapy
Cytokine elaboration is one of the inherent components of the host response to tissue injury. The cytokines, released by the host myocardium plays an active role after myocardial infarction to modulate tissue repair, remodeling and adaptation after injury. They generally activate matrix metalloproteinase and collagen formation, regulation of integrins and angiogenesis. Besides, they also mediate the recruitment and mobilization of bone-marrow-derived primitive stem cells which differentiate into endothelial cells.43 The effect of cytokines can also lead to adverse conditions like acute cardiac rupture or chronic dilatation, paving the way for heart failure.44 Cytokines such as granulocyte colony-stimulating factor (G-CSF),45 stromal-derived growth factor (SDF-1),46 leukemia inhibitory factor (LIF),47 insulin-like growth factor (IGF-1),48 erythropoietin (EPO),49 etc., have critical roles in myocardial-protective effects. Hypoxia, resulted after MI stimulates the expression of hypoxia-inducible factor (HIF) which in turn induce several signaling factors.50 Moreover, the cytokines like TNF-α, IL-1β and IL-6 are also associated with the remodeling process post-myocardial infarction.51
G-CSF plays a critical role in regulation of proliferation, differentiation and survival of myeloid progenitor cells, mobilization of hemopoietic stem cells to the peripheral circulation and also stimulates healing and repair.52 EPO is important for erythrocyte survival and differentiation, vascular auto regulation and attenuation of apoptotic and inflammatory causes of cell death.53 The trafficking and survival of hematopoietic, endothelial progenitors and mesenchymal stem cells, augmentation of vasculogenesis, neovascularization in the ischemic tissues by the recruitment of endothelial progenitor cell (EPC), etc., are the major responsibilities of SDF-1.54 The local functions of various cytokines are given in Table 2. Hyun-Jae Kang et al. conducted clinical studies on 116 human subjects with acute myocardial infarction with a combination of cell and cytokine therapy using erythropoietin analog, darbepoetin and G-CSF. Though these attempts are promising, more studies are needed to correlate the effect of cytokines onto the conventional therapeutic platforms.55
Table 2. Local functions of various cytokine-mediated therapy.
| Cytokine | Local Function |
|---|---|
| G-CSF |
Stimulation of myeloid progenitor cells Mobilization of hemopoietic stem cells Healing and repair |
| EPO |
Erythrocyte survival and differentiation Vascular auto regulation Prevention of apoptosis and inflammation |
| SDF-1 |
Stimulates hematopoietic and endothelial progenitors Activates mesenchymal stem cells Neovascularization |
| TNF-α |
Downregulate myocyte contractility |
| IL-6 | Reduction of systolic cytosolic [Ca2+] |
IGF-1 is responsible for nuclear phospho-Akt and telomerase activity and the delaying of cardiomyocyte aging and death.56 TNF-α and IL-6 can attenuate myocyte contractility by the immediate reduction of systolic cytosolic (Ca2+) via alterations in sarcoplasmic reticulum function and is reversible by the removal of the cytokine signal.57 However, TNF-α can also downregulate myocyte contractility indirectly through nitric oxide-dependent attenuation of myofilament Ca2+ sensitivity.58 The remodeling signals mediated by cytokines and progenitor cells in the infarcted myocardium can also initiate the repair process which includes phagocytosis and resorption of the necrotic tissue, survival of the regenerating myocytes, degradation and synthesis of matrix, proliferation of the myofibroblasts, vasculogenesis and progenitor cell proliferation.59 Taken together, cytokine-mediated therapy is emerging to be a novel strategy for the management of end stage MI.
The anti-cytokine therapeutic agents viz. p75 TNF receptor (Fc construct, etanercept, infliximab and adalimumab) are found to reduce the inflammatory risks of MI. Certolizumab pegol is a novel TNF inhibitor which is having a comparatively high half life, since it is coupled to polyethylene glycol (PEG).60 Anti-TNF therapy was not fully successful. The main drawbacks found during clinical trials are toxicity, racial variations, polymorphism of TNF gene, adverse effects with other medications, etc. Moreover, patients with (NYHA) class III or IV heart failure are not advised to treat with anti-TNF-α medications. The same effect will occur with other cytokines also.61
Relevance and application of growth factor in regenerative therapy
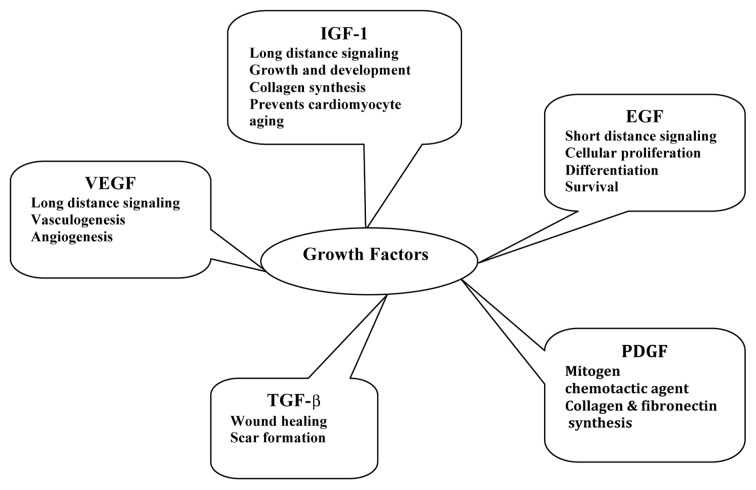
Growth factors are signaling polypeptides capable of provoking specific cellular responses in a biological environment. These can facilitate cell chemotaxis, proliferation, matrix synthesis, cell differentiation and regeneration and so on.62 After their release from platelets, polymorphonuclear leukocytes and macrophages in the injury site, growth factors bind to cell surface receptors to determine the intracellular changes to DNA synthesis and expression which result in tissue repair and regeneration.63,64 Regenerating tissues must have an ordered orchestra of several growth factors. The epidermal growth factor (EGF) confine signaling to only a short distance65 and the insulin-like growth factor-1 (IGF-1) may act at great distances66 along with vascular endothelial growth factor (VEGF).67 Insulin-like growth factor (IGF) and its isoforms exerts its role in various tissue regeneration processes by stimulating protein synthesis, cell proliferation, collagen synthesis and other biological responses. These anabolic effects of IGF have attracted the attention of many researchers.68,69 The nature and role of various growth factors are narrated in Figure 2. The transforming growth factor β (TGF-β) is a multipotent growth factor involved in wound healing and scar formation. During wound healing, TGF-β is released from degranulating platelets and is also secreted by lymphocytes, macrophages, endothelial cells, smooth muscle cells, epithelial cells and/or fibroblasts.70 PDGF is a mitogen and chemotactic agent of fibroblasts and smooth muscle cells which stimulate macrophages to secrete other growth factors important for various stages of the regenerative processes. PDGF also stimulates the production of several ECM molecules, including collagen and fibronectin.71
Figure 2. Nature and role of various growth factors in regenerative therapy.
The growth factors can be delivered as genetic material, which may be a gene or a plasmid, to the target site where it expresses the encoded growth factor. Another promising approach is the direct application of growth factors along with a carrier. The choice of the carrier is very crucial because the delivery kinetics of the growth factor strongly depends on the carrier molecule as it is immobilized in the carrier. Usually environmentally sensitive smart polymeric biomaterials are used for this purpose. But the demerit is that constant, sustained and controlled release could not be achieved in vivo.72 More studies are needed to bring these tissue engineering strategies for human application.
Evolution of Cardiac Tissue Engineering
Dynamic cardiomyoplasty, a novel surgical procedure for the treatment of end-stage heart failure was introduced by Carpentier, Magovern, Stevenson and others in 1985.
This therapy has been applied in more than 600 patients worldwide since its clinical introduction. In cardiomyoplasty, the patient's own latissimus dorsi muscle is mobilized as a pedicle graft, wrapped around the heart, and then stimulated in synchrony with cardiac systole. The key principle behind muscle-powered cardiac assistance is that with chronic electrical stimulation, skeletal muscle can be converted from fatiguing muscle to fatigue-resistant muscle. However the operative mortality in this procedure has been significant. Grafts made of materials such as expanded poly tetrafluoroethylene or glutaraldehyde-treated xeno pericardiums are used as experimental implants by some investigators as an alternate to patient's own latissimus dorsi muscle for repair of cardiac defects. However, these attempts were not successful because these grafts lack growth potential and are non-contractile.
The term “tissue engineering” was introduced in 1987 US National Science Foundation (NSF) in Washington, DC. A definition for tissue engineering was introduced in the NSF organized conference on tissue engineering in Lake Tahoe, CA, “application of principles and methods of engineering and life sciences toward fundamental understanding of structure-function relationship in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain or improve functions.”73
Tissue engineering enables the creation of man-made implantable biological substitutes known as neo-organs to restore, maintain or improve tissue functions.74 In the cardiac tissue engineering approach, the patient receives a functional living tissue fabricated using living cardiomyocytes and associated cells that are incorporated into three-dimensional scaffolds of biodegradable polymeric material in cell culture. The three-dimensional scaffolds morphology guides the tissue development in the matrix. Tissue engineering is more advantageous over cell transplantation since a functional three-dimensional tissue could be developed. The scaffold initially acts as an adhesive and physical support for the cells.75-77 The entire functional living tissue is transplanted into the wound site, where further remodeling can occur. At the same time, the artificial polymeric material breaks down, leaving only a completely natural final product in the body, a neo-organ. Thus the damaged cardiac tissue can be rejuvenated with a newly grown tissue engineered implant, which will alleviate post ischemic complications.
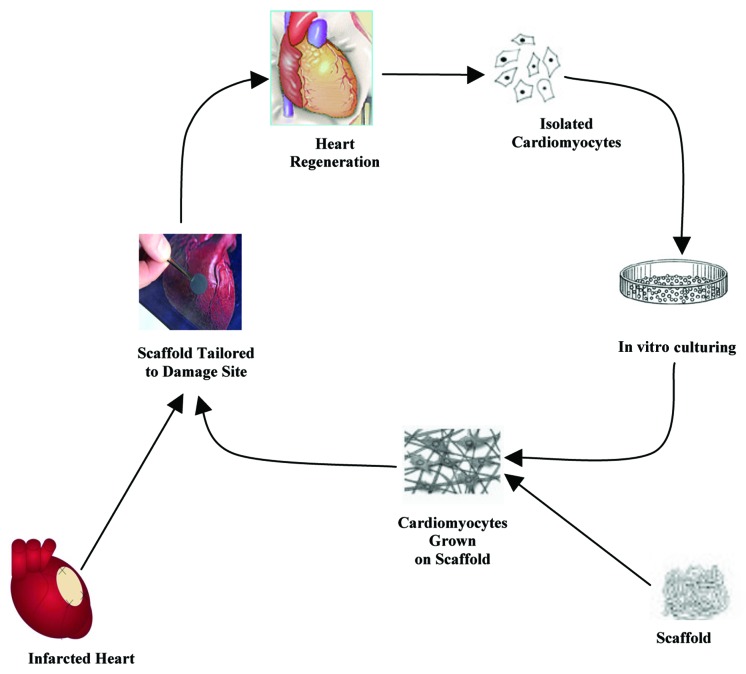
The general procedure for cardiac tissue engineering is portrayed in Figure 3. Researchers have attempted to assemble the organ parts in vitro conditions.78,79 In the late 1950s, Moscona cultivated freshly isolated cells from fetal chicken heart in an Erlenmeyer flask under proper conditions. He obtained an aggregate of cells which resemble the intact heart tissue.80 Similar explorations showed that the isolated cells from immature hearts retain the potency to reform heart-like tissues under favorable cell culture conditions.81 This approach was also applicable to most tissues other than heart. Years later, free-floating monolayer sheets that generate exogenous matrix-free cardiac tissue constructs were developed.82 Simpson and colleagues observed that neonatal rat cardiac myocytes can associate themselves to adopt a tissue-like organization.83
Figure 3. The general procedure for cardiac tissue engineering.
In the late 1980s, Vandenburgh and Terracio and their coworkers introduced computer-controlled instrumentation to study the mechanical stimulation on the heart. They also demonstrated cell orientation and differentiation.84,85 Based on the studies from embryonic fibroblasts, researchers developed an improved in vitro heart model. This model enables the measurement of contractile force and genetic, pharmacological and mechanical properties.86
The introduction of material sciences to the principles of tissue engineering by the researchers from MIT, Cambridge paved new ways to cardiac tissue engineering. They used polyglycolic acid as a scaffold in bioreactor cultures.87 Li et al. cultured fetal rat ventricular cells onto gelatin scaffolds in vitro and tailored them to infarcted rat hearts. Even though they obtained spontaneous contractile activity, they met with poor sarcomere development. The presence of many unknown cells was another demerit.88 Leor (the first to report cardiomyocytes tissue engineering) and colleagues succeeded in generating vascularization in alginate scaffolds seeded with fetal cardiac cells. But these cells failed to integrate with the host myocardium.89 Vunjak-Novakovic and team combined preformed collagen foam with neonatal rat heart cells suspended in Matrigel. With electrical stimulation for extended times they could generate cardiac muscle constructs with improved morphology, contractile function and molecular marker content.90
Cardiac tissue engineering is based on the premise that suitable matrices transmit appropriate signals for cardiac progenitor’s cells to proliferate and form new cardiac tissue. Various techniques have been developed for the engineering of beating 3D cardiac tissues. Many authors reported many advantageous variations from classical methods which guaranteed long-term effects due to biocompatibility and biodegradability of the biomaterial.
Role of tissue matrix/scaffold in tissue engineering
Regeneration of soft tissues like myocardial tissues under in vitro condition needs an appropriate scaffold in the form of a soft and pliable material. The 3D polymeric scaffolds have roles in several aspects of tissue engineering—as a platform for the regeneration of remaining healthy tissues, formation of tissue from seeded cells, modulation of tissue ingrowths from the surroundings and thereby satisfying the physiological and metabolic need of the regenerating tissues. Recent advances in materials science and bioengineering have resulted in a multitude of scaffold options.
Natural and synthetic materials are explored as scaffold material to grow the cardiac cells, the cardiomyocytes. To allow the development of myocardial tissue, the scaffold should be compatible to cell growth. The scaffold should be produced into three-dimensional porous structures that are dimensionally stable under physiological conditions. Furthermore, the mechanical properties of the scaffolding material should be adequate to provide the correct micro-stress-environment for the cells to develop the required phenotype and adaptation. Therefore the scaffold should be flexible enough to allow the contraction of the growing tissue and to withstand the back up pressure of the surrounding myocardium after implantation.
Biodegradable polymeric materials form an attractive choice because of the ease of preparation with required microstructure, bio-mechano-electrical properties and degradation profile. Biodegradable polymer could be designed to degrade in vivo in a controlled manner over a predetermined period. The advantages of biodegradable materials are (1) they do not have to be removed after use by secondary surgery because degradation products formed can be excreted from the body via natural pathways and (2) progressive loss of degradable implant material will lead to regeneration of heart tissue. Because of this convenience, synthesis of various medical devices such as sutures, orthopedic fixation devices, temporary vascular grafts, stents, tissue scaffolds, and drug delivery devices rely on the polymeric materials.91 The commonly used natural and synthetic biomaterials for soft tissue engineering applications are listed in Table 3.
Table 3. Scaffolds materials used in tissue engineering.
| Synthetic scaffolds materials | Natural scaffold materials |
|---|---|
| Poly lactic acid92 |
Fibrin glue96,97 |
| Poly glycolic acid92 |
Gelatin98 |
| Hydroxyethyl methacrylate (HEMA)93 |
Collagen99,100 |
| Methyl methacrylate (MMA)93 |
Alginate101,102 |
| Poly propylene fumarate (PPF)94 |
Chitosan103 |
| Poly(dioxanone)95 |
Hyaluronoc acid104 |
| Poly(e-caprolactone)95 |
Mussel proteins105 |
| Poly(trimethylene carbonate) copolymers96 | Elastin106 |
Poly (propylene fumarate) coploymer has also been prepared and studied for the tissue engineering of bones.107-110 Biodegradable hydrogels based on poly (caprolactone diol-co-propylene fumarate-co-ethylene glycol) with poly (ethylene glycol) and poly (caprolactone diol) chain ends and alginate have been reported for tissue engineering.111,112
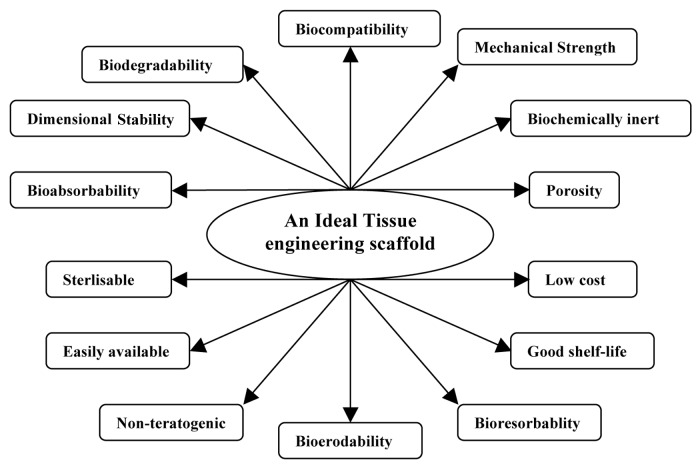
Jayabalan et al. have studied the carboxy terminated-poly(propylene fumarate)-co-ethylene glycol)-acrylamide and polyethylene glycol terminated poly(propylene fumarate)/acrylamide hydrogel scaffolds for cardiac applications. These hydrogels favor adhesion and proliferation of cardiac fibroblast cells of new born rat (Wistar) due to the formation of synergistic hydrophilic-hydrophobic surface-by-surface reorganization.113,114 Apart from the mentioned facts, a typical scaffold for implantation should meet several stringent criteria. The essential characteristics of an ideal tissue engineering scaffold are displayed on Figure 4.
Figure 4. Essential characteristics of an ideal tissue engineering scaffold.
The scaffold should be interconnected with a porous (50 microns) network to enable the migration of nutrients, accommodation of large number of cells inside the pores and their organization into a functioning tissue and removal of waste materials which all are essential for starting vascularization. The porous scaffolds were fabricated by fiber bonding, solvent casting/particulate leaching, gas foaming, freeze drying, phase separation, etc.115-117 Moreover, the scaffold should be able to release the growth factors, biosignals and other bioactive components in a regulated fashion.118 Several cardiomyocyte 3D in vitro culture systems entered the scene with synthetic polymers components as the underlying matrix. Li and associates have shown that cardiac cells can attach to scaffolds to form contractile cell polymer constructs. They constructed a viable cardiac graft that contracted spontaneously in culture conditions.119 Bursac and coworkers made electrophysiologic studies and comparison of the constructs in a cell-polymer bioreactor model system with native cardiac tissue.120 A research team led by Eschenhagen successfully induced a long-term stretch to cultured cardiomyocytes. These studies were able to focus on the important features of cardiac diseases (such as myocardial hypertrophy) associated with heart failure.121-123
Some investigators have attempted to seed and grow cardiomyocytes in a three-dimensional scaffold material to develop an implant for implantation onto the scar tissue. Kofidis et al. and Zhao et al.124,125 have used a commercially available collagen scaffold and studied the growth of cardiomyocytes to develop a contractile bioartificial myocardial tissue. Gonen-Wadmany et al.126 studied the cellular organization of cardiomyocytes within a three-dimensional hydrogel scaffold. Naito et al.127 studied the growth of fetal rat cardiac cells in thermo responsive artificial extra cellular matrix, poly (N-isopropyl acrylamide)-grafted gelatin (PNIPAM-gelatin) scaffold. Sakai et al.128 have studied the growth of cardiomyocytes in vitro in gelatin sponge and observed potent inflammatory reaction after 4 weeks of implantation due to dissolution of the sponge. McDevitt et al.129 examined spatially organized cardiomyocyte cultures on biodegradable, elastomeric polyurethane films patterned by micro contact printing of laminin lanes.
Role of cell adhesion in tissue engineering
An ideal tissue engineering scaffold should provide a substrate for transplanted cell adhesion. This will favor the localization of the cells in vivo, and serves as a stage for the formation of new tissue masses by the integration of transplanted cells and the corresponding host cells in the niche.130 Within the adhesion molecules, a defined spectrum of bioactive molecules, such as ligands for adhesion receptors, functional parts of natural growth factors, cytoskeletal elements, hormones and enzymes or synthetic regulators of cell exist. These are incorporated in defined concentrations and spatial distribution against a bioinert matrix. It would inspire the cells to orchestrate the host response toward the transplanted cells to initiate vascularization, to prevent anoikis, to improve their survival and proper functioning in the host environment.131
One of the two main strategies for the design of biocompatible artificial implants modulating the cell-material interactions is to create an inert surface hindering the adsorption of proteins and adhesion of cells. And this can prevent the activation of the immune system, blood coagulation, thrombosis, extracellular matrix deposition and other biochemical interactions. This approach is exploited for the engineering of blood-contacting devices including heart valves, smooth bioinert vascular prostheses, catheters for hemodialysis, myocardial implants, vesicles for therapeutic drug delivery and so on.132,133 The second and the more general and advanced strategy aims at generation of materials promoting attachment, migration, proliferation, differentiation, long-term viability and desired functions in a controlled fashion. These materials can be constructed “two-dimensionally or three-dimensionally” with their surfaces colonized by cells of interest for the designing of a hybrid bioartificial organ. In the long run the artificial support would be reorganized and resorbed gradually by growing cells and subsequently replaced by the newly formed extracellular matrix and differentiated cells to restore fully functional native tissue existing in the organ prior to damage.134-136
Several research groups have generated scaffold materials having better adhesive properties composed of natural polymers such as collagen.137 Promising results in the development of collagen-based matrix seeded with beating cardiomyocytes having improved adhesive property, morphology and contractile function, were developed. These patches were studied extensively in animal models by Zimmermann et al. The patch could survive with beating cells for eight weeks after engraftment on the heart of immunosuppressed rats.138-140 Similar approaches and results were obtained using alginate, a negatively charged polysaccharide from seaweed, based scaffolds by Cohen et al. They observed an intense neovascularization when compared with the control group.141
A wide variety of naturally derived or synthetic polymers, to which adhesion is regulated by adsorbed proteins, are currently being used as cell vehicles due to their intrinsic cell binding capabilities.142 Synthetic peptides mediating adhesion can also be presented to cells as self-assembling hydrogels coupled with their side chains to polymer backbones or as components of synthetic proteins consisting of the cell interactive domains.143 The utility of cell adhesion approaches should be highly specific; otherwise it can dramatically alter the cell response and enhance or diminish processes such as proliferation or differentiation. Besides the chemistry of the cell-material interface, its mechanics are also recognized to play a key role in the cell response. The stiffness of the adhesion substrate in vitro can control the differentiation of adult tissue-derived progenitor populations.144,145 However, the features of the types of cell population (primitive or committed cell populations) is unobvious.146
Controlling the adhesive cues presented to transplanted cells has an impact on their survival and the formation of new tissue structures or regeneration of damaged tissues. Introduction of hydrogel into the injured site presents specific adhesive molecules of desirable patterns for promoting the formation of complex tissue-specific structures.147 Local angiogenesis in implantable matrices also can be improved by accelerating the homing of progenitor cells via covalent fixation of adhesion molecules that interact with the cell membrane protein. The molecule L-selectin, was proposed recently to be an excellent candidate for improving local angiogenesis.148 The attachment of mesenchymal cells to artificial scaffolds can be achieved by the modification of the scaffold with adhesion molecules or associated with growth factors.149
For obtaining endothelial cell-specific adhesion, covalent bonding of synthetic peptides based on the receptor-binding domains of the cell adhesion proteins on to the graft material is required. For example integrins synthesized and expressed on the surface of the endothelial cell will recognize and bind to the tripeptide Arg-Gly-Asp (RGD).150 In vitro endothelial cell adhesion, spreading, and growth on RGD attached to starch-coated polystyrene and a fibronectin-coated surface was studied by Holland et al.151 A similar approach was made by Hubbell. They immobilized a synthetic tetrapeptide containing the sequence Arg-Glu-Asp-Val (REDV) on the nonadhesive glycophase glass and polyethylene terephthalate modified with polyethylene glycol and observed the attachment and uniform spreading of the endothelial cells. It was found to be nonthrombogenic also. Fibroblasts, vascular smooth muscle cells and platelets failed to do the same.152,153
Role of vascularization in tissue engineering
The tissue engineering enables delivery of associated growth factors to the specific site of injury mostly from the impregnated scaffolds or matrices. Still, matrix degradation and subsequent diffusion-based delivery systems with pre-programmed kinetics are appealing for growth factor delivery. These can provide controlled and sustained release for tunable times.154,155 However, in certain situations there is a need for delivery systems that respond to local environmental signals or externally applied cues in order for controlled release. This discharge on demand can be offered by the introduction of stimuli-responsive components into the delivery systems or scaffold. Various biotic and abiotic factors like pH, temperature, enzymes and ionic strength that can cleave a cross-linker were adopted to immobilize growth factor, drugs or ions or other signals. These factors will trigger the cleavage of an engineered substrate. Light, electric or magnetic fields, ultrasound, etc., can also regulate the release of growth factors.156
One of the most significant requirements for a tissue engineering scaffold is its vulnerability to induce vascular infiltration.157 Once implanted, the engineered heart muscle has to survive the ischemic period until a few new vessels invade the graft to maintain viability, integrity and function. Implanted cardiomyocytes are very sensitive to ischemia and this may lead to necrosis and apoptosis. For proper regeneration a myocardial tissue engineered graft requires persistent and consistent neovascularization or angiogenesis.
The molecular signals released by the grafted cells and the host cells will determine the extent of angiogenesis at the site of implantation. The process of vasculogenesis can occur through either sprouting or nonsprouting mechanisms. The sprouting angiogenesis involves the branching of new capillaries from preexisting vessels. The nonsprouting mechanism results from the hematopoietic precursor cells incorporated into the growing vascular bed. The signaling factors like vascular epithelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) are the key players of the scene to stimulate endothelial cell proliferation, migration and blood vessel formation.158,159 Therefore, it is preferable to incorporate various angiogenic factors into the bioengineered tissues to promote blood vessel growth.
Basic fibroblast growth factor (bFGF)-2 is a member of the FGF families of heparin-binding growth factors. FGF-2 exerts its neovasculogenic function through the interactions with various endothelial cell surface receptors, including tyrosine kinase receptors and integrins. A diverse array of free and extracellular matrix-associated molecules including MAPK via Pritein Kinase C and p21, p53 and other cell cycle regulators are also involved.160,161 In addition, FGF-2 associates with VEGF and cytokines to modulate blood vessel growth in post-infarcted heart.162
VEGF expression is mediated through HIF-1 in ischemic tissues163 and in turn is a regulator of SDF-1 and other major growth factor signals involved in neovascularisation.164 VEGF signaling aids in the retention of progenitor cells in a prevascular niche of the ischemic tissue, which is necessary for the proper development of blood vessels.165 VEGF family of proteins is also thought to stimulate platelet-derived growth factor receptors (PDGF) and thus regulate endothelial tip cell and pericyte recruitment to ischemic sites, which plays a crucial part in vascular permeability and stabilization.166 Pre-vascularization of the implanted scaffold prior to cell seeding167 and growing of endothelial cells can enhance myocyte survival rate and induce a spatial organization in 3D configuration into the bioengineered tissues.168
Summary and Future Directions
Regeneration of myocardium using regenerative therapy involving various cells is promising. Still, encountering challenges and risks like cardiac arrythmias, calcifications, scarring, teratoma and other associated complications have to be rectified. Development of an efficient and reproducible method to control and direct differentiation of stem cells to the desired cell type in vitro is more relevant and needed. A tissue engineering approach on the proper handling of the cytosolic calcium ions on the infarcted cardiomyocyte has to be initiated. Cytokines and growth factors have an important role in the remodeling process of infarcted myocardium. But the short half-life of growth factors is a major challenge. So the sustained release of growth factors is appreciated since the tissue healing mechanism persists for weeks to months. It is also essential to have spatially and temporally orchestrated distributions of several growth factors for the regenerating tissues.
For the cardiac tissue engineering, the ideal cardiac tissue construct should display functional and morphological properties of native heart muscle and remain viable after implantation. Mechanical, electrical and functional integration into the host organ should result in improved contractile function of diseased myocardium. So the construct should maintain its contractibility, electrophysiology, mechanical strength, flexibility and should meet several stringent criteria like biocompatibility, resistance to stress and strain, sterilizablity and maintain the biomechanical properties and dimensional stability until the functionally active extra cellular matrix is regenerated. Unfortunately, such an ideal biomaterial fulfilling all these qualities has not been developed yet. Novel biochemical and molecular concepts and approaches for the betterment of cell adhesion-based strategies from natural or artificial or the combination of both on cardiac tissue engineering have to be evolved. Recent advances in biochemistry and material science have shown improved cell-matrix interactions by coupling adhesive molecules and growth factors to achieve efficiency in the tissue engineering of the heart.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/19429
References
- 1.A & M Mind Power Solutions. Cardiovascular Device & Diagnosis Market January 1, 2011 Pub ID: AMPS6065538.
- 2.de Muinck ED. Gene and cell therapy for heart failure. Antioxid Redox Signal. 2009;11:2025–42. doi: 10.1089/ars.2009.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klimanskaya I, Rosenthal N, Lanza R. Derive and conquer: sourcing and differentiating stem cells for therapeutic applications. Nat Rev Drug Discov. 2008;7:131–42. doi: 10.1038/nrd2403. [DOI] [PubMed] [Google Scholar]
- 4.Lees JG, Lim SA, Croll T, Williams G, Lui S, Cooper-White J, et al. Transplantation of 3D scaffolds seeded with human embryonic stem cells: biological features of surrogate tissue and teratoma-forming potential. Regen Med. 2007;2:289–300. doi: 10.2217/17460751.2.3.289. [DOI] [PubMed] [Google Scholar]
- 5.Weissman IL. Medicine: politic stem cells. Nature. 2006;439:145–7. doi: 10.1038/439145a. [DOI] [PubMed] [Google Scholar]
- 6.Weissman IL. Stem cells--scientific, medical, and political issues. N Engl J Med. 2002;346:1576–9. doi: 10.1056/NEJMsb020693. [DOI] [PubMed] [Google Scholar]
- 7.Ryu JH, Kim IK, Cho SW, Cho MC, Hwang KK, Piao H, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319–26. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 8.Kellar RS, Landeen LK, Shepherd BR, Naughton GK, Ratcliffe A, Williams SK. Scaffold-based three-dimensional human fibroblast culture provides a structural matrix that supports angiogenesis in infarcted heart tissue. Circulation. 2001;104:2063–8. doi: 10.1161/hc4201.097192. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu T. Myocardial Tissue Engineering. In: Eberli D, ed. Tissue Engineering for Tissue and Organ Regeneration. InTech, 2011. [Google Scholar]
- 10.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/S0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 12.Gandia C, Armiñan A, Garci´a-Verdugo JM, Lledo´ E, Ruiz A, Miñana MD, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–45. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 13.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1:333–42. doi: 10.1016/S1534-5807(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 15.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:II63–9. doi: 10.1161/01.CIR.100.suppl_2.II-63. [DOI] [PubMed] [Google Scholar]
- 16.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, et al. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation. 2000;102:III56–61. doi: 10.1161/01.CIR.102.suppl3.III-56. [DOI] [PubMed] [Google Scholar]
- 17.Kamelger FS, Marksteiner R, Margreiter E, Klima G, Wechselberger G, Hering S, et al. A comparative study of three different biomaterials in the engineering of skeletal muscle using a rat animal model. Biomaterials. 2004;25:1649–55. doi: 10.1016/S0142-9612(03)00520-9. [DOI] [PubMed] [Google Scholar]
- 18.Li RK. Cell transplantation to improve heart function: cell or matrix. Yonsei Med J. 2004;45:S72–3. [Google Scholar]
- 19.Krupnick AS, Kreisel D, Szeto WY, Popma SH, Rosengard BR. A murine model of left ventricular tissue engineering. J Heart Lung Transplant. 2001;20:197–8. doi: 10.1016/S1053-2498(00)00417-4. [DOI] [PubMed] [Google Scholar]
- 20.Matsubayashi K, Fedak PW, Mickle DA, Weisel RD, Ozawa T, Li RK. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation. 2003;108(Suppl 1):II219–25. doi: 10.1161/01.cir.0000087450.34497.9a. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Rabkin-Aikawa E, Guleserian KJ, Perry TE, Masuda Y, Sutherland FWH, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–7. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 22.Li RK, Yau TM, Weisel RD, Mickle DA, Sakai T, Choi A, et al. Construction of a bioengineered cardiac graft. J Thorac Cardiovasc Surg. 2000;119:368–75. doi: 10.1016/S0022-5223(00)70193-0. [DOI] [PubMed] [Google Scholar]
- 23.Lanza R, Moore MA, Wakayama T, Perry AC, Shieh JH, Hendrikx J, et al. Regeneration of the infarcted heart with stem cells derived by nuclear transplantation. Circ Res. 2004;94:820–7. doi: 10.1161/01.RES.0000120863.53562.DF. [DOI] [PubMed] [Google Scholar]
- 24.Lee MS, Makkar RR. Stem-cell transplantation in myocardial infarction: a status report. Ann Intern Med. 2004;140:729–37. doi: 10.7326/0003-4819-140-9-200405040-00013. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 27.Forte G, Pietronave S, Nardone G, Zamperone A, Magnani E, Pagliari S, et al. Human cardiac progenitor cell grafts as unrestricted source of supernumerary cardiac cells in healthy murine hearts. Stem Cells. 2011;29:2051–61. doi: 10.1002/stem.763. [DOI] [PubMed] [Google Scholar]
- 28.Branco E, Fioretto ET, Cabral R, Palmera CA, Gregores GB, Stopiglia AJ, et al. Myocardial homing after intrapericardial infusion of bone marrow mononuclear cells. Arq Bras Cardiol. 2009;93:e50–3. doi: 10.1590/S0066-782X2009000900021. [DOI] [PubMed] [Google Scholar]
- 29.Choi YS, Matsuda K, Dusting GJ, Morrison WA, Dilley RJ. Engineering cardiac tissue in vivo from human adipose-derived stem cells. Biomaterials. 2010;31:2236–42. doi: 10.1016/j.biomaterials.2009.11.097. [DOI] [PubMed] [Google Scholar]
- 30.Pojda Z, Machaj EK, Ołdak T, Gajkowska A, Jastrzewska M. Nonhematopoietic stem cells of fetal origin--how much of today’s enthusiasm will pass the time test? Folia Histochem Cytobiol. 2005;43:209–12. [PubMed] [Google Scholar]
- 31.Bui QT, Gertz ZM, Wilensky RL. Intracoronary delivery of bone-marrow-derived stem cells. Stem Cell Res Ther. 2010;1:29. doi: 10.1186/scrt29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 33.Pieske B, Kretschmann B, Meyer M, Holubarsch C, Weirich J, Posival H, et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–78. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 34.Schwinger RH, Böhm M, Schmidt U, Karczewski P, Bavendiek U, Flesch M, et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca(2+)-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92:3220–8. doi: 10.1161/01.cir.92.11.3220. [DOI] [PubMed] [Google Scholar]
- 35.Field ML, Azzawi A, Unitt JF, Seymour AM, Henderson C, Radda GK. Intracellular [Ca2+] staircase in the isovolumic pressure--frequency relationship of Langendorff-perfused rat heart. J Mol Cell Cardiol. 1996;28:65–77. doi: 10.1006/jmcc.1996.0007. [DOI] [PubMed] [Google Scholar]
- 36.Egger M, Niggli E. Regulatory function of Na-Ca exchange in the heart: milestones and outlook. J Membr Biol. 1999;168:107–30. doi: 10.1007/s002329900502. [DOI] [PubMed] [Google Scholar]
- 37.Silverman HS, Stern MD. Ionic basis of ischaemic cardiac injury: insights from cellular studies. Cardiovasc Res. 1994;28:581–97. doi: 10.1093/cvr/28.5.581. [DOI] [PubMed] [Google Scholar]
- 38.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000;47:446–56. doi: 10.1016/S0008-6363(00)00078-X. [DOI] [PubMed] [Google Scholar]
- 39.Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, et al. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–9. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- 40.Burkard N, Rokita AG, Kaufmann SG, Hallhuber M, Wu R, Hu K, et al. Conditional neuronal nitric oxide synthase overexpression impairs myocardial contractility. Circ Res. 2007;100:e32–44. doi: 10.1161/01.RES.0000259042.04576.6a. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–7. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minezaki KK, Suleiman MS, Chapman RA. Changes in mitochondrial function induced in isolated guinea-pig ventricular myocytes by calcium overload. J Physiol. 1994;476:459–71. doi: 10.1113/jphysiol.1994.sp020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minatoguchi S, Takemura G, Chen XH, Wang N, Uno Y, Koda M, et al. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. 2004;109:2572–80. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- 44.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 45.White HD, Norris RM, Brown MA, Brandt PWT, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.CIR.76.1.44. [DOI] [PubMed] [Google Scholar]
- 46.De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, et al. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103:2452–9. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 47.Zou Y, Takano H, Mizukami M, Akazawa H, Qin Y, Toko H, et al. Leukemia inhibitory factor enhances survival of cardiomyocytes and induces regeneration of myocardium after myocardial infarction. Circulation. 2003;108:748–53. doi: 10.1161/01.CIR.0000081773.76337.44. [DOI] [PubMed] [Google Scholar]
- 48.Musarò A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, et al. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2004;101:1206–10. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100:4802–6. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenza GL. Development of novel therapeutic strategies that target HIF-1. Expert Opin Ther Targets. 2006;10:267–80. doi: 10.1517/14728222.10.2.267. [DOI] [PubMed] [Google Scholar]
- 51.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–53. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 52.Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, et al. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004;18:851–3. doi: 10.1096/fj.03-0637fje. [DOI] [PubMed] [Google Scholar]
- 53.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–6. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 54.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 55.Kang HJ, Kim MK, Kim MG, Choi DJ, Yoon JH, Park YB, et al. A multicenter, prospective, randomized, controlled trial evaluating the safety and efficacy of intracoronary cell infusion mobilized with granulocyte colony-stimulating factor and darbepoetin after acute myocardial infarction: study design and rationale of the ‘MAGIC cell-5-combination cytokine trial’. Trials. 2011;12:33. doi: 10.1186/1745-6215-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535–42. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303–12. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–33. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 59.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–3. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 60.Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16:3152–67. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- 61.Sarzi-Puttini P, Atzeni F, Shoenfeld Y, Ferraccioli G. TNF-alpha, rheumatoid arthritis, and heart failure: a rheumatological dilemma. Autoimmun Rev. 2005;4:153–61. doi: 10.1016/j.autrev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell. 1991;64:271–80. doi: 10.1016/0092-8674(91)90638-F. [DOI] [PubMed] [Google Scholar]
- 63.Sharma P, Maffulli N. Basic biology of tendon injury and healing. Surgeon. 2005;3:309–16. doi: 10.1016/S1479-666X(05)80109-X. [DOI] [PubMed] [Google Scholar]
- 64.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–90. [PubMed] [Google Scholar]
- 65.DeWitt A, Iida T, Lam HY, Hill V, Wiley HS, Lauffenburger DA. Affinity regulates spatial range of EGF receptor autocrine ligand binding. Dev Biol. 2002;250:305–16. doi: 10.1006/dbio.2002.0807. [DOI] [PubMed] [Google Scholar]
- 66.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, et al. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–52. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–27. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abrahamsson SO, Lohmander S. Differential effects of insulin-like growth factor-I on matrix and DNA synthesis in various regions and types of rabbit tendons. J Orthop Res. 1996;14:370–6. doi: 10.1002/jor.1100140305. [DOI] [PubMed] [Google Scholar]
- 69.Banes AJ, Tsuzaki M, Hu P, Brigman B, Brown T, Almekinders L, et al. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J Biomech. 1995;28:1505–13. doi: 10.1016/0021-9290(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 70.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–30. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 72.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 74.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280:H168–78. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 75.Zammaretti P, Jaconi M. Cardiac tissue engineering: regeneration of the wounded heart. Curr Opin Biotechnol. 2004;15:430–4. doi: 10.1016/j.copbio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Zimmermann WH, Melnychenko I, Eschenhagen T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 2004;25:1639–47. doi: 10.1016/S0142-9612(03)00521-0. [DOI] [PubMed] [Google Scholar]
- 77.de Muinck ED. Gene and cell therapy for heart failure. Antioxid Redox Signal. 2009;11:2025–42. doi: 10.1089/ars.2009.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fischer A. The interaction of two fragments of pulsating heart tissue. J Exp Med. 1924;39:577–84. doi: 10.1084/jem.39.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lewis MR. Muscular contraction in tissue cultures. Contrib Embryol Carnegie Inst. 1920;9:191–212. [Google Scholar]
- 80.Moscona AA. Tissues from dissociated cells. Sci Am. 1959;200:132–4, passim. doi: 10.1038/scientificamerican0559-132. [DOI] [PubMed] [Google Scholar]
- 81.McDonald TF, Sachs HG, DeHaan RL. Development of sensitivity to tetrodotoxin in beating chick embryo hearts, single cells, and aggregates. Science. 1972;176:1248–50. doi: 10.1126/science.176.4040.1248. [DOI] [PubMed] [Google Scholar]
- 82.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 83.Simpson DG, Terracio L, Terracio M, Price RL, Turner DC, Borg TK. Modulation of cardiac myocyte phenotype in vitro by the composition and orientation of the extracellular matrix. J Cell Physiol. 1994;161:89–105. doi: 10.1002/jcp.1041610112. [DOI] [PubMed] [Google Scholar]
- 84.Terracio L, Miller B, Borg TK. Effects of cyclic mechanical stimulation of the cellular components of the heart: in vitro. In Vitro Cell Dev Biol. 1988;24:53–8. doi: 10.1007/BF02623815. [DOI] [PubMed] [Google Scholar]
- 85.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell Dev Biol. 1988;24:166–74. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 86.Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993;268:23850–5. [PubMed] [Google Scholar]
- 87.Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, et al. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol Bioeng. 1999;64:580–9. doi: 10.1002/(SICI)1097-0290(19990905)64:5<580::AID-BIT8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 88.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100(Suppl):II63–9. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 89.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, et al. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation. 2000;102(Suppl 3):III56–61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 90.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46. doi: 10.1016/S0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 92.Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224–30. doi: 10.1016/S0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 93.van Wachem PB, Hogt AH, Beugeling T, Feijen J, Bantjes A, Detmers JP, et al. Adhesion of cultured human endothelial cells onto methacrylate polymers with varying surface wettability and charge. Biomaterials. 1987;8:323–8. doi: 10.1016/0142-9612(87)90001-9. [DOI] [PubMed] [Google Scholar]
- 94.Krishna L, Jayabalan M. Synthesis and characterization of biodegradable poly (ethylene glycol) and poly (caprolactone diol) end capped poly (propylene fumarate) cross linked amphiphilic hydrogel as tissue engineering scaffold material. J Mater Sci Mater Med. 2009;20(Suppl 1):S115–22. doi: 10.1007/s10856-008-3493-3. [DOI] [PubMed] [Google Scholar]
- 95.Barrows TH. Degradable implant materials: a review of synthetic absorbable polymers and their applications. Clin Mater. 1986;1:233–57. doi: 10.1016/S0267-6605(86)80015-4. [DOI] [Google Scholar]
- 96.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res. 2004;94:1124–32. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 97.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF--fibrin matrices for endothelialization. J Control Release. 2001;72:101–13. doi: 10.1016/S0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 98.Rosellini E, Cristallini C, Barbani N, Vozzi G, Giusti P. Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering. J Biomed Mater Res A. 2009;91:447–53. doi: 10.1002/jbm.a.32216. [DOI] [PubMed] [Google Scholar]
- 99.Steffens GC, Yao C, Pre´vel P, Markowicz M, Schenck P, Noah EM, et al. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng. 2004;10:1502–9. doi: 10.1089/ten.2004.10.1502. [DOI] [PubMed] [Google Scholar]
- 100.Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, et al. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–20. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 101.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/S0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 102.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–23. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 103.Li Z, Guan J. Hydrogels for Cardiac Tissue Engineering. Polymers. 2011;3:740–61. doi: 10.3390/polym3020740. [DOI] [Google Scholar]
- 104.Di Felice V, De Luca A, Serradifalco C, Di Marco P, Verin L, Motta A, et al. Adult stem cells, scaffolds for in vivo and in vitro myocardial tissue engineering. Ital J Anat Embryol. 2010;115:65–9. [PubMed] [Google Scholar]
- 105.Dalsin JL, Hu BH, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125:4253–8. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 106.Lu Q, Ganesan K, Simionescu DT, Vyavahare NR. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials. 2004;25:5227–37. doi: 10.1016/j.biomaterials.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 107.Jayabalan M, Thomas V, Sreelatha PK. Studies on poly(propylene fumarate-co-ethylene glycol) based bone cement. Biomed Mater Eng. 2000;10:57–71. [PubMed] [Google Scholar]
- 108.Timmer MD, Ambrose CG, Mikos AG. In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)-diacrylate. Biomaterials. 2003;24:571–7. doi: 10.1016/S0142-9612(02)00368-X. [DOI] [PubMed] [Google Scholar]
- 109.Jayabalan M. Studies on Poly(propylene fumarate-co-caprolactone diol) Thermoset Composites towards the Development of Biodegradable Bone Fixation Devices. Int J Biomater 2009; 2009:486710. [DOI] [PMC free article] [PubMed]
- 110.Dean D, Topham NS, Meneghetti SC, Wolfe MS, Jepsen K, He S, et al. Poly(propylene fumarate) and poly(DL-lactic-co-glycolic acid) as scaffold materials for solid and foam-coated composite tissue-engineered constructs for cranial reconstruction. Tissue Eng. 2003;9:495–504. doi: 10.1089/107632703322066679. [DOI] [PubMed] [Google Scholar]
- 111.Krishna L, Jayabalan M. Synthesis and characterization of biodegradable poly (ethylene glycol) and poly (caprolactone diol) end capped poly (propylene fumarate) cross linked amphiphilic hydrogel as tissue engineering scaffold material. J Mater Sci Mater Med. 2009;20(Suppl 1):S115–22. doi: 10.1007/s10856-008-3493-3. [DOI] [PubMed] [Google Scholar]
- 112.Krishna L, Jayabalan M. Studies on injectable and biodegradable poly(ethylene glycol) end capped poly(propylene fumarate) – alginate hydrogel as tissue engineering scaffold material. J Mater Sci Mater Med. 2009;20(Suppl 1):S115–22. doi: 10.1007/s10856-008-3493-3. [DOI] [PubMed] [Google Scholar]
- 113.Kallukalam BC, Jayabalan M, Sankar V. Studies on chemically crosslinkable carboxy terminated-poly(propylene fumarate-co-ethylene glycol)-acrylamide hydrogel as an injectable biomaterial. Biomed Mater. 2009;4:015002. doi: 10.1088/1748-6041/4/1/015002. [DOI] [PubMed] [Google Scholar]
- 114.Kallukalam BC, Jayabalan M, Sankar V. Injectable polyethylene glycol terminated poly(propylene fumarate)/acrylamide biodegradable materials for cardiac applications. Hacettepe Journal of Biology and Chemistry. 2008;36:283–90. [Google Scholar]
- 115.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323–30. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 116.Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–22. doi: 10.1016/0142-9612(96)87284-X. [DOI] [PubMed] [Google Scholar]
- 117.Nam YS, Park TG. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials. 1999;20:1783–90. doi: 10.1016/S0142-9612(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 118.Leor J, Cohen S. Myocardial tissue engineering: creating a muscle patch for a wounded heart. Ann N Y Acad Sci. 2004;1015:312–9. doi: 10.1196/annals.1302.026. [DOI] [PubMed] [Google Scholar]
- 119.Li RK, Yau TM, Weisel RD, Mickle DA, Sakai T, Choi A, et al. Construction of a bioengineered cardiac graft. J Thorac Cardiovasc Surg. 2000;119:368–75. doi: 10.1016/S0022-5223(00)70193-0. [DOI] [PubMed] [Google Scholar]
- 120.Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol. 1999;277:H433–44. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 121.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11:683–94. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 122.Fink C, Ergün S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669–79. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 123.Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68:106–14. doi: 10.1002/(SICI)1097-0290(20000405)68:1<106::AID-BIT13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 124.Kofidis T, Akhyari P, Wachsmann B, Boublik J, Mueller-Stahl K, Leyh R, et al. A novel bioartificial myocardial tissue and its prospective use in cardiac surgery. Eur J Cardiothorac Surg. 2002;22:238–43. doi: 10.1016/S1010-7940(02)00256-7. [DOI] [PubMed] [Google Scholar]
- 125.Zhao YS, Wang CY, Guo XM, Zhang XZ, Wang XL, Qiao Y, et al. [Experimental study of tissue-engineered heart tissue using type I collagen as scaffold] Zhonghua Yi Xue Za Zhi. 2004;84:766–70. [PubMed] [Google Scholar]
- 126.Gonen-Wadmany M, Gepstein L, Seliktar D. Controlling the cellular organization of tissue-engineered cardiac constructs. Ann N Y Acad Sci. 2004;1015:299–311. doi: 10.1196/annals.1302.025. [DOI] [PubMed] [Google Scholar]
- 127.Naito H, Takewa Y, Mizuno T, Ohya S, Nakayama Y, Tatsumi E, et al. Three-dimensional cardiac tissue engineering using a thermoresponsive artificial extracellular matrix. ASAIO J. 2004;50:344–8. doi: 10.1097/01.mat.0000132656.42232.e3. [DOI] [PubMed] [Google Scholar]
- 128.Sakai T, Li RK, Weisel RD, Mickle DA, Kim ET, Jia ZQ, et al. The fate of a tissue-engineered cardiac graft in the right ventricular outflow tract of the rat. J Thorac Cardiovasc Surg. 2001;121:932–42. doi: 10.1067/mtc.2001.113600. [DOI] [PubMed] [Google Scholar]
- 129.McDevitt TC, Woodhouse KA, Hauschka SD, Murry CE, Stayton PS. Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. J Biomed Mater Res A. 2003;66:586–95. doi: 10.1002/jbm.a.10504. [DOI] [PubMed] [Google Scholar]
- 130.Hanjaya-Putra D, Bose V, Shen YI, Yee J, Khetan S, Fox-Talbot K, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804–15. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nerem RM. Cell-based therapies: from basic biology to replacement, repair, and regeneration. Biomaterials. 2007;28:5074–7. doi: 10.1016/j.biomaterials.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 132.Photos PJ, Bačáková L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90:323–34. doi: 10.1016/S0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 133.Bernacca GM, Straub I, Wheatley DJ. Mechanical and morphological study of biostable polyurethane heart valve leaflets explanted from sheep. J Biomed Mater Res. 2002;61:138–45. doi: 10.1002/jbm.10149. [DOI] [PubMed] [Google Scholar]
- 134.Tassiopoulos AK, Greisler HP. Angiogenic mechanisms of endothelialization of cardiovascular implants: a review of recent investigative strategies. J Biomater Sci Polym Ed. 2000;11:1275–84. doi: 10.1163/156856200744200. [DOI] [PubMed] [Google Scholar]
- 135.Nöth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002;8:131–44. doi: 10.1089/107632702753503126. [DOI] [PubMed] [Google Scholar]
- 136.Wang DA, Ji J, Sun YH, Shen JC, Feng LX, Elisseeff JH. In situ immobilization of proteins and RGD peptide on polyurethane surfaces via poly(ethylene oxide) coupling polymers for human endothelial cell growth. Biomacromolecules. 2002;3:1286–95. doi: 10.1021/bm0255950. [DOI] [PubMed] [Google Scholar]
- 137.Fromstein JD, Zandstra PW, Alperin C, Rockwood D, Rabolt JF, Woodhouse KA. Seeding bioreactor-produced embryonic stem cell-derived cardiomyocytes on different porous, degradable, polyurethane scaffolds reveals the effect of scaffold architecture on cell morphology. Tissue Eng Part A. 2008;14:369–78. doi: 10.1089/tea.2006.0410. [DOI] [PubMed] [Google Scholar]
- 138.Zimmermann WH, Melnychenko I, Eschenhagen T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 2004;25:1639–47. doi: 10.1016/S0142-9612(03)00521-0. [DOI] [PubMed] [Google Scholar]
- 139.Zimmermann WH, Eschenhagen T. Cardiac tissue engineering for replacement therapy. Heart Fail Rev. 2003;8:259–69. doi: 10.1023/A:1024725818835. [DOI] [PubMed] [Google Scholar]
- 140.Zimmermann WH, Eschenhagen T. Tissue engineering of aortic heart valves. Cardiovasc Res. 2003;60:460–2. doi: 10.1016/j.cardiores.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 141.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80:305–12. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 142.Silva EA, Mooney DJ. Synthetic extracellular matrices for tissue engineering and regeneration. Curr Top Dev Biol. 2004;64:181–205. doi: 10.1016/S0070-2153(04)64008-7. [DOI] [PubMed] [Google Scholar]
- 143.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 144.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 145.Gwak SJ, Bhang SH, Kim IK, Kim SS, Cho SW, Jeon O, et al. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials. 2008;29:844–56. doi: 10.1016/j.biomaterials.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 146.Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85:145–56. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 147.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci U S A. 2006;103:2494–9. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Suuronen EJ, Cao X, Melhuish A, Zhang P, Mckee D, Li F, et al. Cell-based angiogenic therapy without cell transplantation: enhanced engraftment of endogenous circulating progenitor cells using an acellular matrix-bound ligand. FASEB J. 2009;23:1447–58. doi: 10.1096/fj.08-111054. [DOI] [PubMed] [Google Scholar]
- 149.Shi J, Dong N, Sun Z. [Impact of immobilized RGD peptides on cell attachment of decellularized valve scaffolds] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2008;25:388–92. [PubMed] [Google Scholar]
- 150.Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987;84:6471–5. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Holland J, Hersh L, Bryhan M, Onyiriuka E, Ziegler L. Culture of human vascular endothelial cells on an RGD-containing synthetic peptide attached to a starch-coated polystyrene surface: comparison with fibronectin-coated tissue grade polystyrene. Biomaterials. 1996;17:2147–56. doi: 10.1016/0142-9612(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 152.Hubbell JA, Massia SP, Desai NP, Drumheller PD. Endothelial cell-selective materials for tissue engineering in the vascular graft via a new receptor. Biotechnology (N Y) 1991;9:568–72. doi: 10.1038/nbt0691-568. [DOI] [PubMed] [Google Scholar]
- 153.Massia SP, Hubbell JA. Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin alpha 4 beta 1. J Biol Chem. 1992;267:14019–26. [PubMed] [Google Scholar]
- 154.Cao L, Mooney DJ. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Deliv Rev. 2007;59:1340–50. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–12. doi: 10.1023/A:1025034925152. [DOI] [PubMed] [Google Scholar]
- 156.Ulijn RV, Bibi N, Jayawarna V, Thornton PD, Todd SJ, Mart RJ, et al. Bioresponsive hydrogels. Mater Today. 2007;10:40–8. doi: 10.1016/S1369-7021(07)70049-4. [DOI] [Google Scholar]
- 157.Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J Biomater Sci Polym Ed. 2004;15:701–26. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 158.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–91. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 159.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–7. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 160.Ito T, Sawada R, Fujiwara Y, Seyama Y, Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun 2007; 359:108-14. J Cell Physiol. 1952;40:367–81. doi: 10.1016/j.bbrc.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 161.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 162.Kanda S, Miyata Y, Kanetake H. Fibroblast growth factor-2-mediated capillary morphogenesis of endothelial cells requires signals via Flt-1/vascular endothelial growth factor receptor-1: possible involvement of c-Akt. J Biol Chem. 2004;279:4007–16. doi: 10.1074/jbc.M307569200. [DOI] [PubMed] [Google Scholar]
- 163.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–11. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 164.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 166.Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177:489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park HJ, Yoo JJ, Kershen RT, Moreland R, Atala A. Reconstitution of human corporal smooth muscle and endothelial cells in vivo. J Urol. 1999;162:1106–9. doi: 10.1016/S0022-5347(01)68084-4. [DOI] [PubMed] [Google Scholar]
- 168.Wu X, Rabkin-Aikawa E, Guleserian KJ, Perry TE, Masuda Y, Sutherland FWH, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–7. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]