Abstract
Tissue destruction, pain and loss of function in chronically inflamed tissues can result from noxious agents released from myeloperoxidase (MPO) and its highly reactive product hypochlorous acid (HOCl) or proteases such as neutrophil elastase (NE).
Currently there exists a high demand for medications that provide gentle treatments, free from side effects inherent in those prescribed today. One method to circumvent side effects is through the use of locally applied drug delivery. In contrast to systemic therapy, the main advantages of transport systems are the low dosages of drug with a time-controlled delivery.
The aim of this study was to ascertain interactions of NE and its inhibitor α1-antitrypsin (AT), the influence of hypochlorous acid (HOCl), as well as its scavengers, in order to define an effective mixture of drugs acting in a synergistic way which can be applied by means of drug delivery systems. These investigations determine the effective amounts of AT/HOCl-scavengers that drug mixtures need for delivery under inflammatory conditions in order to prevent tissue damage. AT was shown to inhibit NE in a dose-dependent manner, whereas a physiological concentration of 1.14 µM AT caused a significant NE inhibition (78%, pH 7.5). The concomitant existence of MPO/HOCl inactivated AT in a dose-dependent manner as well. To regain AT efficacy, HOCl-scavengers, such as l-methionine, α-aminosalicylic acid and cefoperazone were additionally applied.
Finally, AT was assembled as surface layer onto layer-by-layer biopolymer-coated microcarriers and carrier phagocytosis by polymorphonuclear leukocytes could be shown.
Keywords: chronic inflammation, human neutrophil elastase, hypochlorous acid, microcarrier, myeloperoxidase, α1-antitrypsin
Introduction
The presence of several diseases of civilization with chronic inflammations leads to tissue destruction and pain.1 Current medication principally focuses on systemically applied immunosuppressants, glucocorticoids and non-steroidal antirheumatic agents. These induce significant drawbacks such as immune system impairment, increased risk of infections or cardiovascular toxicity.2–4 The continuing frustration to patients asks for gentle treatment methods with reduced side effects. Therefore, the investigation of the applicability of so called “drug cocktails” by means of colloidal delivery systems, such as nanocarriers or microcarriers and microspheres, which allow a targeted application into the specific cell or tissue for a highly effective treatment can be challenging.5–7
In this context, the modular and multifunctional design of colloidal layer-by-layer (LbL) coated micro- and nanocarrier provides several advantages in contrast to systemic therapy. The application of low doses of several active agents operating as a synergistic drug cocktail, the specific addressing of cells induced by additional surface functionalization and a time-controlled release within the cell due to a decomposing multilayer could be a new and promising approach for the treatment of highly complex processes such as chronic inflammations.
Chronic inflammatory conditions are characterized by imbalances between pro- and anti-inflammatory substances, resulting in an excess of proteases, such as protease 3 (PR3), cathepsin G (CG) and human neutrophil elastase (NE), peroxidases as myeloperoxidase (MPO) and reactive oxygen species (ROS) released from azurophil granules of polymorphonuclear leukocytes (PMNs).8 The increased protease release results in the attack of the host-specific extracellular matrix (ECM), leading to tissue degradation.9
Two important processes leading to chronically inflamed tissue are: (1) increased protease release and (2) oxidative stress. The relative amount of the naturally occurring protease inhibitor α1-antitrypsin (AT) is too low to form sufficient NE-AT complexes.10,11 On the other hand, PMN-mediated enhanced oxidative stress leads to further protease activation by means of AT inactivation. The highly reactive MPO product, hypochlorous acid (HOCl), among other ROS react and subsequently oxidize lipids, nucleic acids, amino acids or proteins and can cause chlorination as well.12–14 As a result, the NE inhibitory activity of AT is abolished by sulfoxidation of its active center.15 Due to significantly increased NE activity and ROS release as well as the additional AT inactivation, chemokine release by macrophages occurs. This prevents a successful clearance of immuno-reactive cells at inflammatory loci.16 A further insufficient termination of inflammation causes the elongation of the process and finally leads to a manifestation of a chronic proceeding process.17
The combined application of AT, HOCl/ROS scavengers as well as MPO inhibitors was supposed to constitute a promising concept in order to re-balance the protease/anti-protease concentration and subsequently to reduce tissue disturbing effects of proteases as well as oxidizing effects of ROS and MPO.
Sohrab et al. and Perlmutter et al. have shown that externally administered AT increases the intra- and inter-cellular concentration and results in NE-AT complex formation.18,19 The simultaneous usage of ROS/HOCl scavengers is supposed to facilitate the maintenance of AT activity in chronic inflammatory regions. Scavengers such as taurine, l-methionine, α-aminosalicylic acid (ASA) or cefoperazone20–23 are intended to “protect” AT activity in an adverse environment. In contrast to conventional methods, the idea of a combined therapy promises to be a highly effective treatment method. Such a concept of combined transport and time-controlled release can be developed with LbL microcarriers focusing on PMN action. PMNs migrate and accumulate in huge numbers in chronically inflamed tissues and are responsible for increased protease and ROS release.24,25 LbL microcarriers can optionally deliver a cocktail of active agents into the extracellular space or directly into the granulocytic phagolysosomes, the place of origin of proteases and ROS.
Our aim was the determination of optimal AT and scavenger concentrations regarding physiologically occurring PMN numbers in chronic inflammatory regions to provide a sufficient basis for the transport of a cocktail of those substances by LbL carriers. The influence of different AT concentrations has been described, as well as how the negative MPO/HOCl effects could be reduced by the addition of HOCl scavengers. The results obtained by means of pure substances were transferred into physiologic conditions by the use of the supernatant of stimulated PMNs. First attempts were made to apply AT supported by microcarriers into PMNs.
Results
An optimal design of effective colloidal drug carriers for the treatment of chronic inflammatory processes requires the knowledge of amounts and interrelated effects of AT and AT-protecting substances. Therefore, this study was designed to establish a cocktail of anti-inflammatory substances which were combined in optimal concentrations in order to inhibit host-damaging NE- as well as MPO/HOCl-effects, respectively.
Due to the fact that these anti-inflammatory microcarriers are supposed to act in phagolysosomes as well as in the extracellular space of inflammatory regions, the experiments were performed at acidic pH (pH 5 and 6), simulating the phagolysosomal environment in PMNs, and at slightly acidic as well as about neutral pH (pH 7 and 7.5), simulating extracellular environment at inflammatory conditions.
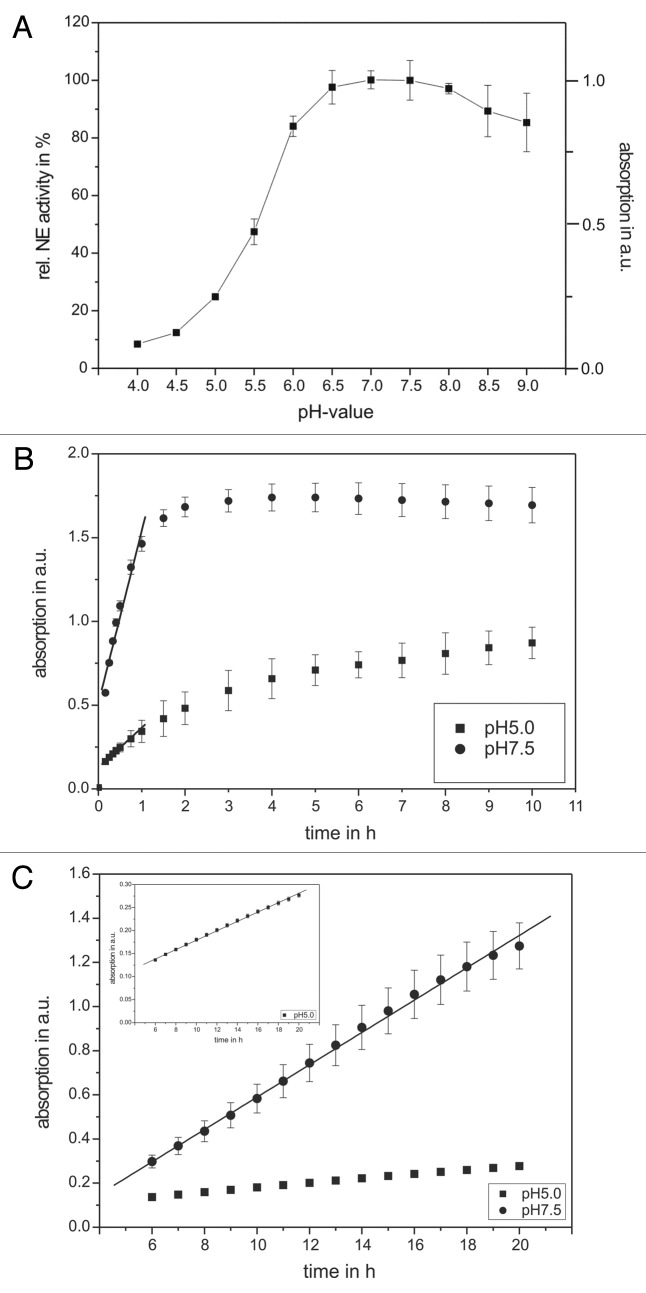
At first, the activity of pure NE as well as of the NE-containing supernatant of stimulated PMNs was pH- and time-dependently investigated (Fig. 1). The NE activity shows highest intensities between pH 6.5 and 8 with a maximal value at pH 7–7.5 which was set to 100%, suggesting an optimal NE activity at pH 7.5 as can be found in normal tissues or under only slightly inflammatory conditions (Fig. 1A). Since chronic inflammations were accompanied with a local pH value decrease, investigations with lower pH values were performed. A minor decrease to pH 6 caused only marginal NE activity decrease to 84 ± 4% whereas pH 5 already provoked a NE activity loss of ~75%.
Figure 1. The pH and time dependency of human neutrophil elastase activity. Shown are the NE activity optima according to the pH value of the buffer solution (A) as well as kinetic studies in the in vitro model system using pure substances (B) and in the supernatant of PMNs (C). An amount of 68 nM NE and 1 mM substrate were incubated with different pH values and in a time-dependent way. The pH optimum of NE activity could be approved in the neutral range (between pH 7–8). The supernatant of 3 × 105 PMNs (PMA-activated) was incubated with 1 mM substrate and NE activity was determined in a time-dependent way. In traces (B and C) results obtained at pH 5 are indicated as squares, wheras results obtained at pH 7.5 are indicated as circles. The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation.
To determine optimal conditions for NE activity measurements, pure NE (Fig. 1B) and NE-containing supernatants of stimulated PMNs (Fig. 1C) were exposed to the specific NE substrate at different pH values. Absorbance was measured vs. time.
As seen in Figure 1B, pure NE linearly increases absorbance until 0.5 h, followed by entire saturation after 2 h (pH 7.5, squares) and after 10 h (pH 5, dots) incubation, respectively. Activity measurements were performed within 0.5 h, providing a linear dependency. Regarding NE in the supernatant of stimulated PMNs, the situation was different. For both pH values a linear absorbance increase can be found after substrate incubation up to 20 h (Fig. 1C), requiring an enhanced incubation time. These results indicate lower NE activity in the supernatant of activated PMNs when compared with applied NE from the manufacterer. One reason may be the presence of additional inhibitory compounds in the degranulation mixture such as several cellular enzymes, proteins and oxidative agents. The preferred binding of NE to surfaces of the neutrophil plasma membrane26 circumvents the availability of the total cell NE amount in the supernatant of stimulated PMNs. Absorbance at 410 nm could be found between 0.3 and 1.3 (pH 7.5, black dots) and 0.14 and 0.27 (pH 5, squares) indicating activity loss at low pH values. For all further investigations the following optimized incubation time frames were used: activity measurements of pure NE were performed after 20 min (pH 7.5) or 1 h (pH 5) substrate incubation, respectively. For all experiments related to the NE of PMN supernatants a longer incubation time of 16 h was chosen for more reproducible results toward absorbance measurements.
AT inhibitory influence toward NE activity
The influence of AT toward NE activity was investigated in a concentration dependent way (0.095–1.9 µM) (Fig. 2) with pure substances (Fig. 2A) as well as in the supernatant of stimulated PMNs (Fig. 2B). For every measurement, NE activity (68 nM as well as the NE containing supernatant of 3 × 105 PMNs) was normalized to 100%. In both investigations, AT reduced the NE activity significantly with increasing concentrations at different pH values (between 5 and 7.5) (Fig. 2A and B). Additionally, pH 6 (dots) shows comparable results to pH 5 (squares), whereas the obtained data set at pH 7 (triangles) displayed similar results to that obtained at pH 7.5. For all further investigations two representative pH values (5 and 7.5, simulating intracellular as well as intercellular conditions, respectively) were chosen.
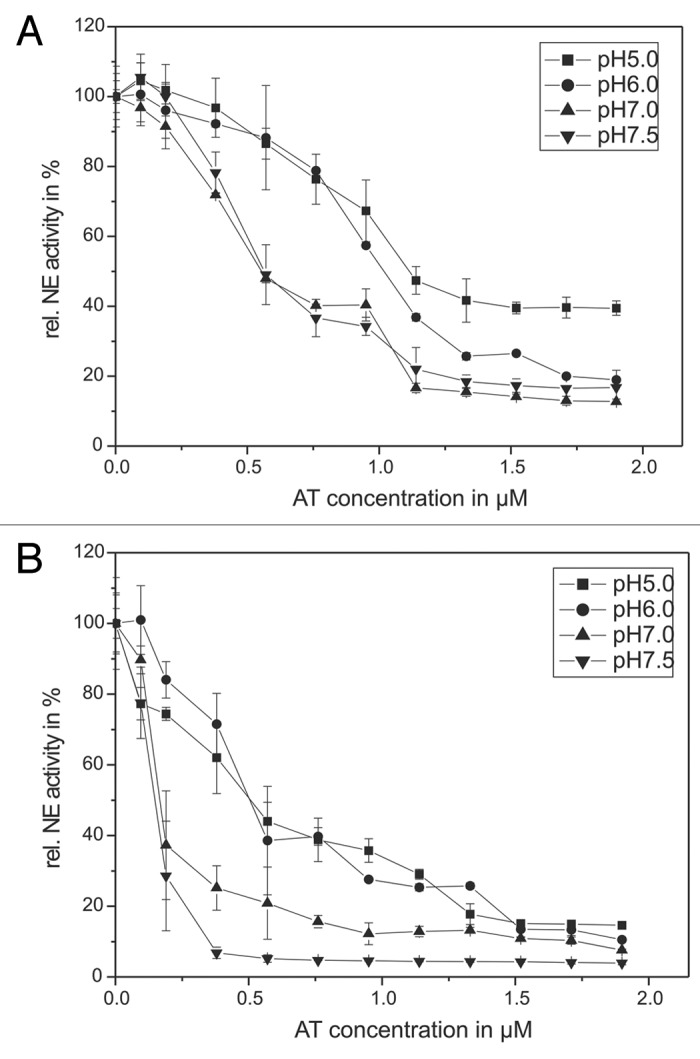
Figure 2. The pH-dependency of human antitrypsin. The pH-dependent influence of AT on the NE activity in an in vitro model system (A) and in the supernatant of activated PMNs (B) are presented. Sixty-eight nanomolars NE and 1 mM substrate were incubated with AT in a concentration dependent way (0.095–1.9 µM) for 20 min at pH 7.5 (inverse triangles) and pH 7 (triangles) as well as for 60 min at pH 6 (circles) and pH 5 (squares), respectively, at RT (trace A). PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate and AT in a concentration dependent way (0.095–1.9 µM) (circles) and pH 5 (squares) at RT (trace B). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
The AT efficacy is twice at pH 7.5 (inverse triangles) compared with pH 5 (squares) when pure substances are applied. In order to induce a 50% NE inhibition 0.57 µM AT are needed at pH 7.5 whereas 1.14 µM AT has to be used at pH 5.
AT application also caused a decreased NE activity in the supernatant of activated PMNs (Fig. 2B). The addition of 0.57 µM AT at pH 5 and 0.14 µM AT at pH 7.5 caused a NE activity decrease to 50%.
In order to determine the AT inactivation by HOCl and the MPO-H2O2-Cl−-system with the pure enzyme 1.14 µM (pH 5) or 0.57 µM AT (pH 7.5) amounts were used. For experiments focused on degranulated NE in the PMN supernatant, 0.095 µM AT (pH 7.5) as well as 0.57 µM AT (pH 5) were added.
The inactivating effects of the MPO-H2O2-Cl−-system /HOCl on the AT activity
During inflammatory processes, PMNs release MPO, leading to the generation of its highly reactive product, HOCl, that may cause AT inactivation by means of methionine-sulfoxidation in the active center of AT. In order to investigate the influence of the MPO-H2O2-Cl−-system toward AT efficacy, MPO as well as HOCl were added to the NE/AT-system in a concentration dependent way (Fig. 3). The mixture was incubated with AT for 5 min and the NE activity was detected immediately after substrate incubation. As a control, NE activity without AT and HOCl/MPO was detected and set to 100%.
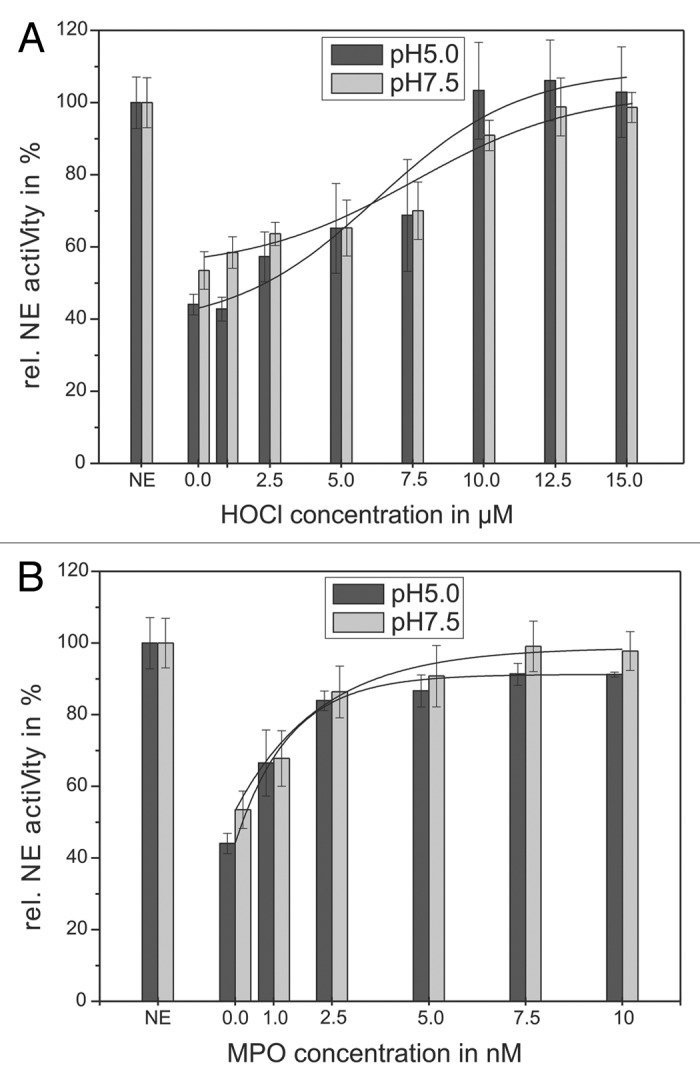
Figure 3. Shown is the impairing influence of HOCl (A) and the MPO-H2O2-Cl−-system (B) on the AT inhibiting activity toward NE. Sixty-eight nanomolars NE, 1 mM substrate and 1.14 µM AT were incubated with HOCl in a concentration dependent way (1–15 µM) for 20 min at pH 7.5 (gray bars) and for 60 min at pH 5 (black bars), respectively, at RT (trace A). An amount of 68 nM NE, 1 mM substrate and 1.14 µM AT were incubated with the MPO-H2O2-Cl−-system in a concentration dependent way (1–10 nM MPO, 2–20 µM H2O2, 140 mM NaCl) for 60 min at pH 7.5 (gray bars) and pH 5 (black bars), respectively, at RT (trace B). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of 3 independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
At first, the influence of HOCl was investigated. Figure 3A shows the influence of different HOCl-concentrations (1–15 µM) on AT efficacy illustrated by the determination of the NE activity. Without HOCl, NE activity (68 nM) was decreased to 44 ± 3% at pH 5 by 1.14 µM AT (black bars) and to 53 ± 5% at pH 7.5 by 0.57 µM AT (gray bars). The addition of HOCl to the mixture resulted in a reduced AT-inhibiting effect on NE. This effect was found strongly depending on the HOCl-concentration. Independent of pH value, a concentration of 12.5 µM HOCl caused a full reconstitution of NE activity.
Next, NE activity was measured in the presence of different concentrations of MPO 1–10 nM (Fig. 3B). As expected, the application of MPO results in a reduced AT effect and increased NE activity, respectively. Here, a concentration of 5 nM MPO also fully reconstitutes NE activity in a pH-independent matter.
These results show the necessity to protect AT from the inactivating effects of the MPO-H2O2-Cl−-system or HOCl, respectively, during the therapeutic application as anti-inflammatory agent.
The influence of HOCl-scavengers
To maintain AT activity in an inflammatory environment, different HOCl-scavengers were investigated regarding a potential reduction of the negative MPO/HOCl effects on AT. Taurine, l-methionine, ASA and cefoperazone were co-incubated with the pure enzyme as well as the supernatant of activated PMNs.
As described in Marcinkiewicz et al.,20 the amino sulfonic acid taurine is a potential HOCl scavenger reacting to long-living taurine-chloramine. However, the application of taurine (25–500 mM) does not result in significant effects on NE activity neither with pure enzyme nor in the supernatant of activated PMNs (data not shown).
l-methionine
l-methionine was also known for its potential as a HOCl scavenger. Externally added l-methionine can compete with the methionine residues in the active center of AT, regarding an occurring sulfoxidation and therefore, reduce the negative effects. The investigation was performed in a concentration-dependent way (Fig. 4). AT concentrations of 1.14 µM (pH 5) and 0.57 µM (pH 7.5) were used, reducing NE activity to 44 ± 3% at pH 5 and to 53 ± 5% at pH 7.5. The inhibitory effect of AT was then suppressed by 12.5 µM HOCl leading to a reconstitution of NE activity (97 ± 11% at pH 5 and 95 ± 4% at pH 7.5, negative control). l-methionine was added (0.1–1 mM) to the incubation mixture at pH 5 (black bars) and pH 7.5 (gray bars; Fig. 4A).
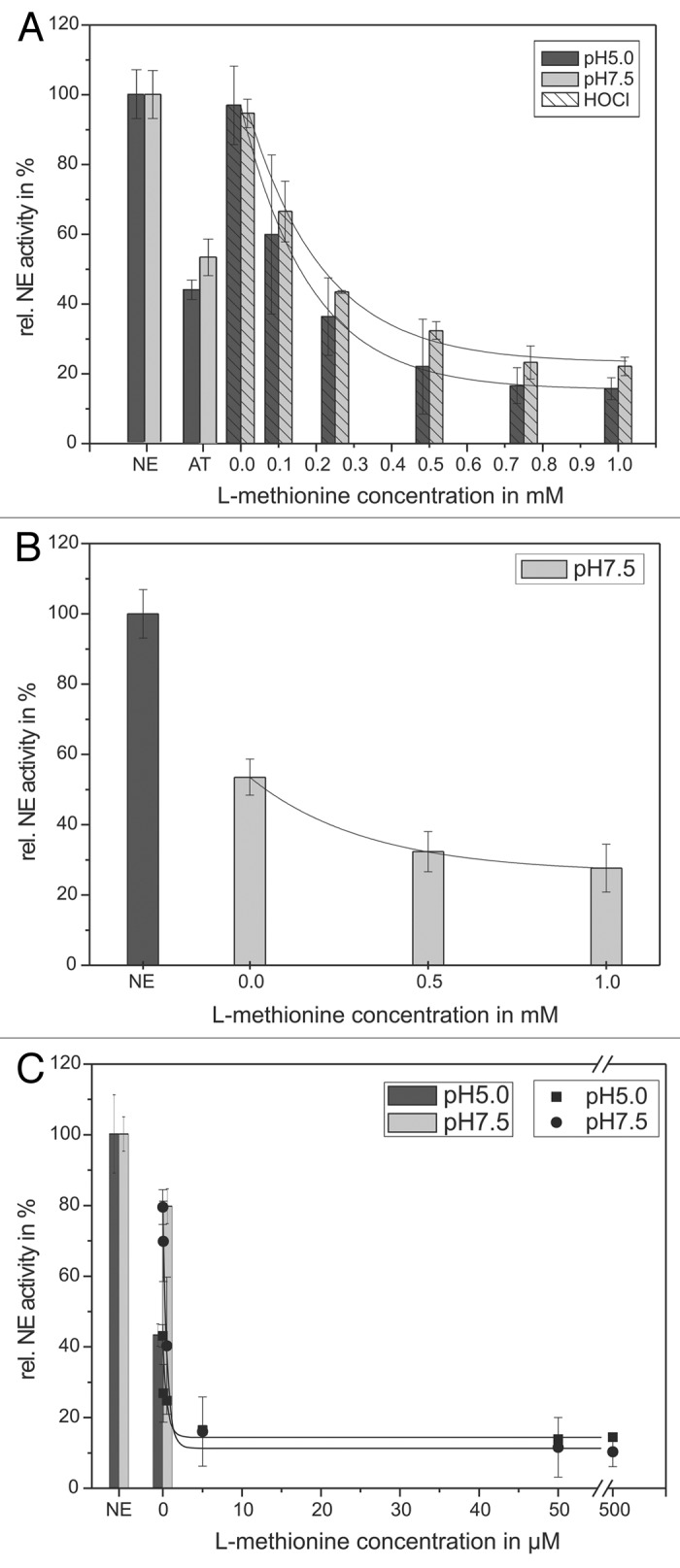
Figure 4. Shown is the protection of the AT inhibitory activity toward NE by means of l-methionine. 68 nM NE, 1 mM substrate, 12.5 µM HOCl and 1.14 µM AT (pH 5, black bars) or 0.57 µM AT (pH 7.5, gray bars), respectively, were incubated with l-methionine in a concentration dependent way (0.1–1 mM) for 20 min at pH 7.5 (gray bars) and 60 min at pH 5 (black bars) at RT (trace A). The HOCl addition is marked with stripes. An additional NE activity reducing effect of l-methionine could be observed without any HOCl addition (trace B). PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate, 1.14 µM AT (pH 5, black bars/squares) or 0.57 µM AT (pH 7.5, gray bars/circles), respectively, and l-methionine in a concentration dependent way (0.05–500 µM) for 16 h at pH 7.5 (circles) and pH 5 (squares) at RT (trace C). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
Based on this experimental set-up, increasing concentrations of l-methionine lead to a decreased NE activity. Already, after the addition of l-methionine, a full recovery of the AT activity can be obtained. Nevertheless, another phenomenon can be observed. A further increase of the l-methionine concentration up to 1 mM results in a more pronounced decrease of the NE activity to 16 ± 3% at pH 5 and to 22 ± 3% at pH 7.5, in comparison to the exclusive AT inhibitory effect. Thus, it can be assumed that the concerted action of AT and l-methionine amplifies the NE inhibition.
However, l-methionine does not influence NE activity. Direct incubation of l-methionine and NE without AT and HOCl does not change NE activity (data not shown).
On the other hand, a mixture containing l-methionine (0.5 or 1 mM) as well as 0.57 µM AT was added to NE without any HOCl addition to study the exclusive effect on AT. A NE/AT mixture without l-methionine served as control and was set as “0 mM l-methionine” in Figure 4B. Hereby, a more pronounced decrease in NE activity to 28 ± 7% can be shown in comparison to AT which reduced NE activity only to 53 ± 5%. This clearly indicates a positive (“protecting”) effect on AT activity.
To compare those results with inflammatory conditions, the “protecting” effect of l-methionine on AT inhibitory activity toward NE (100%) was additionally investigated in the supernatant of activated PMNs without external HOCl-addition (Fig. 4C). The incubation of the supernatant with 0.57 µM AT (at pH 5) and 0.095 µM AT (at pH 7.5) led to a reduced NE activity of 43 ± 3% as well as 80 ± 5% which was used as control and set as “0 mM l-methionine.” The application of (0.05–500 µM) l-methionine caused an enhanced inhibition of the NE activity, too. The use of 0.72 µM (pH 5) and 0.48 µM (pH 7.5) l-methionine reduced the remaining NE activity to 50%. A total effect of NE reduction can be observed at 50 µM l-methionine with decreased NE activities (14 ± 1% at pH 5 as well as 12 ± 8% at pH 7.5).
In summary, in the in vitro model system as well as in supernatants of activated PMNs l-methionine provoked significantly reduced NE activities caused by a decreased HOCl effect toward AT. However, in the supernatant of activated PMNs significant lower amounts of l-methionine are necessary to achieve a NE activity reduction.
ASA
The anti-inflammatory agent ASA is known as ROS-scavenger.27 To investigate whether ASA “protects” AT by decreasing HOCl effects, different ASA concentrations were applied (Fig. 5). Hereby, NE activity was set as 100%. In the model system 1.14 µM AT decreased NE activity to 44 ± 3% at pH 5 and 0.57 µM AT decreased NE activity to 53 ± 5% at pH 7.5 (Fig. 5A). By the use of 12.5 µM HOCl NE activity could be reconstituted to 97 ± 11% at pH 5 and to 95 ± 4% at pH 7.5.
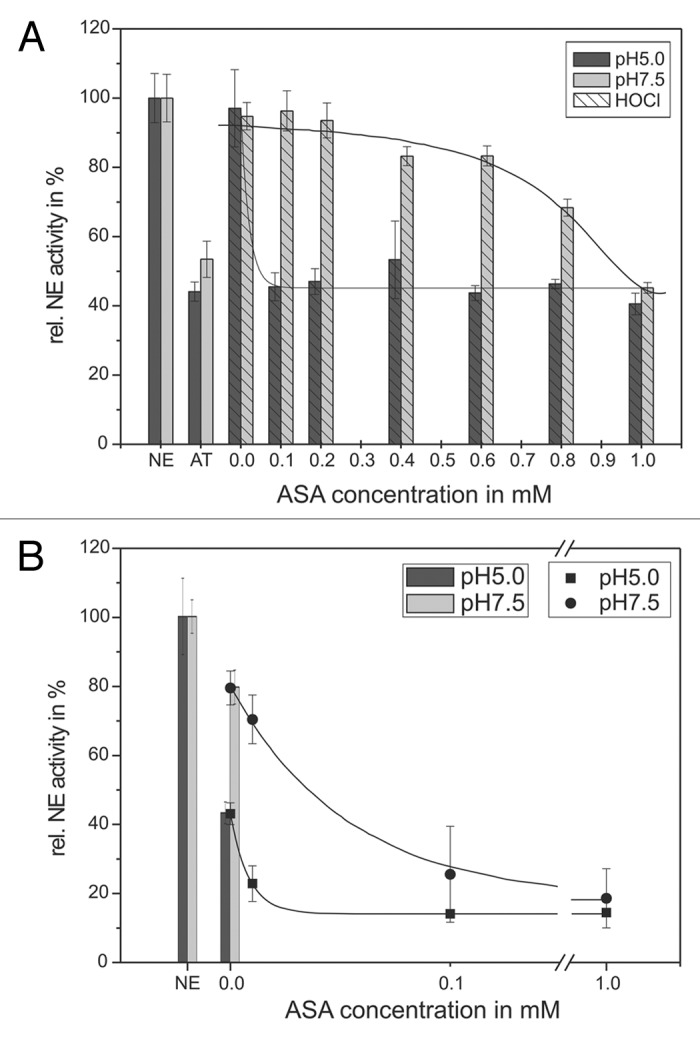
Figure 5. Shown is the protection of the AT inhibitory activity toward NE by means of ASA. 68 nM NE, 1 mM substrate, 12.5 µM HOCl and 1.14 µM AT (pH 5, black bars) or 0.57 µM AT (pH 7.5, gray bars), respectively, were incubated with ASA in a concentration dependent way (0.1–1 mM) for 20 min at pH 7.5 (gray bars) and 60 min at pH 5 (black bars) at RT (trace A). The HOCl addition is marked by stripes. PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate, 1.14 µM AT (pH 5, black bars/squares) or 0.57 µM AT (pH 7.5, gray bars/circles), respectively, and ASA in a concentration dependent way (0.01–1 mM) for 16 h at pH 7.5 (gray bars/circles) and pH 5 (black bars/squares) at RT (trace B). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
With increasing ASA concentration, at pH 7.5 only a slight NE reductive effect could be observed. Here, an application of 949 µM ASA is needed to reduce the NE activity to 50%. In contrast, at pH 5 a much lower concentration of 48 µM ASA was needed. Subsequently, a 20-fold lower ASA concentration is necessary to protect AT by scavenging the inactivating HOCl effects at pH 5 compared with the effects at pH 7.5. The same situation could be found in the supernatant of activated PMNs. NE (set as 100%) was incubated with 0.57 µM AT at pH 5 and with 0.095 µM AT at pH 7.5 (Fig. 5B) constituting the positive control. This led to a reduced NE activity of 43 ± 3% at pH 5 and 80 ± 5% at pH 7.5, respectively. As shown above, a slight NE activity decrease could be detected at pH 7.5. The application of 56 µM (pH 7.5) ASA caused a NE activity decrease of 50%. In contrast, at pH 5 only 12 µM are needed for a 50% NE inhibition. A total effect could be obtained using 100 µM ASA (14 ± 1%) at pH 5 and 26 ± 14% at pH 7.4, respectively.
The capability of ASA to decrease the NE activity is achieved by an improvement of the inactivating effects of HOCl toward AT and could be found at both pH 5 and pH 7.5. The effects were observed in the model system as well as in supernatant of activated PMNs. However, at pH 5 lower amounts of ASA were necessary to reduce the NE activity significantly.
Cefoperazone
The antibiotic cefoperazone acts as both AT “protector” and NE inhibitor.23 It was added to the experimental mixture in order to reduce the inactivating effects of HOCl on AT and to inhibit NE activity at pH 5 (black bars) and pH 7.5 (gray bars) (Fig. 6).
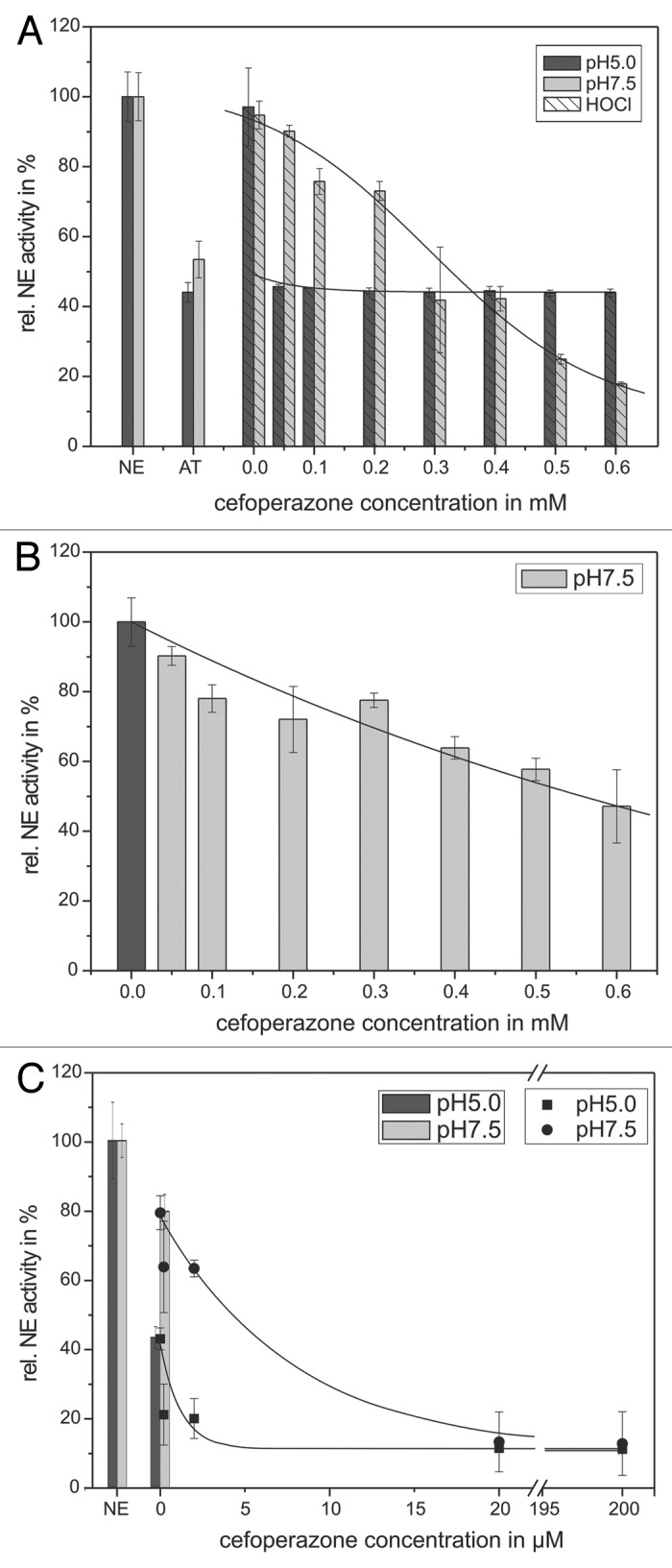
Figure 6. Shown is the protection of the AT inhibitory activity toward NE by means of cefoperazone. 68 nM NE, 1 mM substrate, 12.5 µM HOCl and 1.14 µM AT (pH 5, black bars) or 0.57 µM AT (pH 7.5, gray bars), respectively, were incubated with cefoperazone in a concentration dependent way (0.05–0.6 mM) for 20 min at pH 7.5 (gray bars) and 60 min at pH 5 (black bars) at RT (trace A). An additional NE activity reducing effect of cefoperazone can be observed, without AT addition at pH 7.5 (trace B). PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate, 1.14 µM AT (pH 5, black bars/squares) or 0.57 µM AT (pH 7.5, gray bars/circles), respectively, and cefoperazone in a concentration dependent way (2–200 µM) for 1 6 h at pH 7.5 (gray bars/circles) and pH 5 (black bars/squares) at RT (trace C). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
Compared to the NE control at 100% in the in vitro model system, the application of 1.14 µM AT reduced NE activity to 44 ± 3% at pH 5 and 0.57 µM AT decreased NE activity to 53 ± 5% at pH 7.5, respectively (Fig. 6A). 12.5 µM HOCl reconstituted NE activity to 97 ± 11% at pH 5 and to 95 ± 4% at pH 7.5, respectively. Cefoperazone was added to the NE/NE-substrate/AT/HOCl mixture in a concentration-dependent way (50–600 µM). With increasing concentration a NE activity decrease could be observed. A 50% reduced NE activity could be obtained after application of 1.2 µM (pH 5) and 327 µM (pH 7.5) cefoperazone, respectively. A further increase of the cefoperazone concentration to 600 µM resulted in a significantly pronounced NE activity decrease to 18 ± 1% at pH 7.5. Here, a 50% NE inhibition could be detected at pH 7.5 using nearly 300-fold the amount of cefoperazone used at pH 5.
The direct application of up to 600 µM cefoperazone to NE without AT and HOCl addition caused also a decreased NE activity 47 ± 10%, approving a NE inhibitory effect of cefoperazone (Fig. 6B).
Moreover, the effect of cefoperazone on NE activity and the HOCl scavenging was also determined in the supernatant of activated PMNs. Here, a strong pH-dependent effect in cefoperazone efficacy was found, too. The incubation of the supernatant with 0.57 µM AT at pH 5 and 0.095 µM AT at pH 7.5 resulted in reduced NE activity, 43 ± 3% at pH 5 and 80 ± 5% at pH 7.5, respectively. Compared with the NE inhibition caused by AT, which was set to 100%, the addition of 0.2–200 µM cefoperazone led to an enhanced inhibition of the NE activity. The use of 1.3 µM (at pH 5) and 6.6 µM (at pH 7.5) cefoperazone reduced the NE activity to 50%. Twenty micromolars cefoperazone decreased the NE activity to 11 ± 1% at pH 5 and to 13 ± 7% at pH 7.5, respectively.
The capability of cefoperazone to reduce the NE activity in an in vitro model system as well as in the supernatant of activated PMNs includes both the direct inhibition of NE and a HOCl scavenging effect “protecting” AT from HOCl impairing effects.
PMN vitality after AT co-incubation
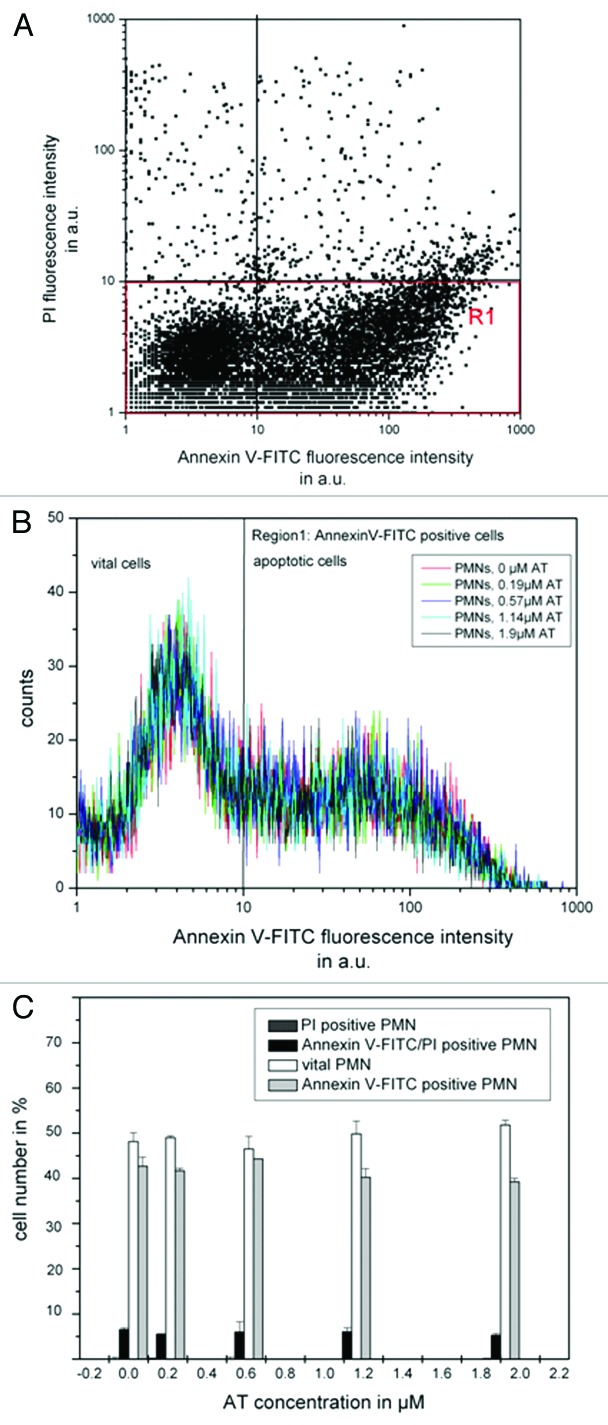
In order to determine if AT by itself has an influencing effect toward cell behavior, PMNs were incubated with AT (0–1.9 µM) for 16 h and analyzed regarding their vitality and induced apoptosis. As can be seen from Figure 7, PMNs show an increased apoptosis rate, visualized by means of increased annexin V-FITC binding [Fig. 7A, Region 1 (R1)], after 16 h incubation in RPMI 1640, even without AT co-incubation. Only marginal amounts of PMNs are PI-positive (necrotic). Figure 7B and C indicate no significant influence of different AT concentrations toward apoptosis as well as vitality of PMNs allowing the application in the proposed drug delivery system.
Figure 7. Shown is the influence of AT on PMN vitality. 106 PMNs were co-incubated with AT (0–1.9 µM) in RPMI1640 medium (10% FBS) for 16 h at 37°C. Apoptosis and vitality were investigated by means of annexin V-FITC and PI binding. PMNs were quantitatively analyzed by flow cytometry. PMNs without treatment show increased annexin V-FITC binding after 16 h inubation [trace (A)], but co-incubation with AT could not cause any additionally effect [trace (B and C)]. The number of PI-positive is cells is marginal for untreated cells as well as for AT-treated PMNs [traces (A and C)]. Shown are the mean values of three independent experiments ± standard deviation.
Development of the drug delivery system
FITC-labeled AT was coated as outermost layer [layer 6, (5 ± 2) pg] onto CaCO3 microcarriers (Fig. 8). The uniform distribution of AT as a microcarrier surface layer was proved by means of CLSM (Fig. 8, green FITC fluorescence). Additionally, an uptake of AT-functionalized microcarriers by PMNs could be shown indicating access of these carriers toward NE-containing phagolysosomes. Multilayer degradation and cytotoxicity of the basis-functionalized particle system was already shown by Rathmann et al.28
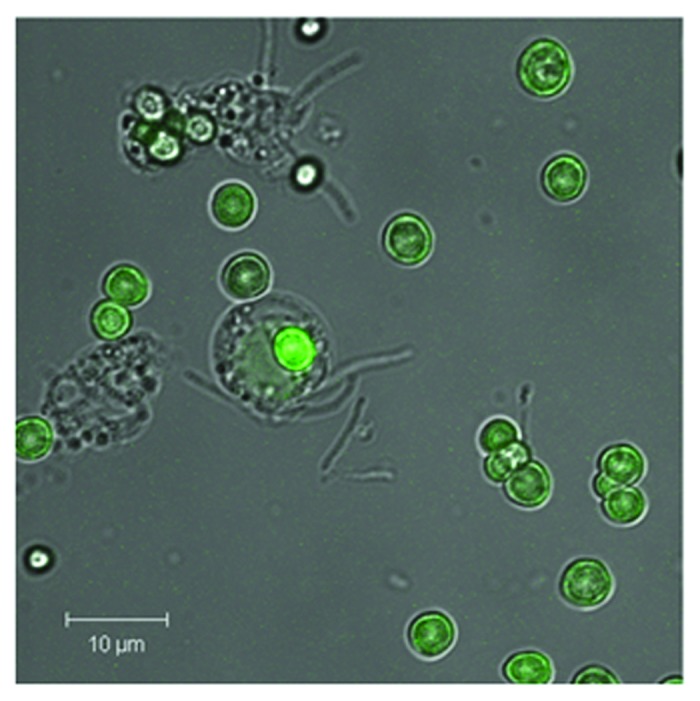
Figure 8. Shown is the phagosomal uptake of AT-functionalized CaCO3 microcarriers by PMNs. CaCO3 microparticles were coated with five layers PRM/DXS and surface-coated with FITC-labeled AT according to the following scheme CaCO3//PRM/DXS/PRM/DXS/PRM/AT-FITC. A uniform AT-FITC coating can be seen by means of CLSM. Microparticles (5 × 105) were co-incubated with PMNs (105) in a ratio of 5:1 for 24 h (37°C, 5% CO2). Uptake by PMNs was visualized by means of CLSM. A representative result of more than five independent measurements is presented.
Discussion
The excessive release of a multiplicity of reactive and degradative substances in chronic processes is supposed to be regulated by protease inhibitors as well as HOCl and ROS scavengers. Therefore, the aim of the study was the determination of an optimal cocktail composition consisting of a specific anti-protease, AT, and “AT-protecting agents” for a highly effective inhibition of NE within PMNs. In further studies, this mixture will be applied using a functionalized LbL microcarrier transport and delivery system for targeted, time-controlled and low-dose medication with reduced side effects.
Thereby, the focus has two aims: first, excessive NE release, causing tissue destruction and loss of function, has to be reduced. NE is the major protease responsible for extracellular proteolysis that is mediated by PMNs. It contributes to tissue damage by catalyzing the hydrolysis of a wide variety of matrix macromolecules, plasma proteins, inflammatory mediators and cell surface receptors with important local and systemic consequences. NE also exerts direct effects on various resident and inflammatory cells.29 And second, the disturbed balance of NE and AT caused by a massive invasion of PMNs into chronically inflamed regions is targeted to be re-adjusted.
The simulation of chronic inflammations by means of a cell model provides the basis for this study. For the application of our proposed “cocktail of anti-inflammatory substances” we focus on a combined application: since serine, cysteine and aspartate proteases are stored within azurophil granules of leukocytes29 and can be released into the extracellular space as a consequence of several stimuli, their simultaneous inhibition in the intra- as well as extra-cellular space seems to be very promising but requires efficacy investigation at acidic (pH 5, phagolysosomal environment) and about neutral (pH 7.5) conditions. Nevertheless, the pH optimum of NE could be approved at neutral pH.29
According to increased PMN numbers in inflamed regions (more than 5 × 106/ml) and a NE content of 1–4 pg/PMN, the NE concentration of 68 nM (equivalent to 3 × 105 PMNs), used in our experiments, corresponds well with the physiologic situation in inflamed regions.30 Moreover, for absorbance measurements, the set-up was adjusted to a linear range in order to find an adequate incubation time frame (20 min for pH 7.5; 60 min for pH 5 as well as 16 h for the supernatant of stimulated PMNs).
Although AT efficacy is significantly reduced at acidic conditions, the application of AT (0.095–1.9 µM) results in a significant NE inhibition. A 50% NE inhibition can be induced by 0.53 µM AT (pH 5) and 0.17 µM AT (pH 7.5) in the supernatant of PMNs. Nevertheless, since AT activity experiments at pH 7.0 and pH 6.0 show comparable results to the data obtained at pH 7.5 and 5, respectively, experiments were focused on minimal pH 5 and maximal pH 7.5. Usually, protease inhibitors such as AT are present in the extracellular milieu confining the proteolytic activity of leukocytes to the immediate pericellular microenvironment.29 Moreover, the results encourage the application of AT not only in the extracellular environment, but additionally as a component of a drug delivery system aiming the cell by the endolysosomal pathway in order to avoid further protease release and subsequent detrimental effects.
Compared with AT serum concentrations (1.3–2.3 mg/ml/25–67 µM),31 the effective concentration range was found to be significant lower, indicating a high efficacy and mildness of the intended local treatment method. Even higher amounts of invaded PMNs (up to 1 × 108 cells/ml) can be regulated—by adjusting the microcarrier number. According to effective AT concentrations in PMN supernatants, a carrier/PMN ratio of about 3:1 or 4:1 is supposed to be sufficient, when only AT-functionalized carriers with one AT layer [(5 ± 2) pg AT] are used. This is in accordance with biocompatibility studies.32 For the advanced drug delivery system using the cocktail of anti-inflammatory agents, the carrier number is assumed to be further reducible due to higher efficacy.
AT activity impairment was found to be induced by MPO mediated HOCl generation, whereas HOCl is responsible for methionine sulfoxidation at its active center.15 The process was approved by the addition of different HOCl concentrations choosen to be related to inflammatory sites (12.5 µM).33,34 A rapid AT inactivation was indirectly measured via the subsequent NE activity reconstitution. Already half of the physiologically available MPO amount (2–4 µg/ml MPO/106 PMNs/ml)30 is sufficient for NE activity recovery (Table 1).
Table 1. Effective concentrations for AT and HOCl-scavengers in the in vitro model system.
| pH 5 | pH 7.5 | |
|---|---|---|
| α1-antitrypsin |
1.14 |
0.57 |
| L-methionine |
156 |
192 |
| ASA |
48 |
949 |
| Cefoperazone | 1.2 | 3.27 |
Concentrations of active agents (in micromolars) which are needed in order to obtain a 50% efficacy of human neutrophil elastase in the molecular model system. The values were determined in a mixture containing pure HNE as provided by the manufacturer, substrate, buffer, HOCl, AT and active agents as stated, by means of absorbance measurements.
In order to simulate physiological conditions, supernatants of activated PMNs were also used in a similar experimental set up. Effective AT concentrations were significantly lower than in the previous model using pure molecules (Table 2). This apparently higher AT efficacy could be caused by the existence of AT and other protease inhibitors in the supernatant of PMNs contributing to NE inhibiting action. PMNs by themselves contain AT to interact with released and potentially destructive NE.35 The consequence is a lower AT amount needed for potential NE/AT interactions. Similarities could be observed for HOCl effects. In the experimental set-up using pure molecules, MPO, as well as HOCl, directly affect AT, whereas MPO in the supernatant released by PMNs affects a huge number of molecules resulting in the formation of chloramines.36
Table 2. Effective concentrations for AT and HOCl-scavengers in vivo.
| pH 5 | pH 7.5 | |
|---|---|---|
| α1-antitrypsin |
0.53 |
0.17 |
|
l-methionine |
0.72 |
0.48 |
| ASA |
12 |
56 |
| Cefoperazone | 1.3 | 6.6 |
Concentrations of active agents (in micromolars) which are needed in order to obtain a 50% efficacy of human neutrophil elastase in the supernatant of stimulated PMNs. The values were determined in the supernatant of PMA-stimulated PMNs by means of substrate as well as active agent addition, as stated, and spectrophotometrical measurement.
For an advanced cocktail directed against NE tissue destruction and imbalances, four additive substances, taurine, l-methionine, α-amino salicylic acid and cefoperazone, were investigated (Tables 1 and 2). Although taurine is described as ROS-Scavenger,20 it did not show any effect in our experiments. Probably, the generation of taurine chloramine could not further contribute to an AT activity improvement and had impairing effects by itself.
However, the existence of a sulfur atom in the l-methionine structures makes it a good choice in order to protect AT from sulfoxidation. Micromolar concentrations used in the model system and even nanomolar concentration used in the PMN supernatant are sufficient to recover AT activity. For pH 5 an additional NE inhibiting effect caused by l-methionine could be observed. This effect could only be detected after AT/l-methionine co-application. l-methionine alone shows no NE inhibition suggesting an AT-stabilizing effect at low pH. Since AT polymerization (resulting in the loss of inhibitory activity) is favored at extreme pH values as occurring in endolysosomes,37 one can suppose that l-methionine supports the biologically active conformational strain of AT by preventing polymerization.
The HOCl scavenging effect of ASA could be approved as already described.38 Its activity at low pH is much higher than at neutral pH, which makes it a suitable agent, especially for the application inside phagolysosomal compartments. Here, micromolar concentrations are sufficient to prevent AT inactivation. HOCl initially reacts with the ASA-amino group, resulting in the formation of short-living chloramine which is spontanously decomposed to an iminoquinone.39 The overall reaction is slowing down with increasing pH.40 Subsequently, ASA exerts its therapeutic effects by reacting with the oxidative species produced by activated neutrophils. However, this reaction can also generate reactive intermediates, which are able to bind covalently to human hemoglobin and potentially other proteins containing thiol groups causing the adverse reactions that have been associated with ASA use.38 For this purpose, the local application by means of a drug delivery system seems to be a good solution. Cefoperazone can be regarded as both HOCl scavenger and NE inhibitor, but it only acts as an additional NE inhibitor at neutral pH. At pH 5 no NE inhibiting effect could be found, making this molecule interesting as an additional AT “protecting” agent that can be effective in the low micromolar range.
In summary, in this study we successfully investigated a mixture of effective substances for the improvement of NE-mediated tissue destruction in chronically proceeding processes. We could show that the application of AT in order to prevent NE-mediated proteolysis can be ameliorated by a number of supporting substances, especially by the scavenging of HOCl which is concomitantly released at inflammatory loci. l-methionine and cefoperazone can be utilized in the low micromolar range in addition to the AT approach. Both substances are supposed to be applied with AT in a LbL-coated microparticle system in the future. AT, what is supposed to be biocompatible toward PMN vitality, was coated as a surface layer onto biopolymer coated microcarriers and phagocytosis by PMNs could be shown providing the basis for a new drug delivery approach in chronic inflammatory processes.
An advanced approach will be designed for a concomitant release of synergistically acting drugs and subsequently, a protection of AT in an adverse environment.
Experimental Section
Materials
α1-antitrypsin (AT), 2-amino-ethane-1-sulfonic acid (taurine), l-methionine, cefoperazone, dextran (~70000 Dalton), Histopaque®, phorbol 12-myristate 13-acetate (PMA), hanks balanced salt solution without CaCl2 (HBSS), Hank's balanced salt solution with CaCl2 (HBSS + CaCl2), citric acid monohydrate, hydrochloric acid (HCl), hydrogen peroxide (H2O2), tris(hydroxymethyl)aminomethane (TRIS) and sodium hypochlorite (NaOCl) were purchased from Sigma-Aldrich Chemical Co. The NE-substrate MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide, NE and heparin were purchased from Merck KGaA. Phosphate buffered saline (PBS) was obtained from PAA and sodium chloride (NaCl), di-sodiumhydrogenphosphate (Na2HPO4) were from VWR International GmbH. Human neutrophil myeloperoxidase (MPO) was a product of Planta Natural Products.
Determination of NE activity
The application of the specific NE substrate MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide allows the spectrophotometric detection of the NE activity at 410 nm. The reaction mixture (50 µl) contained 100 mM TRIS-HCl (pH 7.5) or 50 mM citrate-phosphate-buffer (pH 5) and 1 mM substrate. In order to start the reaction 68 nM NE were added. The experiment was performed at room temperature (RT) for 20 min at pH 7.5 as well as for 1 h at pH 5, respectively. For the quantification of the NE activity the amount of generated yellow p-nitroanilide was determined by means of a microplate reader. The two different time frames were set to provide a sufficient generation of p-nitroanilide but to avoid saturation.
Based on these experimental conditions, the following experiments were performed to investigate the influence of several factors on the NE activity.
NE activity depending on the incubation time and pH value
The determination of the ph-dependent NE activity was performed with 50 mM citrate-phosphate-buffer (pH 4–7) as well as 100 mM TRIS-HCl buffer (pH 7.5–9). The NE activity was measured after 30 min incubation as described above.
For time-dependent measurements at pH 5 and pH 7.5, NE activity was determined periodically after 10 min up to 1 h incubation time and periodically after 1 h up to 20 h incubation time as described above.
AT influence on NE activity
AT inhibitory effects toward NE activity were determined in a concentration-dependent way at pH 5, 6, 7 and 7.5 in order to simulate early and late phagolysosomal as well as extracellular conditions in chronic inflamed tissue. Therefore, AT was added to the reaction mixture in concentrations between 0.095–1.90 µM shortly before the addition of NE and NE-substrate. Incubation procedure and NE activity measurement were performed as described before. The experiment was performed for 20 min at pH 7 and 7.5 as well as 1 h at pH 5 and 6. NE activity was measured as described above.
The influence of hypochlorous acid (HOCl) on AT inhibitory activity
The MPO product HOCl inactivates AT by methionine residue oxidation (Met351 and Met358) in the active center of the enzyme. For the concentration-dependent investigation of AT inactivation at pH 7.5 and pH 5 HOCl concentrations between 1–15 µM were used for pre-incubation with AT (1.14 µM at pH 5 and 0.57 µM at pH 7.5). This concentration range can be regarded as relevant at sites of inflammation.33,41 The pre-incubated reaction mixture was added to the NE-substrate mixture and incubated as described before. The determination of the NE activity was performed as described above.
In order to adjust the NaOCl concentration, NaOCl was spectrophotometrically determined immediately prior to use at pH 12 (ε290 = 350 M−1 cm−1),24 while H2O2 concentration was checked in distilled water (ε230 = 74 M−1 cm−1).25
The influence of the MPO-H2O2-Cl−-system on the AT inhibitory activity
Moreover, the potentially inactivating effect of MPO toward AT was also investigated. 1.14 µM (pH 5) or 0.57 µM (pH 7.5) AT was incubated with 140 mM NaCl and 1–10 nM MPO. To start MPO-mediated HOCl-production 2–20 µM H2O2 was added. H2O2 addition was performed every 3 min (1 µl) for a time frame of 30 min to avoid enzyme inactivation. After the pre-incubation of the MPO-H2O2-Cl−-system and AT, the mixture was added to the NE/NE-substrate containing reaction mixture, incubated and NE activity was measured as described above.
Taurine and l-methionine influence on HOCl effects
In order to reduce the negative effects of MPO and its product HOCl, several protective and scavenging substances were investigated toward their capability to maintain AT effects.
The scavengers taurine 25–500 mM and l-methionine 0.1–1 mM were pre-incubated with 12.5 µM HOCl for 5 min. Afterwards 1.14 µM AT (pH 5) or 0.57 µM AT (pH 7.5) was added to the reaction mixture and incubated for 5 min. This pre-incubation mixture of scavengers/AT and HOCl was added to the NE/NE-substrate containing reaction mixture and incubated. The NE activity was determined as described above.
Cefoperazone and α-aminosalicylic acid (ASA) influence on HOCl effects
The influence of cefoperazone, known as NE-inhibitor and ROS scavenger,23 as well as ASA, known as anti-inflammatory agent,42 were investigated too. Cefoperazone (50–600 µM) and ASA (100–1,000 µM) were incubated with 12.5 µM HOCl for 5 min. Afterwards, 1.14 µM AT (pH 5) or 0.57 µM AT (pH 7.5) was added to the reaction mixture and incubated for 5 min before the NE/NE-substrate containing mixture was added and incubated as described before. NE activity was measured as described above.
The determination of NE activity in the supernatant of stimulated PMNs
Isolation of polymorphonuclear leukocytes (PMNs) from human blood43
Human whole blood of healthy donors was incubated with heparin (100 U/ml) to avoid coagulation and then treated with dextran (20% m/v, 30 min, RT) for erythrocyte separation. Blood was obtained from a blood bank. Donors were informed and signed consents were obtained from the patients. After erythrocyte sedimentation, PMNs were separated using density gradient centrifugation (400 g; 4°C; 25 min) with Histopaque®1077. By washing with distilled water, residual erythrocytes could be removed by hypotonic lysis. PMNs were counted and resuspended in HBSS without Ca2+. PMNs were used for further experiments immediately after isolation taking into account their natural life span.44,45
PMN stimulation and degranulation
PMNs (5 million cells/ml HBSS + Ca2+) were activated with PMA (10−7 M) for 30 min at 37°C. After centrifugation (5 min, 400 g), the supernatant containing the released highly reactive enzymes and substances was used for the NE assay. Finally for each experiment 60 µl were used containing supernatant of 3 × 105 cells.
NE activity determination
NE activity in the supernatant of activated PMNs was measured by the use of NE-substrate. The reaction mixture (100 µl) contained 60 µl supernatant and 30 µl PBS (pH 7.5) or 50 mM citrate-phosphate-buffer (pH 5), respectively. In order to start the reaction 1 mM NE-substrate was added. After 16 h an adequate NE activity was measured as described before.
AT influence on NE activity
For the investigation of AT effects in the supernatant of activated PMNs AT was added in a concentration-dependent (0.095–1.90 µM) way to the supernatant/buffer-mixture before NE substrate addition. NE activity was measured as described before.
The influence of HOCl scavengers
To avoid AT inactivation in the supernatant of activated PMNs, different HOCl-scavengers were investigated. Taurine (5–500 mM), l-methionine (5–500 µM), cefoperazone (2–200 µM) and ASA (10–1,000 µM) were mixed with the supernatant/buffer solution before AT was added. For this purpose 0.57 µM AT were used at pH 5 and 0.095 µM AT at pH 7.5, respectively. NE activity was determined as described before.
The influence of applied AT toward PMN viability
The local application of different concentrations of AT as an active drug in human tissue by means of drug delivery systems requires the investigation of its own potential negative influence on cell behavior. Therefore, AT (0–1.9 µM) was co-incubated with 106 PMNs in RPMI medium (1 ml, containing 10% FBS, 24 well plate) for 16 h and investigated for increased apoptosis and necrosis by means of Annexin V-FITC and Propidium Iodide (PI) binding. The staining was performed according to manufacturer’s instructions (Annexin V-FITC Apoptosis Detection Kit, Calbiochem). The measurement and quantitative analysis was done by flow cytometry (FACS Calibur, BD) and WinMDI software.
Development of the drug delivery system
CaCO3 microparticle preparation
CaCO3 particles with 5 ± 1 µm in diameter were prepared by rapid mixing of equal volumes of 0.33 M CaCl2 and 0.33 M Na2CO3 (both Sigma-Aldrich) solutions followed by a sedimentation step as described previously.46 Afterwards, the particles were washed five times in double distilled water and subsequently coated with polyelectrolyte multilayer.
Labeling of AT
To establish AT as a multilayer constituent, the protein was labeled with fluorescein isothiocyanate (FITC) according to the manufacturer’s instruction (Pierce FITC Antibody Labeling Kit, Fisher Scientific GmbH). Briefly, 500 µl AT (4 mg/ml, borate buffer pH 8.5) were incubated with FITC reagent for 60 min at room temperature. The unbound FITC was separated using centrifuge columns. The label degree and AT concentration were calculated according to absorption at 280 nm and 495 nm. Aliquots of labeled AT were then stored at -20°C until usage.
Layer-by-layer (LbL)-coating of CaCO3 particles
The multilayer consisting of the oppositely charged biocompatible and biodegradable polyelectrolytes protamine sulfate salt from herring (PRM, MW 4 kDa; Sigma-Aldrich) and dextran sulfate sodium salt (DXS, MW 40 kDa; INC Biochemicals) was performed by alternating adsorption of five layers PRM and DXS. Both PRM and DXS were dissolved at a concentration of 4 mg/ml in 0.1 M sodium chloride (NaCl; Sigma-Aldrich) solution. After particle incubation for 10 min under rapid shaking at room temperature, the carriers were centrifuged and three times washed in 0.1 M NaCl solution to remove unbound polyelectrolytes.
For AT-FITC (54 kDa; Sigma-Aldrich), assembed as outermost layer (layer 6), the carriers were 30 min incubated with AT dissolved in borate buffer (50 mM sodium borate, 0.2 M NaCl, pH 8.5) at a concentration of 4 mg/ml and 15°C followed by washing three times with 0.1 M NaCl solution.
Cell/carrier interaction
For carrier uptake experiments by PMNs, cells were isolated as described above. About 105 cells were incubated with 5 × 105 carriers (carrier/cell ratio 1:5) in RPMI with 5% FBS in 8-well plates (Lab-Tek, Chambered Coverglass system, Nalgene Nunc/Thermo Fisher Scientific, Roskilde) for 24 h at 37°C and 5% CO2. Afterwards, medium was exchanged by PBS and samples were visualized by CLSM.
Flow Cytometry (FCM)
FCM (FACSCalibur, BD Biosciences) was used for the quantitative analysis of the vitality state of PMNs after AT influence. FITC fluorescence was detected in fluorescence channel 1 (FL1) using the 530/30 nm bandpass filter. PI fluorescence was detected in fluorescence channel 2 (FL2) using the 585/42 bandpass filter.
Confocal Laser Scanning Microscopy (CLSM)
Confocal laser scanning fluorescence microscopy (Zeiss, LSM 510 META) was applied to visualize the homogeneous distribution of AT as multilayer constituent as well as the uptake of AT-functionalized microcarriers by PMNs. A laser excitation of 488 nm (argon laser) was used to detect FITC fluorescence of labeled AT by a bandpass filter of 505–525.
Statistics
Each experiment was repeated at least three times. All data are expressed as mean ± standard deviation (SD). NE activity without any influencing factors was set to 100% as positive reference value.
Acknowledgments
The work presented in this paper was made possible by funding from the German Federal Ministry of Education and Research (BMBF, PtJ-Bio, 0313909).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/19190
References
- 1.Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. 2008;121(Suppl 1):S21–31. doi: 10.1016/j.amjmed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Doan T, Massarotti E. Rheumatoid arthritis: an overview of new and emerging therapies. J Clin Pharmacol. 2005;45:751–62. doi: 10.1177/0091270005277938. [DOI] [PubMed] [Google Scholar]
- 3.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007;26:1228–33. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- 4.Dubertret L. Retinoids, methotrexate and cyclosporine. Curr Probl Dermatol. 2009;38:79–94. doi: 10.1159/000232305. [DOI] [PubMed] [Google Scholar]
- 5.Cortez C, Tomaskovic-Crook E, Johnston APR, Scott AM, Nice EC, Heath JK, et al. Influence of size, surface, cell line, and kinetic properties on the specific binding of A33 antigen-targeted multilayered particles and capsules to colorectal cancer cells. ACS Nano. 2007;1:93–102. doi: 10.1021/nn700060m. [DOI] [PubMed] [Google Scholar]
- 6.Reibetanz U, Claus C, Typlt E, Hofmann J, Donath E. Defoliation and plasmid delivery with layer-by-layer coated colloids. Macromol Biosci. 2006;6:153–60. doi: 10.1002/mabi.200500163. [DOI] [PubMed] [Google Scholar]
- 7.DeGeest BG, Vandendroucke RE, Guenther AM, Sukhorukov GB, Hennink WE, Sanders NN, et al. Intracellularly Degradable Polyelectrolyte Microcapsules. Adv Mater (Deerfield Beach Fla) 2006;18:1005–9. doi: 10.1002/adma.200502128. [DOI] [Google Scholar]
- 8.Galle J. [Atherosclerosis and arteriitis: implications for therapy of cardiovascular disease] Herz. 2004;29:4–11. doi: 10.1007/s00059-004-2520-5. [DOI] [PubMed] [Google Scholar]
- 9.Sandholm L. Proteases and their inhibitors in chronic inflammatory periodontal disease. J Clin Periodontol. 1986;13:19–26. doi: 10.1111/j.1600-051X.1986.tb01409.x. [DOI] [PubMed] [Google Scholar]
- 10.Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Köhnlein T, Welte T. The discovery of α1-antitrypsin and its role in health and disease. Respir Med. 2011;105:1129–39. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Brower MS, Harpel PC. Alpha-1-antitrypsin-human leukocyte elastase complexes in blood: quantification by an enzyme-linked differential antibody immunosorbent assay and comparison with alpha-2-plasmin inhibitor-plasmin complexes. Blood. 1983;61:842–9. [PubMed] [Google Scholar]
- 12.Matheson NR, Wong PS, Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979;88:402–9. doi: 10.1016/0006-291X(79)92062-X. [DOI] [PubMed] [Google Scholar]
- 13.Panasenko OM, Spalteholz H, Schiller J, Arnhold J. Myeloperoxidase-induced formation of chlorohydrins and lysophospholipids from unsaturated phosphatidylcholines. Free Radic Biol Med. 2003;34:553–62. doi: 10.1016/S0891-5849(02)01358-8. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–74. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 15.Clark RA, Stone PJ, El Hag A, Calore JD, Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981;256:3348–53. [PubMed] [Google Scholar]
- 16.Meyer-Hoffert U. Neutrophil-derived serine proteases modulate innate immune responses. Front Biosci. 2009;14:3409–18. doi: 10.2741/3462. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 18.Sohrab S, Petrusca DN, Lockett AD, Schweitzer KS, Rush NI, Gu Y, et al. Mechanism of alpha-1 antitrypsin endocytosis by lung endothelium. FASEB J. 2009;23:3149–58. doi: 10.1096/fj.09-129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlmutter DH, Joslin G, Nelson P, Schasteen C, Adams SP, Fallon RJ. Endocytosis and degradation of alpha 1-antitrypsin-protease complexes is mediated by the serpin-enzyme complex (SEC) receptor. J Biol Chem. 1990;265:16713–6. [PubMed] [Google Scholar]
- 20.Marcinkiewicz J, Grabowska A, Bereta J, Stelmaszynska T. Taurine chloramine, a product of activated neutrophils, inhibits in vitro the generation of nitric oxide and other macrophage inflammatory mediators. J Leukoc Biol. 1995;58:667–74. doi: 10.1002/jlb.58.6.667. [DOI] [PubMed] [Google Scholar]
- 21.Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc Natl Acad Sci U S A. 2009;106:18686–91. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JG, Hallett MB. The reaction of 5-amino-salicylic acid with hypochlorite. Implications for its mode of action in inflammatory bowel disease. Biochem Pharmacol. 1989;38:149–54. doi: 10.1016/0006-2952(89)90161-5. [DOI] [PubMed] [Google Scholar]
- 23.Dallegri F, Dapino P, Arduino N, Bertolotto M, Ottonello L. Cefoperazone prevents the inactivation of alpha(1)-antitrypsin by activated neutrophils. Antimicrob Agents Chemother. 1999;43:2307–10. doi: 10.1128/aac.43.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC. The acid ionization constant of HClO from 5 to 35. J Phys Chem. 1966;70:3798–805. doi: 10.1021/j100884a007. [DOI] [Google Scholar]
- 25.Beers RF, Jr., Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 26.Campbell EJ, Owen CA. The sulfate groups of chondroitin sulfate- and heparan sulfate-containing proteoglycans in neutrophil plasma membranes are novel binding sites for human leukocyte elastase and cathepsin G. J Biol Chem. 2007;282:14645–54. doi: 10.1074/jbc.M608346200. [DOI] [PubMed] [Google Scholar]
- 27.Pro´nai L, Yukinobu I, L´ng I, Fehe´r J. The oxygen-centered radicals scavenging activity of sulfasalazine and its metabolites. A direct protection of the bowel. Acta Physiol Hung. 1992;80:317–23. [PubMed] [Google Scholar]
- 28.Rathmann S, Schönberg M, Leßig J, Reibetanz U. Interaction, uptake, and processing of LbL-coated microcarriers by PMNs. Cytometry A. 2011;79A:979–89. doi: 10.1002/cyto.a.21145. [DOI] [PubMed] [Google Scholar]
- 29.Owen CA, Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol. 1999;65:137–50. doi: 10.1002/jlb.65.2.137. [DOI] [PubMed] [Google Scholar]
- 30.Lehrer RI, Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990;76:2169–81. [PubMed] [Google Scholar]
- 31.Russo AJ, Neville L, Wroge C. Low Serum Alpha-1 Antitrypsin (AAT) in Family Members of Individuals with Autism Correlates with PiMZ Genotype. Biomark Insights. 2009;4:45–56. doi: 10.4137/bmi.s1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leßig J, Neu B, Reibetanz U. Influence of layer-by-layer (LbL) assembled CaCO(3)-carriers on macrophage signaling cascades. Biomacromolecules. 2011;12:105–15. doi: 10.1021/bm101069s. [DOI] [PubMed] [Google Scholar]
- 33.Vogt W, Hesse D. Oxidants generated by the myeloperoxidase-halide system activate the fifth component of human complement, C5. Immunobiology. 1994;192:1–9. doi: 10.1016/S0171-2985(11)80403-1. [DOI] [PubMed] [Google Scholar]
- 34.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson B, Malm J, Persson T, Janciauskiene S, Andersson P, Carlson J, et al. Alpha-1-antitrypsin is present in the specific granules of human eosinophilic granulocytes. Clin Exp Allergy. 2001;31:379–86. doi: 10.1046/j.1365-2222.2001.01017.x. [DOI] [PubMed] [Google Scholar]
- 36.Naskalski JW, Marcinkiewicz J, Drozdz R. Myeloperoxidase-mediated protein oxidation: Its possible biological functions. Clin Chem Lab Med. 2002;40:463–8. doi: 10.1515/CCLM.2002.080. [DOI] [PubMed] [Google Scholar]
- 37.Dafforn TR, Mahadeva R, Elliott PR, Sivasothy P, Lomas DA. A kinetic mechanism for the polymerization of alpha1-antitrypsin. J Biol Chem. 1999;274:9548–55. doi: 10.1074/jbc.274.14.9548. [DOI] [PubMed] [Google Scholar]
- 38.Liu ZC, McClelland RA, Uetrecht JP. Oxidation of 5-aminosalicylic acid by hypochlorous acid to a reactive iminoquinone. Possible role in the treatment of inflammatory bowel diseases. Drug Metab Dispos. 1995;23:246–50. [PubMed] [Google Scholar]
- 39.McKenzie SM, Doe WF, Buffinton GD. 5-aminosalicylic acid prevents oxidant mediated damage of glyceraldehyde-3-phosphate dehydrogenase in colon epithelial cells. Gut. 1999;44:180–5. doi: 10.1136/gut.44.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magsam TG, Winkler ZR, DiCesare JC, Purser GH, 5-Aminosalicylic acid as an antioxidant: Mechanism of reaction with chloramine. CHED 279.
- 41.McKenzie SM, Doe WF, Buffinton GD. 5-aminosalicylic acid prevents oxidant mediated damage of glyceraldehyde-3-phosphate dehydrogenase in colon epithelial cells. Gut. 1999;44:180–5. doi: 10.1136/gut.44.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKenzie SM, Doe WF, Buffinton GD. 5-aminosalicylic acid prevents oxidant mediated damage of glyceraldehyde-3-phosphate dehydrogenase in colon epithelial cells. Gut. 1999;44:180–5. doi: 10.1136/gut.44.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7. [PubMed] [Google Scholar]
- 44.Haslett C. Granulocyte apoptosis and inflammatory disease. Br Med Bull. 1997;53:669–83. doi: 10.1093/oxfordjournals.bmb.a011638. [DOI] [PubMed] [Google Scholar]
- 45.Webb PR, Wang K-Q, Scheel-Toellner D, Pongracz J, Salmon M, Lord JM. Regulation of neutrophil apoptosis: a role for protein kinase C and phosphatidylinositol-3-kinase. Apoptosis. 2000;5:451–8. doi: 10.1023/A:1009601220552. [DOI] [PubMed] [Google Scholar]
- 46.Volodkin DV, Larionova NI, Sukhorukov GB. Protein encapsulation via porous CaCO3 microparticles templating. Biomacromolecules. 2004;5:1962–72. doi: 10.1021/bm049669e. [DOI] [PubMed] [Google Scholar]