Abstract
In this work we describe the fabrication of a biocompatible hydrophilic scaffold composed of cross-linked gelatin that mimics the porous three-dimensional structure of pulmonary tissue as well as its water content and mechanical properties. The lung replica also reproduces the characteristic sonographic signs of pulmonary interstitial syndrome, the B-lines or ultrasound lung comets.
Keywords: B-lines, echographic signals, gelatin, hydrogels, lung replica, mechanical properties, porosity
Introduction
Recreating a functional physiological model is important not only to study the normal state, but also in the perspective of reproducing a specific pathology in vitro. As far as pulmonary tissue is concerned, there are several reports of lung models in the literature. The majority of these are based on the culture of pulmonary epithelial cells at the air-liquid interface for the study of translocation across alveolar epithelial barrier during inhalation. Some of the models reported are based on thin three-dimensional (3D) constructs composed of collagen hydrogels. However, replicating the complex and soft structure of the lung has always been recognized as a major challenge,1 and many researchers appear to concur that a natural matrix, derived from cell-stripped pulmonary tissue is the optimal scaffold for recapitulating lung architecture.
On the other hand, very few studies focus on the replication of a 3D lung-like matrix as a phantom for diagnostic purposes using ultrasound or magnetic resonance imaging. Since pulmonary tissue contains air, which attenuates the propagation of acoustic waves, its response to ultrasound is very specific. It is in fact possible to detect the pathologic presence of extravascular lung water by observing the so-called B-lines (previously called ultrasound lung comets). Sonographic B-lines consist of multiple comet-like tails originating from water thickened interlobular septa2,3 and are the sonographic manifestations of pulmonary interstitial syndrome, including extravascular lung water.4 Lung ultrasound has always been intriguing and attracted the interest of many researchers, however, it is often difficult to quantify or qualify the origin and nature of specific features in an image for the lack of adequate models. A simple and reproducible phantom that recapitulates the acoustic impedance of pulmonary tissue could be an extremely useful tool for modeling healthy and pathological lung ultrasonography. The aim of this study was thus to identify and optimize a porous gel-like biomaterial capable of reproducing the mechanical, architectural and acoustic properties of pulmonary tissue.
Elastic and collagen fibers are the main structural components of pulmonary connective tissue matrix, but their elastic properties are essentially different. Elastic fibers have a very low elastic modulus, while collagen's modulus is almost three orders of magnitude greater. Thus the sonographic response of soft tissues is mainly determined by the compressive/tensile modulus at low deformations of structural collagen containing components.5 Gelatin, a mixture of protein chains derived from thermal denaturation and physicochemical degradation of collagen6-8 was chosen as a bulk material due to its low cost compared with collagen. Moreover, gelatin can be easily processed: it is soluble in most polar solvents (e.g., water) and gels at room temperature. While dissolved in aqueous solutions, gelatin can form physical thermo-reversible gels: during the cooling phase the polymer chains undergo a disordered transition and tend to recover the collagen triple-helix structure.9,10 Several cross-linking agents can be used to stabilize gelatin hydrogels, enhancing their physicochemical and mechanical properties with the increase of the number of cross-links.11 Owing to its high affinity and efficiency, glutaraldehyde (GTA) is commonly used for cross-linking collagen-derived materials and greatly improves the mechanical characteristics of gelatin gels.12 The degree of cross-linking is determined by the concentration of GTA. To reproduce the viscoelastic properties and incompressibility of pulmonary tissue, a water-filled porous structure was realized using freeze-drying and subsequent re-swelling. Freeze-drying is a well-known technique for creating pores, and pore size can be controlled by an appropriate choice of solvent and freezing conditions.13 The porosity, swelling and mechanical properties of cross-linked, freeze-dried gelatin sponges were first characterized as a function of GTA concentration in an aqueous environment. The structure, which most closely resembled the pulmonary tissue, was used as a physiological model to recreate characteristic echographic B-lines of diseased or injured lungs.
Results and Discussion
Morphology
Histological images reveal that the average pore area in gelatin samples is not affected by the cross-linking reaction (as shown in Fig. 1); moreover this porous structure is similar to the alveolar parenchyma, with a range of pore sizes from a few microns to a maximum of 500 µm.
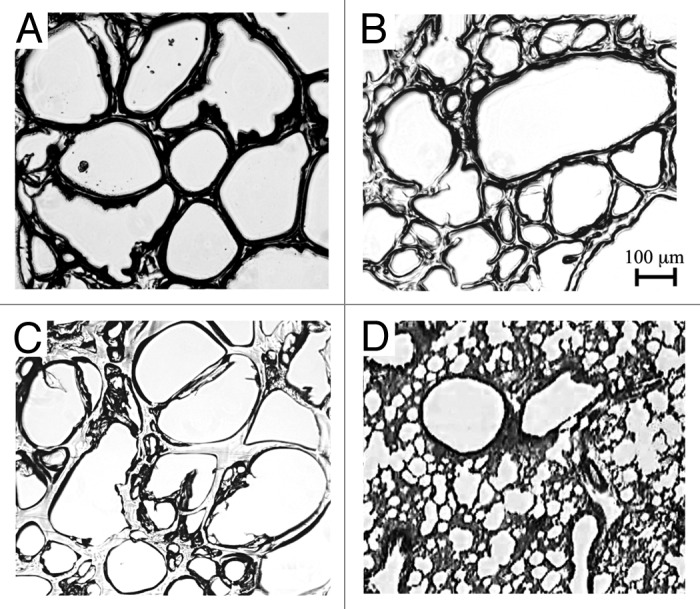
Figure 1. Micrographs of gelatin 5% cross-linked with glutaraldehyde: (A) 10 mM, (B) 1 mM, (C) 0.1 mM and (D) lung histology.14 The scale bar reported is the same for all pictures.
Similarly, SEM images of the freeze-dried gels show an interconnected porous matrix which resembles the micro-architecture of lung tissue matrix (Fig. 2).
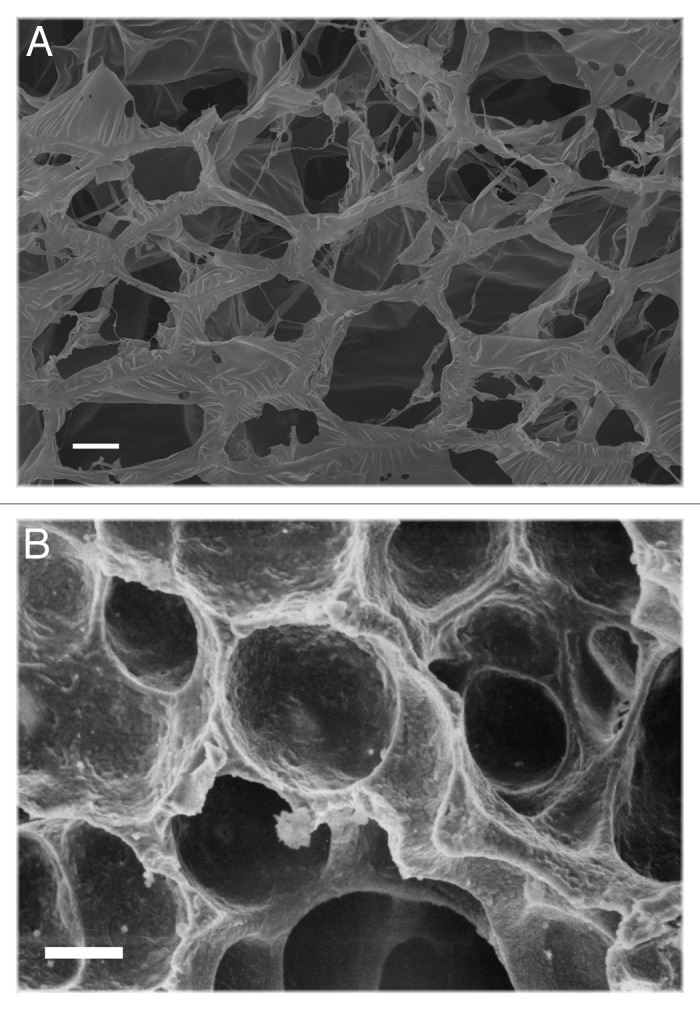
Figure 2. SEM images of (A) lung replica (5% w/v gelatin cross-linked with 1 mM glutaraldehyde and then freeze-dried) and (B) natural tissue: pore dimension and distribution are similar. Scale bar, 20 μm.
Measurement of pores
Pore dimension was analyzed by acquiring digital images using an optical microscope. Pore diameters were measured using ImageJ to analyze the influence of the cross-linking reaction. As reported in Table 1, pore diameter is independent of GTA concentrations, showing that freeze-drying, rather than cross-linking, plays a role in pore generation. Freeze-drying is basically a phase separation process in which the fluid phase sublimes leaving empty spaces in the structure: pore features are therefore strictly dependent on freeze-drying parameters such as temperature and pressure. Moreover, the freezing temperature, which was kept constant during sample preparation, is important in determining the dimension of water crystals. For comparison the table also reports the average pore size of human pulmonary tissue.
Table 1. Average pore size of realized samples and parenchyma.
| Sample | Average diameter (μm) |
|---|---|
| Gelatin 5% w/v + GTA 10 mM |
274 ± 109 |
| Gelatin 5% w/v + GTA 1 mM |
243 ± 151 |
| Gelatin 5% w/v + GTA 0.1 mM |
274 ± 131 |
| Human lung alveolar parenchyma | 20015 |
Pore size was evaluated using imaging analysis, while the tissue value reported is taken from reference 15.
Porosity of scaffolds
In order to measure the porosity and the imbibition properties of cross-linked gelatin samples, swelling measurements were performed. The porosity or weight fraction of samples was evaluated as the weight ratio between dry and wet samples (Eqs. 1 and 2). Sample porosity was measured from the swelling data at 1, 24 and 48 h of swelling. As reported in Table 2, like the pore size, the porosity of samples is also independent of the cross-linking reaction; in particular the measured porosity is similar to that of the healthy lung (0.99), as reported by Lande and Mitzner.7
Table 2. Porosity of gelatin samples (with different degrees of cross-linking) and lung tissue.
| Sample | 1 h swelling | 24 h swelling | 48 h swelling |
|---|---|---|---|
| Gelatin 5% w/v + GTA 10 mM |
0.877 ± 0.016 |
0.921 ± 0.002 |
0.935 ± 0.004 |
| Gelatin 5% w/v + GTA 1 mM |
0.890 ± 0.010 |
0.951 ± 0.002 |
0.949 ± 0.012 |
| Gelatin 5% w/v + GTA 0.1 mM |
0.887 ± 0.007 |
0.956 ± 0.002 |
0.888 ± 0.067 |
| Healthy human lung | 0.997 | ||
Measurements were performed at different time points (1, 24 and 48 h). For the sake of comparison the porosity of healthy lung tissue is reported.7
Swelling properties
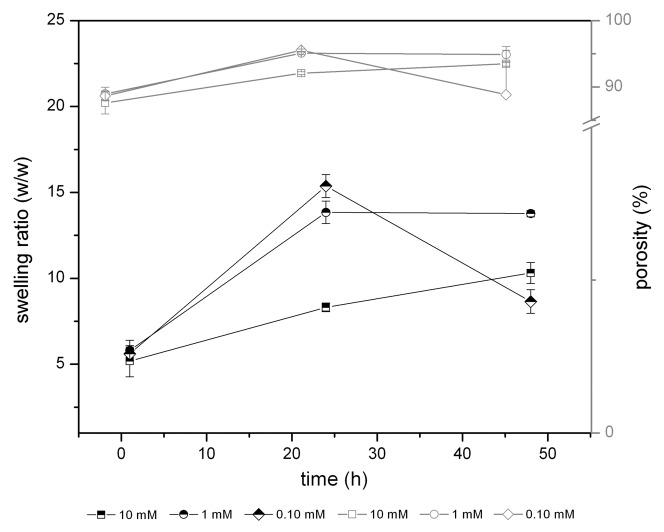
While pore size and porosity are not related to the degree of cross-linking, the hydrophilic nature and consequent interaction with water of the scaffolds is correlated with GTA concentration. As reported in Figure 3, swelling tests reveal that cross-linking reduces the rate of water absorption. In fact, GTA reacts with the amino group of gelatin, thus the interactions between water and the polymeric network are reduced.6,16 Samples prepared with low GTA concentrations swell absorbing almost 15 times their dry weight in water after 24 h, while samples prepared with higher GTA swell to only 8 times as they have less water retention capacity. In general, after 48 h all samples are fully hydrated to the same extent.
Figure 3. Swelling ratio (black lines) vs. porosity (gray lines) behavior in time.
Mechanical properties
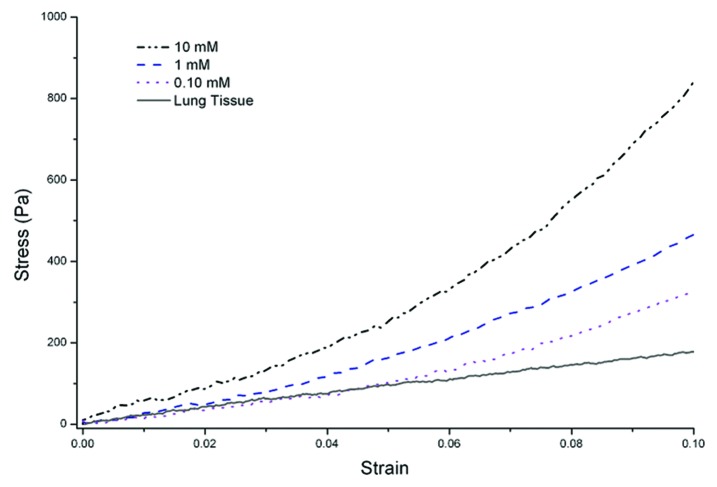
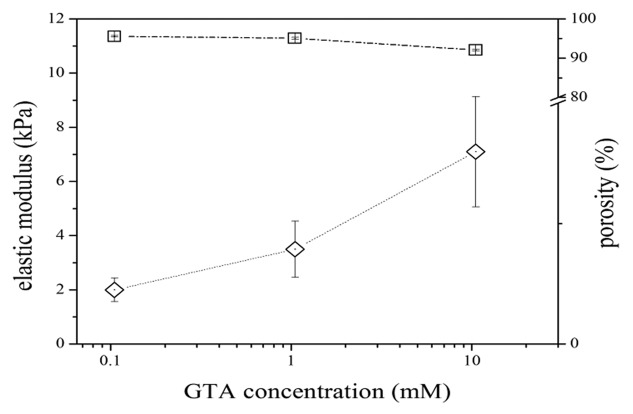
The compressive stress-strain plots are used to evaluate the elastic properties of gelatin porous samples. Stress-strain plots reported in Figure 4 show a nonlinear behavior, typical of soft tissues7 while Figure 5 summarizes the relationship between degree of cross-linking and resultant stiffness of porous gelatin and its porosity. There is clearly a proportional relation between the degree of cross-links and stiffness, underlining that GTA concentration modulates the mechanical properties of the matrix, but does not contribute to the porosity.
Figure 4. Compressive stress-strain plots of porous structures (GTA 10 mM, 1 mM and 0.1 mM) and adult bovine pulmonary tissue.
Figure 5. Influence of cross-linking degree on elastic modulus (squares) and water absorption (diamonds) properties of cross-linked gelatin. For the sake of comparison, pulmonary tissue has an elastic modulus of 1.4 kPa (experimental data).
Reproduction of B-lines
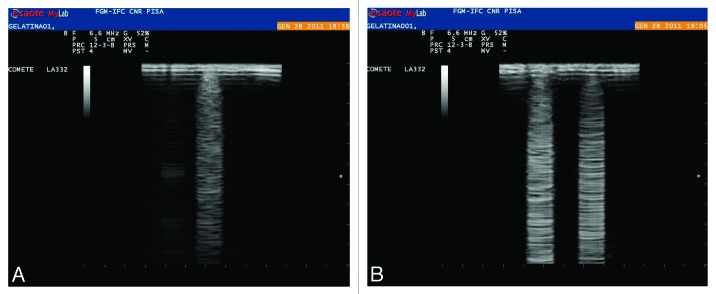
A simple method was used to reproduce B-lines: a controlled volume of (dH2O) was deposited on the surface of porous gelatin samples. The water droplet absorbs into the surface of the material and does not penetrate significantly during the time of observation (a few minutes), giving rise to a comet-tail shaped sign, typical of B-lines as observed using an ultrasound probe. We did not observe any B-lines in uncross-linked gelatin because the gel is very hydrophilic and the water drop immediately spreads throughout the gel. Furthermore the formation of B-lines was independent of the concentration of GTA used in our studies. Using this method it is possible to distinguish the presence of discrete drops and mimic different states of lung disease characterized by different degrees of water accumulation (Fig. 6).
Figure 6. B-line reproduction by hydration of gelatin samples using different controlled water volumes. One 10 μL drop (A) and two drops (B) spaced about 1 cm apart.
Materials and Methods
Materials
All materials were purchased from Sigma-Aldrich. A 5% w/v gelatin solution was prepared by dissolving gelatin (Type A) in deionized water dH2O stirring the solution for 1 h at 50°C. A batch cross-linking solution of glutaraldehyde (GTA) in water was prepared with a concentration of 0.1 M and used for sequential dilution. A 40% v/v ethanol: dH2O solution was used to rinse samples.
Preparation of porous gelatin matrices
Gelatin sponges were prepared to evaluate the porosity and mechanical properties as functions of cross-linking conditions as well as to recreate B-lines in an in vitro model. In particular, the preparation method was divided into two steps. In the first step gelatin was cross-linked using GTA with different concentration (nominated GC); then, in order to obtain a porous matrix, a freeze-drying process was used as described by Lien et al.17 Briefly GTA was added to a 5% w/v gelatin solution to obtain a final volume of 1 mL and 0.1, 1 and 10 mM GC scaffolds were fabricated. The scaffolds were kept in a plastic tube (internal diameter 12 mm) at 25°C for 12 h, until the cross-link reaction had occurred. Two cooling steps were used to freeze the samples; the first step in a refrigerator at 4°C for 6 h and then the second step in a -20°C freezer over-night. Finally samples were freeze-dried (-50°C, 150 mBar) until all water content was removed.
Measurement of swelling ratio
The water absorption capability of porous gelatin structures was determined by immersing freeze-dried samples in water for 1, 24 and 48 h. The swelling ratio was calculated according the following equation (Eq. 1):
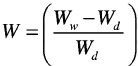 |
In which Wd is the air-dried scaffold weight and Ww is the weight of the wet scaffold.10
Porosity evaluation
The porosity was evaluated by imbibition method and was assumed as the gelatin volume fraction in the swollen samples (φ). Through the water saturation, pore volume was evaluated by weighing swollen and dried samples. The gelatin volume fraction was calculated according to Equation 2:18,19
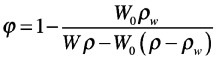 |
in which W0 is the dry weight of the sample, W is the weight of the swollen sample, ρw is the density of the water at RT (room temperature), and ρ is the density of the dry gelatin sample. Pore dimension was evaluated through histological analysis. Samples were embedded and fixed in Tissue-Tek O.C.T. before cryo-sectioning. Horizontal sections of 10 μm thickness were obtained from the cylindrical scaffolds and then observed with an optical microscope (Olympus IX81, Olympus Italia, 4X objective).
Measurement of mechanical properties
Compressive mechanical tests were performed using a twin column testing machine Zwick-Roell Z005 Instron (Zwick Testing Machines, Ltd.). The samples were tested at RT and immersed in water to mimic the aqueous physiological environment and hydrated in dH2O before measurements. Hydrated scaffolds were compressed at 10% of the initial length using a 0.05 mm·s−1 strain rate. Data were then post-processed and stress-strain curves were obtained. The resultant stiffness of the sponges was evaluated within the first linear zone of the stress-strain curve (i.e., within 2% strain). Fresh bovine adult lung tissue was obtained from a local abattoir and small cubes of about 8 cm3 were also tested as a comparative control.
Ultrasound measurements
The echo-ultrasonic signal is the reflection signature of tissues with different acoustic impedance. To reproduce sonographic B-lines, which indicate the presence of extravascular lung water,3 freeze-dried samples were hydrated using a controlled and confined volume of dH2O, drop by drop on the sample surface. The freeze-dried gelatin sponges were prepared in 5 cm diameter Petri dishes to allow contact with the ultrasonic probe. A thin piece of cellophane film placed between the probe and sample was used to protect the hydrated sample from ultrasound gel and mimic the presence of the lung pleura. Scans of samples were performed with a commercial instrument (MyLab30Gold, Esaote), with allowed frequency ranges 3–11 MHz, using a 6.6 MHz linear probe. The linear probe was chosen in order to obtain rectangular images and a reduced penetration into the surface of sample (5 cm of depth penetration). Tests were performed by depositing a 10 µL drop in the center of the dish and recording the sonographic signal, as illustrated in Figure 7. The test was then repeated depositing two drops spaced about 1 cm apart.
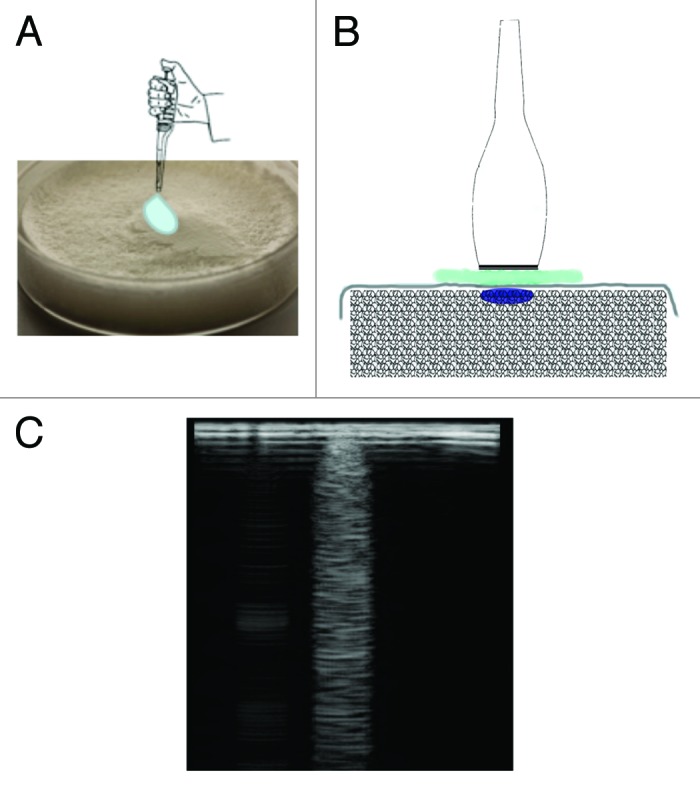
Figure 7. A controlled volume of dH2O on the surface of a freeze-dried sample generates a characteristic B-line: deposition of a drop of water (A), ultrasound probe placed over the drop (B) and detection of the B-line (C).
Conclusion
A poro-elastic replica of the lung that reproduces its elastic and morphologic structure as well as its response to ultrasound through the reproduction of B-lines was realized. The reproduction of B-lines allows the in vitro investigation of the presence of extravascular lung water and can enable the extraction of parameters for the quantitative evaluation of the cardiogenic or pneumogenic pathology interstitial syndrome. Future studies will focus on the modulation of B-line intensity by changing hydration levels in order to better mimic the evolution of lung disease. This simple and reproducible phantom mimics the acoustic impedance of pulmonary tissue and could be an extremely useful tool for modeling healthy and pathological lung ultrasonography.
Acknowledgments
The authors thank Prof. William A. Olexik and Lawrence Berkeley National Lab for the permission to publish respectively lung microscope and alveolar SEM micrographs.
Disclosure of Potential Conflicts of Interest
All the authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/19835
References
- 1.Chen P, Marsilio E, Goldstein RH, Yannas IV, Spector M. Formation of lung alveolar-like structures in collagen-glycosaminoglycan scaffolds in vitro. Tissue Eng. 2005;11:1436–48. doi: 10.1089/ten.2005.11.1436. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein D, Me´zière G, Biderman P, Gepner A, Barre´ O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–6. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 3.Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265–70. doi: 10.1016/j.amjcard.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extra-vascular lung water. Am J Echocardiogr. 2006;19:356–62. doi: 10.1016/j.echo.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Fields S, Dunn F. Letter: Correlation of echographic visualizability of tissue with biological composition and physiological state. J Acoust Soc Am. 1973;54:809–12. doi: 10.1121/1.1913668. [DOI] [PubMed] [Google Scholar]
- 6.Mark HF,ed. Encyclopedia of Polymer Science and Technology. 3. Wiley-Interscience, 2004: 1484-515. [Google Scholar]
- 7.Lande B, Mitzner W. Analysis of lung parenchyma as a parametric porous medium. J Appl Physiol. 2006;101:926–33. doi: 10.1152/japplphysiol.01548.2005. [DOI] [PubMed] [Google Scholar]
- 8.Veis A. The macromolecular chemistry of gelatin. New York, London. Academy. 1964 In press. [Google Scholar]
- 9.Pezron I, Djabourov M, Leblond J. Conformation of gelatin chains in aqueous solutions: 1. A light and small-angle neutron scattering study. Polymer (Guildf) 1991;32:3201–10. doi: 10.1016/0032-3861(91)90143-7. [DOI] [Google Scholar]
- 10.Ross-Murphy SB. Structure and rheology of gelatin gels: recent progress. Polymer (Guildf) 1992;33:2622–7. doi: 10.1016/0032-3861(92)91146-S. [DOI] [Google Scholar]
- 11.Bigi A, Cojazzi G, Panzavolta S, Rubini K, Roveri N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials. 2001;22:763–8. doi: 10.1016/S0142-9612(00)00236-2. [DOI] [PubMed] [Google Scholar]
- 12.Olde Damink LHH, Dijkstra PJ, Luyn MJA, Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Mater Sci Mater Med. 1995;6:460–72. doi: 10.1007/BF00123371. [DOI] [Google Scholar]
- 13.Schoof H, Apel J, Heschel I, Rau G. Control of pore structure and size in freeze-dried collagen sponges. J Biomed Mater Res. 2001;58:352–7. doi: 10.1002/jbm.1028. [DOI] [PubMed] [Google Scholar]
- 14.Microscopic Pictures (Internet). Montgomery, MD USA: Montgomery College; August 2011. http://www.montgomerycollege.edu/~wolexik/205_histology__page.htm
- 15.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–4. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka TT. Gels. Sci Am. 1981;244:110–23. doi: 10.1038/scientificamerican0181-124. [DOI] [PubMed] [Google Scholar]
- 17.Lien SM, Li WT, Huang TJ. Genepin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method. Mater Sci Eng C. 2007;208:36–43. [Google Scholar]
- 18.Martucci JF, Ruseckaite RA, Vàzquez A. Creep of glutaraldehyde-crosslinked gelatin films. Mater Sci Eng. 2006;435:681–6. doi: 10.1016/j.msea.2006.07.097. [DOI] [Google Scholar]
- 19.Mwangi JW, Ofner CM., III Crosslinked gelatin matrices: release of a random coil macromolecular solute. Int J Pharm. 2004;278:319–27. doi: 10.1016/j.ijpharm.2004.03.024. [DOI] [PubMed] [Google Scholar]