Abstract
Human serum albumin (HSA) is an ideal natural colloid that has been widely used in clinical practice for supplemental albumin or as a plasma substitute during therapeutic plasma exchanges to redress hypoproteinemia. However, a paucity of well-designed clinical trials, a lack of a clear cut survival benefit, and frequent case reports of adverse drug reaction (ADR) make the use of HSA controversial. This study aims to review and to comment on the reported ADRs of HSA in the People’s Republic of China, so as to provide the basis for rational HSA use in clinical settings. Data on the ADR case reports from HSA administration between January 1990 and December 2012 available from the China National Knowledge Infrastructure (CNKI) database, Wanfang data (WF), and Chinese Biomedical Literature (CBM) were reviewed. The reasons for using HSA, the types of ADRs, the causality of ADRs and the rationality for HSA administration were extracted and analyzed. In total, 61 cases of ADR reports were identified of which the primary disease of patients using HSA was malignant tumor (34.42%). The primary ADR was anaphylaxis (59.02%). Of the 61 cases, 30 were caused by irrational use of HSA. The most common irrational use was off-label use (56.67%), followed by inappropriate infusion rate. Therefore, we conclude that to avoid the occurrence of ADRs, guidelines for using HSA are needed to guarantee its rational use and HSA should be used strictly according to these guidelines. In addition, medical staff, including clinical pharmacists and nurses, should pay more attention to the patients who inject HSA to ensure its safe use in the clinic.
Keywords: HSA, off-label use, ADR, plasma substitute, albumin, hypoproteinemia
Introduction
Albumin is the most abundant plasma protein in healthy individuals with normal values of approximately 40–55 g/L, accounting for 50%–60% of the measured serum protein.1 Albumin plays an important role in maintaining colloid osmotic pressure, metabolite transport, as well as possessing circulatory protective and anti-oxidant properties.2 Human serum albumin (HSA) is a plasma product that was first purified and prepared in the laboratory in the late nineteenth century and the early twentieth century, and it has been used in the clinical practice for expanding blood volume and supplying plasma-albumin for more than 60 years.3 HSA is supplied for the management of patients with serious medical conditions including hypovolemia, shock, surgical blood loss, burns, trauma, acute respiratory distress syndrome, hemorrhage, acute liver failure, chronic liver disease, cardiopulmonary bypass, hemodialysis, nutrition support, resuscitation, and hypoalbuminemia.4–6 However, the use of HSA is controversial and it has been hotly debated over the past 20 years whether critically ill patients benefit from its administration. In spite of this, HSA is widely used in clinical practice in the People’s Republic of China, which has resulted in increased adverse drug reaction (ADR) case reports. In this article, we reviewed data on ADR case reports from HSA administration in primary Chinese databases, analyzed the possible factors for the occurrence of these ADRs, and aimed to petition HSA’s rational use in order to ensure patient safety.
Methods
Research strategy
A comprehensive search for published ADR case reports of HSA was performed in the Chinese National Knowledge Infrastructure (CNKI), Wanfang data (WF), and Chinese Biomedical Literature (CBM). The search keywords used were as follows: albumin, human serum albumin, adverse drug reaction, side effect, toxicity, case report (in Chinese). The search timespan was from January 1990 to December 2012. In addition, the references in any retrieved case reports were traced to identify any case report that might be missed out.
Inclusion and exclusion criteria
Case reports were included as long as they reported any side effect or toxicity of HSA application and were published in a Chinese journal. Others were excluded if: 1) it was a literature systematic review; 2) it was a Chinese translation of an ADR case reported by non-Chinese authors; 3) it was not Chinese literature; 4) it was a duplicate publication; or 5) it was not a journal article.
Selection process
An initial screening was conducted based on titles or abstracts, following by selection based on full-text review. Case reports were considered eligible if they met the inclusion criteria.
Data extraction
Patient characteristics, including allergic history, serum albumin level, infusion rate, onset of ADRs, outcome, types of ADRs, and primary diseases were extracted from each case report. The rationality of HSA administration was analyzed and ADR causality was assessed using the Naranjo scale.7
Results
Patient characteristics
A total of 61 cases of ADR were reported using our search criteria in the above Chinese databases. Table 1 lists the primary information of the patients. More reports of ADRs involved male (65.57%) versus female (34.43%) patients. Their ages ranged from 5 days to 82 years old. In general, the onset of ADRs was acute (52.46% within 1-hour), but 78.69% patients recovered after symptomatic treatment. According to the Naranjo scale, 10 (16.39%) reactions were assessed to be definite and 45 (73.77%) as probable, while the others could not be assessed due to a lack of detailed descriptions.
Table 1.
Primary patient information
| Characteristic | Numbers (%) |
|---|---|
| Sex | |
| Male | 40 (65.57%) |
| Female | 21 (34.43%) |
| Age | |
| ≤15 | 4 (6.56%) |
| 15–45 | 16 (26.23%) |
| 45–60 | 17 (27.87%) |
| >60 | 24 (39.34%) |
| Allergic history | |
| Yes | 3 (4.91%) |
| No | 58 (95.09%) |
| Serum albumin level | |
| 25–35 g/L | 7 (11.48%) |
| <25 g/L | 1 (1.64%) |
| Unknown | 53 (86.88%) |
| Infusion rate | |
| ≤2 mL/minute | 11 (18.03%) |
| >2 mL/minute | 4 (6.56%) |
| Unknown | 46 (75.41%) |
| Onset of adverse drug reactions | |
| Acute (<1 hour) | 32 (52.46%) |
| Sub-acute (1–24 hours) | 28 (45.90%) |
| Latent (>2 days) | 1 (1.64%) |
| Causality | |
| Definite | 10 (16.39%) |
| Probable | 45 (73.77%) |
| Unknown | 6 (9.84%) |
| Outcome | |
| Fatal | 6 (9.84%) |
| Recovering | 48 (78.69%) |
| Unknown | 7 (11.47%) |
Adverse drug reaction types
The total number of serious non-fatal and fatal adverse events spontaneously reported was 55 and 6, respectively (Table 2). The primary adverse event reports were anaphylaxis (59.02%), followed by pyrogenic reaction and cardiac insufficiency (11.47% each). Of the 59.02% anaphylactic ADRs, anaphylactic shock accounted for 37.70% (n=23), including 9.84% (n=6) who died of shock, followed by 21.31% general allergic reactions.
Table 2.
Human serum albumin adverse drug reaction (ADR) cases (n=61)
| ADRs | Cases (%) (n=61) |
|---|---|
| Anaphylaxis | |
| Shock | 23 (37.70%) |
| Allergic reaction | 13 (21.31%) |
| Pyrogenic reaction | 7 (11.47%) |
| Cardiac insufficiency | 7 (11.47%) |
| Hemolysis | 3 (4.92%) |
| Kidney damage | 3 (4.92%) |
| Pneumonedema | 1 (1.64%) |
| Others | |
| Swelling of parotid gland | 1 (1.64%) |
| Swelling of larynx | 1 (1.64%) |
| Limb anesthesia, convulsion | 1 (1.64%) |
| Mental disorders | 1 (1.64%) |
Role of HSA in these patients
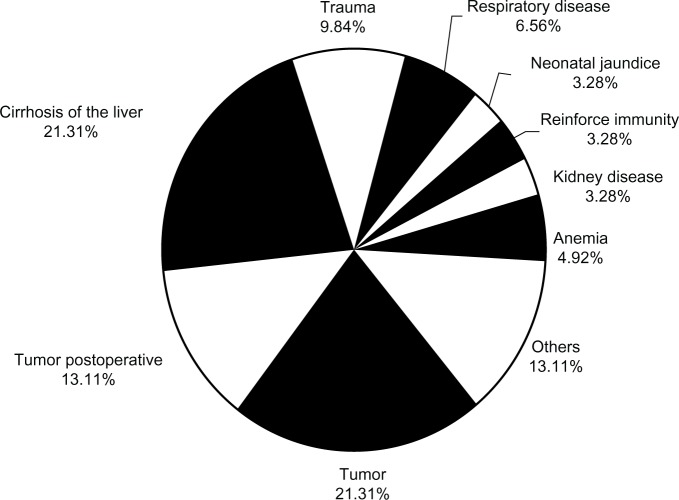
HSA was mainly administrated as a supplementary treatment for various diseases. Primary diseases of patients who use HSA are presented in Figure 1. Malignant tumor accounted for the most frequent use (34.42% in total, 21.31% for primary tumor treatment and 13.11% for postoperative tumor treatment), followed by liver diseases (21.31%) and trauma (9.84%).
Figure 1.
Primary diseases of patients who use HSA (human serum albumin). Primary diseases, rate (n=61).
Irrational use of HSA
Irrational use of HSA is shown in Table 3. Among the 61 case reports, 30 (49.18%) ADRs were caused by irrational use of HSA, and of the 30 cases irrational use of HSA, off-label use contributed 56.67% of the irrational drug use rate, followed by inappropriate infusion rate. The other 31 case of ADRs could not be evaluated due to a lack of detailed descriptions in these reports.
Table 3.
Irrational use of human serum albumin (n=30)
| Reasons for ADRs | Cases (%) (n=30) |
|---|---|
| Off-label use (abuse) | 17 (56.67%) |
| Long time infusion | 1 (3.33%) |
| Infusion too fast | 4 (13.33%) |
| Drug quality | 4 (13.33%) |
| Patients’ physique | 2 (6.67%) |
| Adverse drug interaction | 2 (6.67%) |
Abbreviation: ADR, adverse drug reaction.
Discussion
In recent years, many reports of the wide use and even abuse of HSA have attracted medical workers’ attention to evaluate its safety and efficacy. The present work covering 1990–2012 evaluated reports related to HSA administration and found 61 ADRs occurred during this period in the People’s Republic of China.
However, adverse reactions to HSA occurring outside of the People’s Republic of China are rare.8 Worldwide, there was a total of 211 ADRs reported from 1998 to 2000 and no reports of death related to albumin were documented from 1990 to the end of 2000.9,10 Unfortunately, the situation is not so optimistic in the People’s Republic of China: case reports of HSA adverse reactions are continuously emerging.
The widespread irrational and unnecessary use of HSA is rampant in the People’s Republic of China. Specifically, HSA is inappropriately prescribed in both the elderly and young. In our search, the oldest patient was 82 years old while the youngest was just 5 days; 39.34% ADRs occurred on elderly patients (>60 years). It is critical that the elderly and children should be administrated HSA using an appropriately reduced dosage.
There are some misunderstandings regarding HSA use. First, traditional Chinese medicine advocates food and medicine tonic and most Chinese believe that HSA is a primary medicine tonic and consider HSA an omnipotent medicine. They consider HSA a health care product and administration it for nutrition support or immunity enhancement. However, the University Hospital Consortium (UHC) recommend HSA only for critically ill patients with seralbumin levels lower than 15 g/L, and conditionally when seralbumin levels are between 15–20 g/L.11,12 Unfortunately, adherence to these recommendations and guidelines for the indication of HSA use in the People’s Republic of China is low; standards for treatment are considered lower than 25 g/L, which not only increases the number of people treated with HSA, but also offers no consideration for the primary disease. Even more surprising is the fact that there are patients who are administered HSA with seralbumin levels higher than 25 g/L. In our survey, only 22 (36.07%) cases reported hypoproteinemia, but just eight cases had clearly defined seralbumin values and of these eight patients, seven had seralbumin levels higher than 25 g/L.
To date, no guidelines or standards for the rational use of HSA have been proposed in the People’s Republic of China. Evidence-based medicine and specialists’ opinions suggest that albumin can be indicated in acute conditions for the purpose of expanding the volume and maintaining proper circulation, and in some chronic states, such as hypoalbuminemia. The widely shared and fully agreed indications for the appropriate use of human albumin are paracentesis, therapeutic plasmapheresis, and spontaneous bacterial peritonitis. Indications that are occasionally appropriate when other criteria are fulfilled include cirrhosis of the liver with refractory ascites inpatients with serum albumin <20 g/L, contraindications for the use of non-protein colloids; nephrotic syndrome with albumin <20 g/L; and burns after the first 24 hours when the burned surface area is >30% of the body.13,14 Similarly, based on clinical evidence, HSA is not indicated for the following conditions: albuminemia >25 g/L; malnutrition; hypoalbuminemia without edema and acute hypotension; burns within the first 24 hours; wound healing; non-hemorrhagic shock; hemodialysis; ascites responsive to diuretics; or cerebral ischemia.15,16 According to this standard, 17 cases of off-label use were found in this study and eight cases were infused for nutritional support, two for hepatitis, two for fracture with albumin >25 g/L, and others for infusion for acute appendicitis, suppurative arthritis, cholecystitis, pneumonia, and wound healing.
The quality of HSA may be an important factor for inducing ADRs. As a blood product, although HSA is pasteurized to reduce the risk of infection, it may be contaminated by human immunodeficiency virus (HIV) and hepatitis B virus resulting from being missed at the time of screening.17 The quality of albumin preparations may also differ between manufacturers. Many factors, such as production technology, flowsheet, purity level of the environment, reservoir and packaging vessels, and so on, may lead to differing HSA quality. Even different batches from the same manufacturer may have discrepancies, which may result in the occurrence of ADRs.18 Moreover, as a biological product, it is not as pure as it is labeled.19 The presence of endotoxins, oligomers, polymers, heme compounds, bradykinin, prekallikrein, and other albumin-bound proteins can alter the quality of HSA,20 and even result in anaphylaxis. Endotoxins, for instance, cause a series of adverse reactions including fever, hemolytic reaction, acute kidney failure, shock, and even death.21
Rapid infusion (>2 mL/minute) of HSA may cause a sudden drop in systemic blood pressure, especially in elderly patients and those at risk of congestive heart failure, and this can manifest as congestive heart failure. Slow infusion of HSA is not recommended as it is clearly indicated that HSA should be used with in 4 hours of opening.22 A review by Wang23 reports the death of a neonate from a slow infusion. The recommended infusion rate is 2 mL·min−1 in 4 hours.
In fact, some meta-analyses demonstrate that HSA does not reduce mortality when compared to cheaper expansion agents, such as hydroxyethyl starch or saline in critically ill patients with hypoalbuminemia or hypovolemia (from 1998).24,25 In fact, HSA even increased the mortality rate among critically ill patients.26 Later, many clinical trials found that HSA is not more effective or safer than any other colloid or crystalloid solution and may be detrimental in trauma patients, and albumin will not reduce the risk of dying in patients with large amounts of blood loss.27–32 All these controversies have put the safety and rational use of HSA n the spotlight. In addition, HSA is costly and excessively consumed worldwide; it even uses up 10% of the income of some hospitals of Bologna, Italy.33 The situation is similar in big Chinese hospitals in which the consumption of HSA is large and demand exceeding supply, though the price was over 300 RMB ($50 US dollars) per 10 g in 2012.
Based on the frequency of ADRs and for the reasons mentioned above, we appeal for guidelines to be issued to ensure the rational use of HSA in the People’s Republic of China. Pharmaceutical care from the clinical pharmacists needs to be implemented in big hospitals in the People’s Republic of China and medication education should be conducted for the Chinese society as a whole. Health professionals should educate patients and their relatives on the fact that HSA is not an omnipotent drug and not restorative and that it is a biological product with serious risks.34,35
Limitations of this study are due to our inclusion and exclusion criteria; the data is solely based on the cases that are published in journals, which is less than the number obtained from the national center for ADRs.
Conclusion
HSA is not only expensive but also an unsafe biological product. When using HSA, attention should be paid to potential ADRs. Guidelines for using HSA are needed to guarantee its rational use, and future efforts are needed to define HSA indications to avoid the irrational use and to ensure patient safety.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33(3):209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Mendez CM, McClain CJ, Marsano LS. Albumin therapy in clinical practice. Nutr Clin Pract. 2005;20(3):314–320. doi: 10.1177/0115426505020003314. [DOI] [PubMed] [Google Scholar]
- 3.Doweiko JP, Nompleggi DJ. Use of albumin as a volume expander. JPEN J Parenter Enteral Nutr. 1991;15(4):484–487. doi: 10.1177/0148607191015004484. [DOI] [PubMed] [Google Scholar]
- 4.Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998;53(8):789–803. doi: 10.1046/j.1365-2044.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 5.Liberati A, Moja L, Moschetti I, Gensini GF, Gusinu R. Human albumin solution for resuscitation and volume expansion in critically ill patients. Intern Emerg Med. 2006;1(3):243–245. doi: 10.1007/BF02934748. [DOI] [PubMed] [Google Scholar]
- 6.Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossettias G, Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7(3):216–234. doi: 10.2450/2009.0094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmac Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 8.von Hoegen I, Waller C. Safety of human albumin based on spontaneously reported serious adverse events. Crit Care Med. 2001;29(5):994–996. doi: 10.1097/00003246-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Wilkes MM, Navickis RJ. Safety of human albumin – serious adverse events reported worldwide in 1998–2000. Br J Anaesth. 2003;91(5):625–630. doi: 10.1093/bja/aeg233. [DOI] [PubMed] [Google Scholar]
- 10.Vincent JL. Resuscitation using albumin in critically ill patients: research in patients at high risk of complications is now needed. BMJ. 2006;333(7577):1029–1030. doi: 10.1136/bmj.39029.490081.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen LC, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med. 1995;155(4):373–379. doi: 10.1001/archinte.155.4.373. [DOI] [PubMed] [Google Scholar]
- 12.University HealthSystem Consortium . Technology assessment: albumin, non-protein colloid, and crystalloid solutions. Oak Brook: University Health System Consortium; 2000. [Google Scholar]
- 13.Prinoth O, Strada P. Proposta di linee guida al corretto uso dell’albumina [Proposal for guidelines on the correct use of albumin] Il Servizio Trasfusionale. 2002;3:5–10. Italian. [Google Scholar]
- 14.Vermeulen LC, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med. 1995;155(4):373–379. doi: 10.1001/archinte.155.4.373. [DOI] [PubMed] [Google Scholar]
- 15.Fleck A, Raines G, Hawker F, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1(8432):781–784. doi: 10.1016/s0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 16.Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients [review] Cochrane Database Syst Rev. 2007:CD000567. doi: 10.1002/14651858.CD000567.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 18.Evans TW. Review article: albumin as a drug– biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002;16(Suppl 5):6–11. doi: 10.1046/j.1365-2036.16.s5.2.x. [DOI] [PubMed] [Google Scholar]
- 19.Naderi M, Eshghi P, Saneei Moghaddam E, et al. Safety of human blood products in rare bleeding disorders in southeast of Iran. Haemophilia. 2013;19(2):e90–e92. doi: 10.1111/hae.12068. [DOI] [PubMed] [Google Scholar]
- 20.Yamey G. Albumin industry launches global promotion. BMJ. 2000;320(7234):533. [PMC free article] [PubMed] [Google Scholar]
- 21.Van Liedekerke BM, Nelis HJ, Kint JA, Vanneste FW, De Leenheer AP. Quality control of albumin solutions by size-exclusion high-performance liquid chromatography, isoelectric focusing, and two-dimensional immunoelectrophoresis. J Pharm Sci. 1991;80(1):11–16. doi: 10.1002/jps.2600800104. [DOI] [PubMed] [Google Scholar]
- 22.Grgicevic D. Blood safety. Nat Med. 1995;1(6):493. doi: 10.1038/nm0695-493b. [DOI] [PubMed] [Google Scholar]
- 23.Wang MY. Two fatal allergic reactions caused by human serum albumin. China Pharmaceuticals. 2011;20:83. [Google Scholar]
- 24.Cochrane Injuries Group Albumin Reviewers Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317(7153):235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkes MM, Navickis RJ. Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135(3):149–164. doi: 10.7326/0003-4819-135-3-200108070-00007. [DOI] [PubMed] [Google Scholar]
- 26.Offringa M, Gemke RJ, Henny CP. [Excess mortality in critically ill patients after treatment with human albumin] Ned Tijdschr Geneeskd. 1998;142(33):1855–1858. Dutch. [PubMed] [Google Scholar]
- 27.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 28.Aramwit P, Kasettratat N. Evaluation of serum albumin utilization in inpatient at a private hospital in Bangkok. Yakugaku Zasshi. 2004;124(9):631–634. doi: 10.1248/yakushi.124.631. [DOI] [PubMed] [Google Scholar]
- 29.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, SAFE Study Investigators A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 30.Alderson P, Bunn F, Lefebvre C, et al. Albumin Reviewers Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2004:CD001208. doi: 10.1002/14651858.CD001208.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Liberati A, Moja L, Moschetti I, Gensini GF, Gusinu R. Human albumin solution for resuscitation and volume expansion in critically ill patients. Intern Emerg Med. 2006;1(3):243–245. doi: 10.1007/BF02934748. [DOI] [PubMed] [Google Scholar]
- 32.Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients [review] Cochrane Database Syst Rev. 2011:CD001208. doi: 10.1002/14651858.CD001208.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirici-Cappa F, Caraceni P, Domenicali M, et al. How albumin administration for cirrhosis impacts on hospital albumin consumption and expenditure. World J Gastroenterol. 2011;17(30):3479–3486. doi: 10.3748/wjg.v17.i30.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sikuler E, Shapira A, Mor-Yosef S, et al. [Rational use of albumin] Harefuah. 2000;139(5–6):190–3. 246. Hebrew. [PubMed] [Google Scholar]
- 35.Woodman R. Doctors advised to take special care with human albumin. BMJ. 1999;318(7199):1643. doi: 10.1136/bmj.318.7199.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]