Abstract
Purpose
To assess the safety of duloxetine with regards to bleeding-related events in patients who concomitantly did, versus did not, use nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin.
Methods
Safety data from all placebo-controlled trials of duloxetine conducted between December 1993 and December 2010, and post-marketing reports from duloxetine-treated patients in the US Food and Drug Administration Adverse Event Reporting System (FAERS), were searched for bleeding-related treatment-emergent adverse events (TEAEs). The percentage of patients with bleeding-related TEAEs was summarized and compared between treatment groups in all the placebo-controlled studies. Differences between NSAID user and non-user subgroups from clinical trial data were analyzed by a logistic regression model that included therapy, NSAID use, and therapy-by-NSAID subgroup interaction. In addition, to determine if higher duloxetine doses are associated with an increased incidence of bleeding-related TEAEs, and whether the use of concomitant NSAIDs might influence the dose effect if one exists, placebo-controlled clinical trials with duloxetine fixed doses of 60 mg, 120 mg, and placebo were analyzed. Also, the incidence of bleeding-related TEAEs reported for duloxetine alone was compared with the incidence in patients treated with duloxetine and concomitant NSAIDs. Finally, the number of bleeding-related cases reported for duloxetine in the FAERS database was compared with the numbers reported for all other drugs.
Results
Across duloxetine clinical trials, there was a significantly greater incidence of bleeding-related TEAEs in duloxetine- versus placebo-treated patients overall and also in those patients who did not take concomitant NSAIDS, but no significant difference was seen among those patients who did take concomitant NSAIDS. There was no significant difference in the incidence of bleeding-related TEAEs in the subset of patients treated with duloxetine 120 mg once daily versus those treated with 60 mg once daily regardless of concomitant NSAID use. The combination of duloxetine and NSAIDs was associated with a statistically significantly higher incidence of bleeding-related TEAEs compared with duloxetine alone. A similarly higher incidence of bleeding-related TEAEs was seen in patients treated with placebo and concomitant NSAIDs compared with placebo alone. Bleeding-related TEAEs reported in the FAERS database were disproportionally more frequent for duloxetine taken with NSAIDs compared with the full FAERS background, but there was no difference in the reporting of bleeding-related TEAEs when the cases reported for duloxetine taken with NSAIDs were compared against the cases reported for NSAIDs alone.
Conclusion
Concomitant use of NSAIDs was associated with a higher incidence of bleeding-related TEAEs in clinical trials regardless of whether patients were taking duloxetine or placebo; bleeding-related TEAEs did not appear to increase along with duloxetine dose regardless of NSAID use. In spontaneously reported post-marketing data, the combination of duloxetine and NSAID use was not associated with an increased reporting of bleeding-related events when compared to NSAID use alone.
Keywords: antidepressant, gastrointestinal bleeding, NSAID, aspirin
Introduction
Medications that modulate serotoninergic neurotransmission, like selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs), are commonly prescribed to treat depression, anxiety disorders, and premenstrual dysphoria,1 as well as chronic pain conditions. During the past decade, numerous reports have been published on the risk of abnormal gastrointestinal (GI) bleeding as an infrequent adverse event (AE) associated with serotonin-modulating drugs.2 Patients with musculoskeletal pain conditions, as well as patients with depression and anxiety who experience chronic pain, may also be treated with nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin, which are also associated with GI bleeding.3,4 Concomitant use of NSAIDs with SSRIs or SNRIs may potentiate bleeding-related AEs.5
The tendency for GI bleeding during treatment with serotonin-modulating drugs is linked to a decrease in blood platelet function that is dependent on serotonin. Platelets release serotonin at sites of vascular injury, which signals vasoconstriction and amplification of platelet aggregation for hemostatic thrombus formation.6 However, platelets accumulate serotonin via transporters.7 Treatment with therapeutic doses of serotonin-modulating agents blocks these transporters,8 ultimately impairing serotonin accumulation and thereby rendering platelets dysfunctional.
Duloxetine (Cymbalta®; Eli Lilly and Company, Indianapolis, IN, USA) is an SNRI that is approved by the US Food and Drug Administration (FDA) for the treatment of major depressive disorder and generalized anxiety disorder and the management of neuropathic pain associated with diabetic peripheral neuropathy, fibromyalgia, and chronic musculoskeletal pain associated with osteoarthritis and chronic low back pain. Outside of the United States, it is also approved for lower urinary tract disorders in some countries. A safety profile of duloxetine across these indications has been published that analyzed rates of common treatment-emergent AEs (TEAEs) (defined as AEs that were experienced by 5% or more of duloxetine-treated patients) comparing duloxetine with placebo.9 In those analyses, the frequency of any single bleeding-related event did not meet the threshold for being classified as a common TEAE. The objective of the current analyses was to gain a better understanding of the risk of bleeding-related TEAEs associated with duloxetine treatment in patients who used concomitant NSAIDs. We analyzed the incidence of these TEAEs from placebo-controlled trials of duloxetine across indications. In addition, we analyzed the incidence of these events reported in the FDA Adverse Event Reporting System (FAERS), which receives reports from health care professionals, consumers, and pharmaceutical manufacturers.
Methods
Characteristics of included studies
Safety data were pooled from all randomized, double-blind, placebo-controlled clinical trials of duloxetine conducted between December 1993 and December 2010. All studies were conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. National or institutional review boards at each study site approved the protocols, and written informed consent was obtained from each participant prior to entrance into a study. All patients were at least 18 years of age or older. The acute treatment phase duration of most studies was 3 months or less, and duloxetine doses ranged between 5 mg/day and 120 mg/day. Dosing schedules were fixed or flexible, and the majority of patients received 60 mg/day, 80 mg/day, or 120 mg/day. For all analyses apart from those examining the effect of dosing on bleeding outcomes, duloxetine dose groups were pooled.
Analysis of clinical trial data
Bleeding-related TEAEs were those events that newly occurred or worsened during the treatment phase as compared to the pre-randomization period. These events were coded into a preferred term using the Medical Dictionary for Regulatory Activities (MedDRA)10 version 12.0, which could then be searched using preferred terms from the Standardized MedDRA Queries (SMQs [version 12.0]),11 that included hemorrhage non-laboratory terms (SMQ20000039) and hemorrhage laboratory terms (SMQ20000040). GI bleeding-related TEAEs were searched using specific GI bleeding-related preferred terms.
During each clinical trial, NSAID (including aspirin) use was recorded on electronic case report forms. The information on these forms lacked consistent comprehensive dose and/or duration of use, so concomitant NSAID use was defined as taking an NSAID at any time during the treatment phase. Patients in both treatment groups were then categorized as an NSAID user or non-user.
Differences in the incidence of bleeding-related TEAEs between NSAID subgroups (user versus non-user) were analyzed by a logistic regression model that included therapy, NSAID use, and therapy-by-NSAID subgroup interaction. A statistically significant treatment-by-NSAID subgroup interaction was defined as P≤0.1. Within-subgroup group comparisons were conducted using Fisher’s exact test and were significant at P≤0.05.
To determine whether higher duloxetine doses were associated with an increased incidence of bleeding-related TEAEs, and also to assess if use of NSAIDs modify the dose effect of duloxetine, if such an effect exists, the 55 clinical trials were searched to identify fixed-dose studies with at least two fixed doses of duloxetine in the same study, allowing a direct comparison of two fixed-dose groups. Eight placebo-controlled clinical trials including fixed duloxetine doses of 60 mg, 120 mg, and placebo were found and analyzed. To examine for the presence or absence of a dose effect of duloxetine on the incidence of bleeding-related TEAEs, duloxetine 60 mg and 120 mg were compared with the Cochran–Mantel– Haenszel test for general association controlling for study. Furthermore, to evaluate if the use of concomitant NSAIDs modify the dose effect of duloxetine, if such an effect exists, a subgroup analysis was conducted using the same logistic regression model that included therapy, NSAID use, and therapy-by-NSAID subgroup interaction, with the exception that three treatment arms instead of two were examined.
Finally, to examine whether patients were exposed to a higher bleeding risk when taking duloxetine together with an NSAID compared with taking duloxetine on its own, the incidence of bleeding-related TEAEs was compared between NSAID user subgroups within treatment groups using the Cochran–Mantel–Haenszel test controlling for study.
FAERS data analysis
The FAERS database was searched through March 31, 2012 to ascertain whether there was disproportional reporting of bleeding-related TEAEs associated with duloxetine treatment and concomitant NSAID use (including aspirin). Bleeding-related TEAEs were combined into two groups based upon preferred terms SMQ (excluding laboratory terms). Upper GI bleeding events were combined into one preferred-term group, and another preferred-term group contained all other bleeding-related events. Among cases with duloxetine or NSAIDs as either suspected or concomitant drug(s), the following case groups were utilized: duloxetine without NSAIDs (duloxetine) and duloxetine + NSAIDs. A disproportionality analysis based on the empirical Bayes geometric mean (EBGM)12 was employed to analyze the case groups. The number of cases in the duloxetine group for each group of bleeding-related preferred terms was compared against the full FAERS background, which was comprised of the number of cases reported for each group of preferred terms for drugs other than duloxetine. A similar comparison was made for the number of cases in the duloxetine + NSAIDs group. In addition, the duloxetine + NSAIDs group was compared against the number of cases reported for each group of preferred terms for NSAIDs taken alone. The lower bound of a 90% confidence interval of EBGM (EB05) ≥1 was used as the criterion to signify that the disproportionality of the number of cases reported were higher than in the comparison groups.
Results
Placebo-controlled trials
A total of 19,529 patients (duloxetine, N=11,305; placebo, N=8,224) participated in 55 studies across duloxetine indications that included five studies in chronic musculoskeletal pain (three in chronic low back pain13–15 and two in osteoarthritis knee pain16,17); four in diabetic peripheral neuropathic pain;18–21 five in fibromyalgia;22–26 four in generalized anxiety disorder;27–30 20 in lower urinary tract disorder;31–44 and 17 in major depressive disorder.45–57 Across these trials among NSAID users, 2,580 received placebo and 3,357 received duloxetine; among NSAID non-users, 5,644 received placebo and 7,948 received duloxetine.
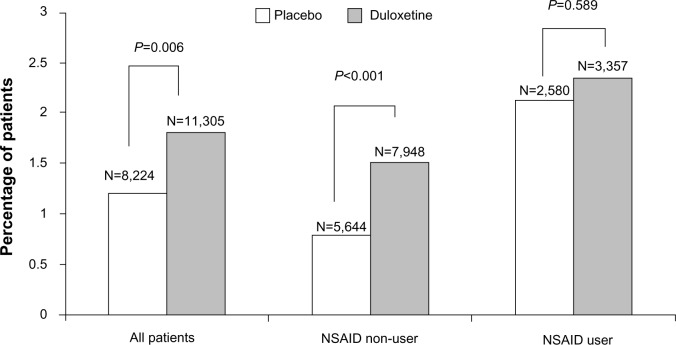
Bleeding-related TEAEs, including GI bleeding-related events, are summarized in Table 1. Overall, the proportion of duloxetine-treated patients experiencing at least one bleeding-related TEAE was significantly greater than that in placebo-treated patients (1.8% versus [vs] 1.2%; P=0.006).
Table 1.
Bleeding-related treatment-emergent adverse events that occurred in five or more duloxetine-treated patients overall and across NSAID user subgroups
| Bleeding-related adverse events | Duloxetine total N=11,305 n (%) | Placebo total N=8,224 n (%) | P-value | NSAID non-user
|
NSAID user
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Duloxetine N=7,948 n (%) | Placebo N=5,644 n (%) | Within subgroup P-value | Duloxetine N=3,357 n (%) | Placebo N=2,580 n (%) | Within subgroup P-value | ||||
| At least 1 event | 199 (1.8) | 100 (1.2) | 0.006 | 120 (1.51) | 45 (0.80) | <0.001 | 79 (2.35) | 55 (2.13) | 0.598 |
| Ecchymosis | 8 (0.1) | 4 (0.0) | 0.425 | 5 (0.06) | 0 | 0.081 | 3 (0.09) | 4 (0.16) | 0.477 |
| Epistaxis | 32 (0.3) | 18 (0.2) | 0.521 | 19 (0.24) | 10 (0.18) | 0.572 | 13 (0.39) | 8 (0.31) | 0.666 |
| Gingival bleeding | 5 (0.0) | 0 (0.0) | 0.073 | 3 (0.04) | 0 | 0.271 | 2 (0.06) | 0 | 0.508 |
| Hematochezia | 5 (0.0) | 5 (0.1) | 0.575 | 3 (0.04) | 2 (0.04) | 1.00 | 2 (0.06) | 3 (0.12) | 0.658 |
| Hematoma | 9 (0.1) | 6 (0.1) | 0.694 | 4 (0.05) | 2 (0.04) | 1.00 | 5 (0.15) | 4 (0.16) | 1.00 |
| Bruising | 14 (0.1) | 4 (0.0) | 0.096 | 8 (0.10) | 1 (0.02) | 0.090 | 6 (0.18) | 3 (0.12) | 0.740 |
| Menorrhagia | 28 (0.2) | 19 (0.2) | 0.901 | 20 (0.25) | 8 (0.14) | 0.183 | 8 (0.24) | 11 (0.43) | 0.248 |
| Metrorrhagia | 15 (0.1) | 6 (0.1) | 0.204 | 11 (0.14) | 3 (0.05) | 0.176 | 4 (0.12) | 3 (0.12) | 1.00 |
| Postmenopausal hemorrhage | 5 (0.0) | 1 (0.0) | 0.199 | 3 (0.04) | 0 | 0.271 | 2 (0.06) | 1 (0.04) | 1.00 |
| Rectal hemorrhage | 11 (0.1) | 5 (0.1) | 0.346 | 7 (0.09) | 3 (0.05) | 0.538 | 4 (0.12) | 2 (0.08) | 0.703 |
| Vaginal hemorrhage | 16 (0.1) | 8 (0.1) | 0.400 | 9 (0.11) | 5 (0.09) | 0.789 | 7 (0.21) | 3 (0.12) | 0.529 |
Abbreviations: N, total number in group; n, number of patients with the event; NSAID, non-steroidal anti-inflammatory drug.
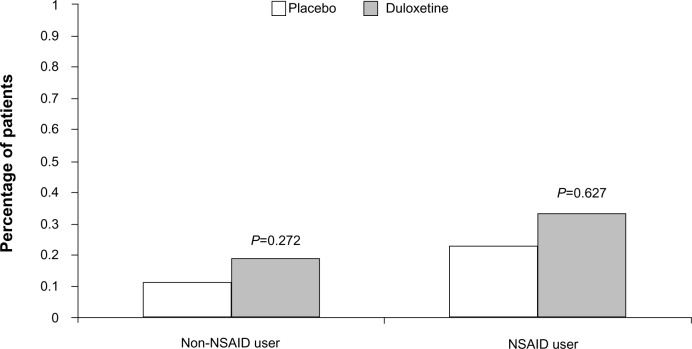
There was a significant treatment-by-NSAID-use subgroup interaction for the occurrence of at least one bleeding event (P=0.029), with a statistically significant duloxetine/placebo difference of 0.71% (1.51% vs 0.80%) in the NSAID non-user group as compared to a nonsignificant 0.22% difference (2.35% vs 2.13%) in the NSAID user group (Figure 1). This indicates the presence of a smaller duloxetine/placebo difference in patients taking concomitant NSAIDs compared with patients not taking NSAIDs. A significant treatment-by-subgroup interaction was not seen for the group of GI bleeding-related events, with a nonsignificant duloxetine/placebo difference of 0.08% (0.19% vs 0.11%) seen in the NSAID non-user group and a nonsignificant 0.10% duloxetine/placebo difference (0.33% vs 0.23%) seen in the NSAID user group (Figure 2).
Figure 1.
Percentage of patients in the nonsteroidal anti-inflammatory drug (NSAID) user/non-user subgroups that reported any treatment-emergent bleeding-related adverse event during placebo-controlled trials of duloxetine.
Notes: NSAID non-user group versus NSAID user group, P<0.001. Treatment-by-subgroup interaction, P=0.029.
Figure 2.
Percentage of patients in the nonsteroidal anti-inflammatory drug (NSAID) user/non-user subgroups that reported treatment-emergent gastrointestinal bleeding-related adverse event during placebo-controlled trials of duloxetine.
Notes: Non-NSAID user group versus NSAID user group, P<0.057. Treatment-by-subgroup interaction, P=0.742.
The dose analyses included eight fixed-dose studies across different disease states; within these studies, 930 patients were randomized to placebo, 913 to duloxetine 60 mg/day, and 904 to duloxetine 120 mg/day. Comparison of the incidence of bleeding-related TEAEs in patients treated with duloxetine 60 mg compared with duloxetine 120 mg revealed no statistically significant differences between the two dose groups for all-bleeding-related TEAEs combined or GI bleeding-related events only. For all-bleeding-related TEAEs combined, there was no statistically significant dose-by-NSAID interaction, indicating that use of NSAIDs did not modify the effect of duloxetine dose. For GI bleeding-related events, a dose-by-NSAID interaction could not be calculated because there were no events reported in at least one treatment arm in each of the NSAID user subgroups.
To examine whether patients were exposed to a higher bleeding risk when taking duloxetine together with an NSAID compared with taking duloxetine on its own, statistical comparisons were conducted for the comparison of duloxetine + NSAID versus duloxetine alone, as well as the comparison of placebo + NSAID versus placebo alone. Regarding all bleeding-related TEAEs, 2.35% of duloxetine-treated patients who used concomitant NSAIDs experienced a bleeding-related event versus 1.51% of duloxetine-treated patients who did not take an NSAID (P<0.044), while 2.13% of placebo-treated patients who also took an NSAID experienced a bleeding-related event versus 0.8% of patients treated with placebo alone. The incidence of GI bleeding-related events within treatment groups was not statistically significantly greater for NSAID users compared with non-users. In duloxetine-treated patients, 0.33% of NSAID users versus 0.19% of NSAID non-users (P=0.536) experienced a GI bleeding-related event, and in placebo-treated patients, 0.23% of NSAID users versus 0.11% of NSAID non-users (P=0.488) experienced a GI bleeding-related event.
FAERS
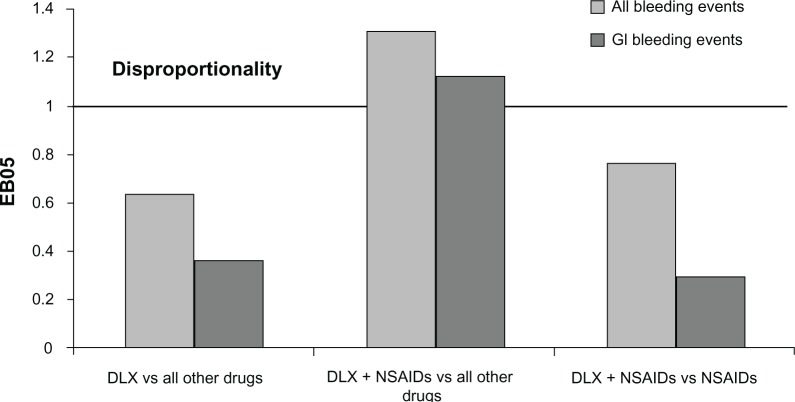
Cases for the all-bleeding-related TEAEs and upper GI bleeding-related TEAEs are summarized in Table 2. The results of the disproportionality analysis for these events are shown in Table 2 and Figure 3. None of the bleeding events, including upper GI bleeding events, were disproportionally reported for duloxetine monotherapy when compared against the full FAERS background that included cases for all other drugs. The reporting of the all-bleeding-related events group (EB05=1.31) and the upper GI bleeding group (EB05=1.12) was more frequent in the cases reported for duloxetine taken with NSAIDs than in the cases reported for drugs other than duloxetine. However, there was no difference in reporting of either group of events when the cases reported for duloxetine taken with NSAIDs were compared against the cases reported for NSAIDs without duloxetine.
Table 2.
Bleeding events from the FAERS up to March 31, 2012 and the results of disproportionality analysis
| Case groups | Group of preferred terms | Cases for duloxetine
|
Cases for all other drugs
|
EB05 |
|---|---|---|---|---|
| N | N | |||
| Duloxetine on the full FAERS drugs background | All bleeding events | 1,112 | 345,876 | 0.63 |
| Upper GI bleeding | 116 | 57,307 | 0.36 | |
| Duloxetine + NSAIDs on the full | All bleeding events | 504 | 346,484 | 1.31 |
| FAERS drugs background | Upper GI bleeding | 80 | 57,343 | 1.12 |
| Duloxetine + NSAIDs on the full | All bleeding events | 504 | 74,397 | 0.75 |
| FAERS NSAIDs background | Upper GI bleeding | 80 | 25,892 | 0.31 |
Abbreviations: FAERS, US Food and Drug Administration Adverse Event Reporting System; EB05, the lower bound of 90% confidence interval of empirical Bayes geometric mean; GI, gastrointestinal; N, total number in group; NSAIDs, nonsteroidal anti-inflammatory drugs.
Figure 3.
FAERS relative reporting of gastrointestinal bleeding events in patients taking duloxetine versus (vs) those not taking duloxetine.
Abbreviations: DLX, duloxetine; NSAIDs, nonsteroidal anti-inflammatory drugs; EB05, the lower bound of 90% confidence interval of empirical Bayes geometric mean; FAERS, US Food and Drug Administration Adverse Event Reporting System; GI, gastrointestinal.
Discussion
To our knowledge, this is the first study to investigate GI and other bleeding-related TEAEs that may be associated with a single SNRI, duloxetine, when taken concomitantly with, and without, NSAIDs. Previous studies have reported on serotonin reuptake inhibitors as a class (SSRIs or SNRIs or both classes combined)5,58–62 without regard to individual differences among these medications with respect to their affinity for the serotonin reuptake receptor, which may affect the level of risk for bleeding-related TEAEs when taken alone or in combination with NSAIDs.63,64
Consistent with duloxetine’s mechanism of action, more duloxetine-treated patients in clinical trials experienced a bleeding-related AE compared with placebo-treated patients. A significantly greater risk of bleeding-related events was seen among duloxetine- versus placebo-treated clinical trial patients who did not use NSAIDs; interestingly, however, while rates of bleeding-related TEAEs were higher in both the duloxetine and placebo treatment groups in patients who also took concomitant NSAIDs, the duloxetine/placebo difference in these rates was smaller than that in the NSAID non-user group and not statistically significant. We hypothesize that the bleeding risk associated with NSAID treatment is greater than that of duloxetine alone, thereby diluting the duloxetine/placebo difference when duloxetine is combined with NSAIDs. These results do not indicate that the combination of duloxetine and NSAIDs significantly increases the bleeding risk beyond the individual risks of each agent.
There was no statistically significant difference between the duloxetine 60 mg and 120 mg doses with respect to the incidence of either all-bleeding-related events or GI bleeding-related events. While this suggests that the use of higher doses of duloxetine is not associated with an increased risk of bleeding events, the results should be interpreted with caution, as relatively few patients were included in the analyses due to the need to include studies with at least two fixed duloxetine-dose arms and a placebo control. There were no duloxetine dose-by-NSAIDs interactions to suggest that the use of concomitant NSAIDs might influence the effect of duloxetine dose.
To address the question of whether patients are exposed to a higher bleeding risk when taking duloxetine together with NSAIDs compared to taking duloxetine alone, between-subgroup comparisons were conducted within treatment groups. The combination of duloxetine and NSAIDs was associated with a statistically significantly higher incidence rate of all bleeding TEAEs compared with duloxetine alone, suggesting an increased risk of bleeding with the combination. Given the well-known risks of bleeding associated with NSAIDs, the finding of a greater incidence of bleeding events for the combination of duloxetine and an NSAID compared with duloxetine alone seems unsurprising. It must, however, be remembered that patients were not randomly assigned to take duloxetine plus NSAIDs or duloxetine alone, so the finding should be treated with caution due to the potential for selection bias.
The results of the disproportionality analysis of bleeding events reported in the FAERS were not significant based on a conservative threshold of EB05 ≥1.0 when duloxetine cases, without NSAIDs as suspected or concomitant drugs, were compared with cases reported for all other drugs. Disproportional reporting was found when duloxetine cases with NSAIDs as suspected or concomitant drugs were compared to cases reported for all other drugs, excluding duloxetine and NSAIDs. However, the disproportionality of reporting for these events disappeared when compared to cases reported for NSAIDs taken alone, suggesting that the finding was driven by concomitant NSAID use rather than by duloxetine. These results are supported by other researchers who did not find an increased risk for bleeding events when NSAIDs were taken with SSRIs, especially when compared to the risk of taking NSAIDs alone.64,65
There are limitations to the analyses of placebo-controlled data. First, the clinical trial data are limited by incomplete information regarding dosing and frequency of concomitant NSAID use. Because of this challenge, we were unable to discern whether patients were taking a therapeutic dose every day or less frequently during the study. Based on what is known about the risk factors for bleeding events, these scenarios could have very different risks. The short duration of most of these studies may also limit the occurrence and detection of bleeding events that develop with prolonged concomitant NSAID use. Regarding the dose analyses, the results should be interpreted with caution – relatively few patients were included in the analyses due to the need to include studies with at least two fixed duloxetine-dose arms and a placebo control.
It is also important to understand the limitations of analyses based on the FAERS data. The rate of spontaneous reporting of any selected TEAE may not reflect the true incidence of that event in the population due to recognized underreporting of these events. In addition, the analyses based on spontaneous datasets like the FAERS are also hampered by duplicate case listings and a large number of false-positive results.
Conclusion
Duloxetine-treated patients in clinical trials had a higher incidence of bleeding-related TEAEs compared with placebo-treated patients, although the duloxetine/placebo difference was smaller in patients using concomitant NSAIDs than it was in non-NSAID users; concomitant use of NSAIDs was associated with a higher incidence of bleeding-related TEAEs in clinical trial patients regardless of whether they were taking duloxetine or placebo. Use of a higher (120 mg once daily) dose of duloxetine was not associated with a higher incidence of bleeding-related events than a lower (60 mg once daily) dose, regardless of concomitant NSAID use; the dose analyses should, however, be treated with caution due to the small sample size. The combination of duloxetine and NSAIDs was associated with a statistically significantly higher incidence rate of all bleeding TEAEs compared with duloxetine alone, suggesting an increased risk of bleeding with the combination. In spontaneously reported post-marketing data, duloxetine and concurrent NSAID use was not associated with significant disproportional reporting of bleeding events when compared with NSAID use alone.
Acknowledgments
This work was supported by Eli Lilly and Company, India-napolis, IN, USA. The studies included in the analyses were sponsored and/or supported by Eli Lilly and Company and Boehringer Ingelheim, GmbH.
Footnotes
Disclosure
All authors own stock in and are employees of Eli Lilly and Company or a subsidiary. The authors report no other conflicts of interest in this work.
References
- 1.Goldberg RJ. Selective serotonin reuptake inhibitors: infrequent medical adverse effects. Arch Fam Med. 1998;7(1):78–84. doi: 10.1001/archfami.7.1.78. [DOI] [PubMed] [Google Scholar]
- 2.Andrade C, Sandarsh S, Chethan KB, Nagesha KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565–1575. doi: 10.4088/JCP.09r05786blu. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald TM, Morant SV, Robinson GC, et al. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. BMJ. 1997;315(7119):1333–1337. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvo F, Fourrier-Réglat A, Bazin F, et al. Investigators of Safety of Non-Steroidal Anti-Inflammatory Drugs: SOS Project Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89(6):855–866. doi: 10.1038/clpt.2011.45. [DOI] [PubMed] [Google Scholar]
- 5.Dalton SO, Johansen C, Mellemkjær L, Nørgård B, Sørensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med. 2003;163(1):59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 6.De Clerck F. The role of serotonin in thrombogenesis. Clin Physiol Biochem. 1990;8(Suppl 3):40–49. [PubMed] [Google Scholar]
- 7.Lesch KP, Wolozin BL, Murphy DL, Reiderer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993;60(6):2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 8.Sallee FR, Hilal R, Dougherty D, Beach K, Nesbitt L. Platelet serotonin transporter in depressed children and adolescents: 3H-paroxetine platelet binding before and after sertraline. J Am Aad Child Adolesc Psychiatry. 1998;37(7):777–784. doi: 10.1097/00004583-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Brunton S, Wang F, Edwards SB, et al. Profile of adverse events with duloxetine treatment: a pooled analysis of placebo-controlled studies. Drug Saf. 2010;33(5):393–407. doi: 10.2165/11319200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999 Feb;20(2):109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mozzicato P. Standardised MedDRA queries: their role in signal detection. Drug Saf. 2007;30(7):617–619. doi: 10.2165/00002018-200730070-00009. [DOI] [PubMed] [Google Scholar]
- 12.Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25(6):381–392. doi: 10.2165/00002018-200225060-00001. [DOI] [PubMed] [Google Scholar]
- 13.Skljarevski V, Desaiah D, Liu-Seifert H, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976) 2010;35(13):E578–E585. doi: 10.1097/BRS.0b013e3181d3cef6. [DOI] [PubMed] [Google Scholar]
- 14.Skljarevski V, Ossanna M, Liu-Seifert H, et al. A double-blind, randomized trial of duloxetine versus placebo in the management of chronic low back pain. Eur J Neurol. 2009;16(9):1041–1048. doi: 10.1111/j.1468-1331.2009.02648.x. [DOI] [PubMed] [Google Scholar]
- 15.Skljarevski V, Zhang S, Desaiah D, et al. Duloxetine versus placebo in patients with chronic low back pain: a 12-week, fixed-dose, randomized, double-blind trial. J Pain. 2010;11(12):1282–1290. doi: 10.1016/j.jpain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Chappell AS, Ossanna MJ, Liu-Seifert H, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, controlled trial. Pain. 2009;146(3):253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Chappell AS, Desaiah D, Liu-Seifert H, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011;11(1):33–41. doi: 10.1111/j.1533-2500.2010.00401.x. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1–2):109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 20.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Ning G, Jia WP, et al. Duloxetine versus placebo in the treatment of patients with diabetic neuropathic pain in China. Chin Med J (Engl) 2010;123(22):3184–3192. [PubMed] [Google Scholar]
- 22.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 23.Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119(1–3):5–15. doi: 10.1016/j.pain.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136(3):432–444. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Chappell AS, Bradley LA, Wiltse C, Detke MJ, D’Souza DN, Spaeth M. A six-month double-blind, placebo-controlled, randomized clinical trial of duloxetine for the treatment of fibromyalgia. Int J Gen Med. 2008;1:91–102. doi: 10.2147/ijgm.s3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold LM, Clauw DJ, Wang F, et al. Flexible-dosed duloxetine in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2010;37(12):2578–2586. doi: 10.3899/jrheum.100365. [DOI] [PubMed] [Google Scholar]
- 27.Koponen H, Allgulander C, Erickson J, et al. Efficacy of duloxetine for the treatment of generalized anxiety disorder: implications for primary care physicians. Prim Care Companion J Clin Psychiatry. 2007;9(2):100–107. doi: 10.4088/pcc.v09n0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartford J, Korstein S, Liebowitz M, et al. Duloxetine as an SNRI treatment for generalized anxiety disorder: results from a placebo and active-controlled trial. Int Clin Psychopharmacol. 2007;22(3):167–174. doi: 10.1097/YIC.0b013e32807fb1b2. [DOI] [PubMed] [Google Scholar]
- 29.Rynn M, Russell J, Erickson J, et al. Efficacy and safety of duloxetine in the treatment of generalized anxiety disorder: a flexible-dose, progressive-titration, placebo-controlled trial. Depress Anxiety. 2008;25(3):182–189. doi: 10.1002/da.20271. [DOI] [PubMed] [Google Scholar]
- 30.Nicolini H, Bakish D, Duenas H, et al. Improvement of psychic and somatic symptoms in adult patients with generalized anxiety disorder: examination from a duloxetine, venlafaxine extended-release and placebo-controlled trial. Psychol Med. 2009;39(2):267–276. doi: 10.1017/S0033291708003401. [DOI] [PubMed] [Google Scholar]
- 31.Norton PA, Zinner NR, Yalcin I, Bump RC, Duloxetine Urinary Incontinence Study Group Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol. 2002;187(1):40–48. doi: 10.1067/mob.2002.124840. [DOI] [PubMed] [Google Scholar]
- 32.Ghoniem GM, Van Leeuwen JS, Elser DM, et al. Duloxetine/Pelvic Floor Muscle Training Clinical Trial Group A randomized controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment and no active treatment in women with stress urinary incontinence. J Urol. 2005;173(5):1647–1653. doi: 10.1097/01.ju.0000154167.90600.c6. [DOI] [PubMed] [Google Scholar]
- 33.Cardozo L, Drutz HP, Baygani SK, Bump RC. Pharmacological treatment for women awaiting surgery for stress urinary incontinence. Obstet Gynecol. 2004;104(3):511–519. doi: 10.1097/01.AOG.0000134525.86480.0f. [DOI] [PubMed] [Google Scholar]
- 34.van Kerrebroeck P, Abrams P, Lange R, et al. Duloxetine Urinary Incontinence Study Group Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG. 2004;111(3):249–257. doi: 10.1111/j.1471-0528.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 35.Dmochowski RR, Miklos JR, Norton PA, Zinner RN, Yalcin I, Bump RC, Duloxetine Urinary Incontinence Study Group Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170(4 Pt 1):1259–1263. doi: 10.1097/01.ju.0000080708.87092.cc. [DOI] [PubMed] [Google Scholar]
- 36.Millard RJ, Moore K, Rencken R, Yalcin I, Bump RC, Duloxetine UI Study Group Duloxetine vs placebo in the treatment of stress urinary incontinence: a four-continent randomized clinical trial. BJU Int. 2004;93(3):311–318. doi: 10.1111/j.1464-410x.2004.04607.x. [DOI] [PubMed] [Google Scholar]
- 37.Kinchen KS, Obenchain R, Swindle R, Duloxetine OAB Study Group Impact of duloxetine on quality of life for women with symptoms of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(5):337–344. doi: 10.1007/s00192-004-1270-5. [DOI] [PubMed] [Google Scholar]
- 38.Steers WD, Herschorn S, Kredert KJ, et al. Duloxetine OAB Study Group Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int. 2007;100(2):337–345. doi: 10.1111/j.1464-410X.2007.06980.x. [DOI] [PubMed] [Google Scholar]
- 39.Bent AE, Gousse AE, Hendrix SL, et al. Duloxetine compared with placebo for the treatment of women with mixed urinary incontinence. Neurourol Urodyn. 2008;27(3):212–221. doi: 10.1002/nau.20471. [DOI] [PubMed] [Google Scholar]
- 40.Castro-Diaz D, Palma PC, Bouchard C, et al. Duloxetine Dose Escalation Study Group Effect of dose escalation on the tolerability and efficacy of duloxetine in the treatment of women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(8):919–929. doi: 10.1007/s00192-006-0256-x. [DOI] [PubMed] [Google Scholar]
- 41.Lin AT, Sun MJ, Tai HL, et al. Duloxetine versus placebo for the treatment of women with stress predominant urinary incontinence in Taiwan: a double-blind, randomized, placebo-controlled trial. BMC Urol. 2008;8:2. doi: 10.1186/1471-2490-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mah SY, Lee KS, Choo MS, et al. Duloxetine versus placebo for the treatment of Korean women with stress predominant urinary incontinence. Korean J Urol. 2006;47:527–535. [Google Scholar]
- 43.Cardozo L, Lange R, Voss S, et al. Short- and long-term efficacy and safety of duloxetine in women with predominant stress urinary incontinence. Curr Med Res Opin. 2010;26(2):253–261. doi: 10.1185/03007990903438295. [DOI] [PubMed] [Google Scholar]
- 44.Schagen van Leeuwen JH, Lange RR, Jonasson AF, Chen WJ, Viktrup L. Efficacy and safety of duloxetine in elderly women with stress urinary incontinence or stress-predominant mixed urinary incontinence. Maturitas. 2008;60(2):138–147. doi: 10.1016/j.maturitas.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein DJ, Mallinckrodt C, Lu Y, Demitrack MA. Duloxetine in the treatment of major depressive disorder: a double-blind clinical trial. J Clin Psychiatry. 2002;63(3):225–231. doi: 10.4088/jcp.v63n0309. [DOI] [PubMed] [Google Scholar]
- 46.Nemeroff CB, Schatzberg AF, Goldstein DJ, et al. Duloxetine for the treatment of major depressive disorder. Psychopharmacol Bull. 2002;36(4):106–132. [PubMed] [Google Scholar]
- 47.Goldstein DJ, Lu Y, Detke MJ, Wiltse C, Mallinckrodr C, Demitrack MA. Duloxetine in the treatment of depression: a double-blind placebo-controlled comparison with paroxetine. J Clin Psychopharmacol. 2004;24(4):389–399. doi: 10.1097/01.jcp.0000132448.65972.d9. [DOI] [PubMed] [Google Scholar]
- 48.Detke MJ, Wiltse CG, Mallinckrodt CH, McNamara RK, Demitrack MA, Bitter I. Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Neuropsychopharmacol. 2004;14(6):457–470. doi: 10.1016/j.euroneuro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Perahia DGS, Wang F, Mallinckrodt CH, Walker DJ, Detke MJ. Duloxetine in the treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Psychiatry. 2006;21(6):367–378. doi: 10.1016/j.eurpsy.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA. Duloxetine, 60 mg once daily for major depressive disorder: a randomized double-blind placebo-controlled trial. J Clin Psychiatry. 2002;63(4):308–315. doi: 10.4088/jcp.v63n0407. [DOI] [PubMed] [Google Scholar]
- 51.Detke MJ, Lu Y, Goldstein DJ, McNamara RK, Demitrack MA. Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psychiatr Res. 2002;36(6):383–390. doi: 10.1016/s0022-3956(02)00060-2. [DOI] [PubMed] [Google Scholar]
- 52.Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- 53.Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. 2005;39(1):43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Nierenberg AA, Greist JH, Mallinckrodt CH, et al. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Curr Med Res Opin. 2007;23(2):401–416. doi: 10.1185/030079906X167453. [DOI] [PubMed] [Google Scholar]
- 55.Brecht S, Courtecuisse C, Debieuvre C, et al. Efficacy and safety of duloxetine 60 mg once daily in the treatment of pain in patients with major depressive disorder and at least moderate pain of unknown etiology: a randomized controlled trial. J Clin Psychiatry. 2007;68(11):1707–1716. doi: 10.4088/jcp.v68n1110. [DOI] [PubMed] [Google Scholar]
- 56.Oakes TM, Myers AL, Marangell LB, et al. Assessment of depressive symptoms and functional outcomes in patients with major depressive disorder treated with duloxetine versus placebo: primary outcomes from two trials conducted under the same protocol. Hum Psychopharmacol. 2012;27(1):47–56. doi: 10.1002/hup.1262. [DOI] [PubMed] [Google Scholar]
- 57.Mundt JC, Debrota DJ, Greist JH. Anchoring perceptions of clinical change on accurate recollection of the past: memory enhanced retrospective evaluation of treatment (MERET) Psychiatry (Edgmont) 2007;4(3):39–45. [PMC free article] [PubMed] [Google Scholar]
- 58.de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population-based case-control study. BMJ. 1999;319(7217):1106–1109. doi: 10.1136/bmj.319.7217.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Jong JC, van den Berg PB, Tobi H, de Jong-van den Berg LT. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55(6):591–595. doi: 10.1046/j.0306-5251.2002.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helin-Salmivaara A, Huttunen T, Grönroos JM, Klaukka T, Huupponen R. Risk of serious upper gastrointestinal events with concurrent use of NSAIDs and SSRIs: a case-control study in the general population. Eur J Clin Pharmacol. 2007;63(4):403–408. doi: 10.1007/s00228-007-0263-y. [DOI] [PubMed] [Google Scholar]
- 61.van Walraven C, Mamdani MM, Wells PS, Williams JI. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ. 2001;323(7314):655–658. doi: 10.1136/bmj.323.7314.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wessinger S, Kaplan M, Choi L, et al. Increased use of selective serotonin reuptake inhibitors in patients admitted with gastrointestinal haemorrhage: a multicentre retrospective analysis. Aliment Pharmacol Ther. 2006;23(7):937–944. doi: 10.1111/j.1365-2036.2006.02859.x. [DOI] [PubMed] [Google Scholar]
- 63.Lewis JD, Strom BL, Localio AR, et al. Moderate and high affinity serotonin reuptake inhibitors increase the risk of upper gastrointestinal toxicity. Pharmacoepidemiol Drug Saf. 2008;17(4):328–335. doi: 10.1002/pds.1546. [DOI] [PubMed] [Google Scholar]
- 64.Vidal X, Ibáñez L, Vendrell L, Conforti A, Laporte JR, Spanish-Italian Collaborative Group for the Epidemiology of Gastrointestinal Bleeding Risk of upper gastrointestinal bleeding and the degree of serotonin reuptake inhibition by antidepressants: a case-control study. Drug Saf. 2008;31(2):159–168. doi: 10.2165/00002018-200831020-00005. [DOI] [PubMed] [Google Scholar]
- 65.Targownik LE, Bolton JM, Metge CJ, Leung S, Sareen J. Selective serotonin reuptake inhibitors are associated with a modest increase in the risk of upper gastrointestinal bleeding. Am J Gastroenterol. 2009;104(6):1475–1482. doi: 10.1038/ajg.2009.128. [DOI] [PubMed] [Google Scholar]