Abstract
Tropomyosins (Tms) are a family of highly conserved actin-binding proteins that play critical roles in a variety of processes, most notably, in the regulation of muscle contraction and relaxation. It is well known that different Tm isoforms have distinct functions and that altered expression of Tm isoforms could lead to changes in cardiac structure and function. To precisely define Tm isoform expression in the human heart, towards a better understanding of their functional roles, we have employed top-down mass spectrometry for in-depth proteomic characterization of Tm isoforms. Using a minimal amount of human heart tissue from rejected donor organs, we confirmed the presence of multiple Tm isoforms including α-Tm, β-Tm and κ-Tm in the human heart, with α-Tm being the predominant isoform, followed by minor isoforms of β-Tm and κ-Tm. Interestingly, our data revealed regional variations of Tm isoforms and posttranslational modifications in the human heart. Specifically, the expression level of κ-Tm was highest in the left atrium but nearly undetectable in the left ventricle. The phosphorylation level of α-Tm (pα-Tm) was significantly higher in the atria than it was in the ventricles. The sequences of all Tm isoforms were characterized and the sites of post-translational modifications were localized. Clearly, top-down mass spectrometry is an attractive method for comprehensive characterization of Tm isoforms and post-translational modifications since it can universally detect and quantify all types of protein modifications without a priori knowledge and without the need for specific antibodies.
Keywords: Tropomyosin, Muscle Contraction, Isoforms, Mass Spectrometry, Post-translational modification
Introduction
Tms are a family of actin-binding proteins found in virtually all eukaryotic cells, playing critical roles in a variety of processes including striated muscle contraction and relaxation (Gunning, 2008; Perry, 2001). Tm is a dimer with α-helical coiled-coil structure that forms a head-to-tail polymer along the major groove of actin filaments. Tm, actin and troponin complex (troponin I, troponin T, troponin C) are five major proteins composing the thin filament in striated muscle. As one of the regulatory proteins in the thin filament, Tm, together with troponin complex, blocks the myosin binding sites on the actin filament, preventing crossbridge formation and, consequently, regulating muscle contraction in a calcium-dependent manner (Gunning, 2008; Jagatheesan et al., 2010a; Jagatheesan et al., 2010b; Perry, 2001).
Human Tm exhibits significant complexity with a number of isoforms arising from different genes and alternative splicing events in addition to post-translational modifications (PTMs) (Denz et al., 2004; Gunning, 2008; Perry, 2001; Wieczorek et al., 1988). It is encoded by four different genes (TPM1, TPM2, TPM3, and TPM4) each of which is known to give rise to multiple isoforms via alternative splicing events (Marston and Redwood, 2003; Perry, 2001; Rajan et al., 2010; Wieczorek, 1988). In this gene family, TPM1 being the most versatile, comprises 15 exons and encodes a variety of tissue specific isoforms. Two major striated and smooth muscle isoforms, α-Tm and β-Tm are encoded by TPM1 and TPM2, respectively, whereas γ-Tm encoded by TPM3 is expressed in slow-twitch skeletal muscles (Perry, 2001; Rajan et al., 2010).
A variety of human diseases have been linked to mutations of Tm encoding genes and differential expression of Tm isoforms (Gunning, 2008; Muthuchamy et al., 1998; Muthuchamy et al., 1995; Perry, 2001; Wieczorek et al., 1988) . Recently, Denz et al. discovered a novel isoform, κ-Tm, also encoded by the TPM1 gene, in human cardiac muscle using an RT-PCR-based strategy (Denz et al., 2004). Subsequently, this cardiac-specific protein isoform was found to be up-regulated in the left ventricle of patients with dilated cardiomyopathy (DCM) and end-stage heart failure (Rajan et al., 2010). The data from Vieczorek’s group also indicated that altered phosphorylation may be a significant factor when linking Tm mutation (Glu54Lys) to dilated cardiomyopathy (Warren et al., 2008). Moreover, a recent study showed that p38-MAPK-mediated dephosphorylation of α-Tm in murine hearts resulted in decreased contractility (Vahebi et al., 2007). Although α-Tm (encoded by TMP1) has been confirmed to be the predominant Tm isoform in the human heart (Rajan et al., 2010), a thorough analysis of Tm isoforms and their associated PTMs is still lacking. Hence, to better understand the role of Tm in cardiac function, complete characterization of human Tm isoforms and their PTMs is needed.
Recently, our group developed a top-down mass spectrometry (MS)-based targeted proteomics approach that enables comprehensive study of Tm isoforms and PTMs from swine heart tissue (Peng et al., 2013). Unlike bottom-up proteomics, top-down MS detects intact proteins without digestion, thereby offering an “bird’s eye view” of all protein modifications including isoforms, mutations, and PTMs simultaneously in one MS spectrum without a priori knowledge (Ayaz-Guner et al., 2009; Dong et al., 2012; Ge et al., 2009; Peng et al., 2012; Peng et al., 2013; Roth et al., 2005; Ryan et al., 2010; Siuti and Kelleher, 2007; Xu et al., 2011; Zabrouskov et al., 2008; Zhang and Ge, 2011; Zhang et al., 2011a; Zhang et al., 2011b). Subsequently, individual protein species (isoforms or post-translationally modified species) can be “gas-phase” isolated and fragmented by tandem MS techniques (MS/MS) such as collisioninduced dissociation (CID) (Senko et al., 1994) and electron capture dissociation (ECD) (Zubarev et al., 2000) in order to obtain sequence data or localize PTM sites.
Herein, we have employed top-down MS-based targeted proteomics to characterize Tm purified from human donor hearts with normal cardiac function. We have improved our Tm purification method and now only ~ 5 mg of cardiac tissue is needed to purify Tm in less than 3 h. Our MS data identified several human Tm isoforms expressed in human heart tissue and mapped PTMs including acetylation and phosphorylation. Moreover, we discovered regional variations in the expression of specific Tm isoforms in human donor hearts.
Methods
Chemicals and reagents
All the reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless noted otherwise. All the solutions were made in Milli-Q water (Millipore, Corp., Billerica, MA).
Human heart tissue samples
All the human heart tissue samples (clinical characteristics in Supplemental Table 1, Supporting information) were collected from the donor hearts of Wisconsin Donor Networking/Wisconsin Tissue Bank. Hearts were collected and immediately placed in ice cold cardioplegic solution. All tissue samples were excised from different chambers of the donor hearts, snap frozen in liquid nitrogen, and stored in −80 °C freezer. The use of heart tissue samples was approved by the Institutional Review Boards of Medical College of Wisconsin and University of Wisconsin-Madison.
Purification of human Tm
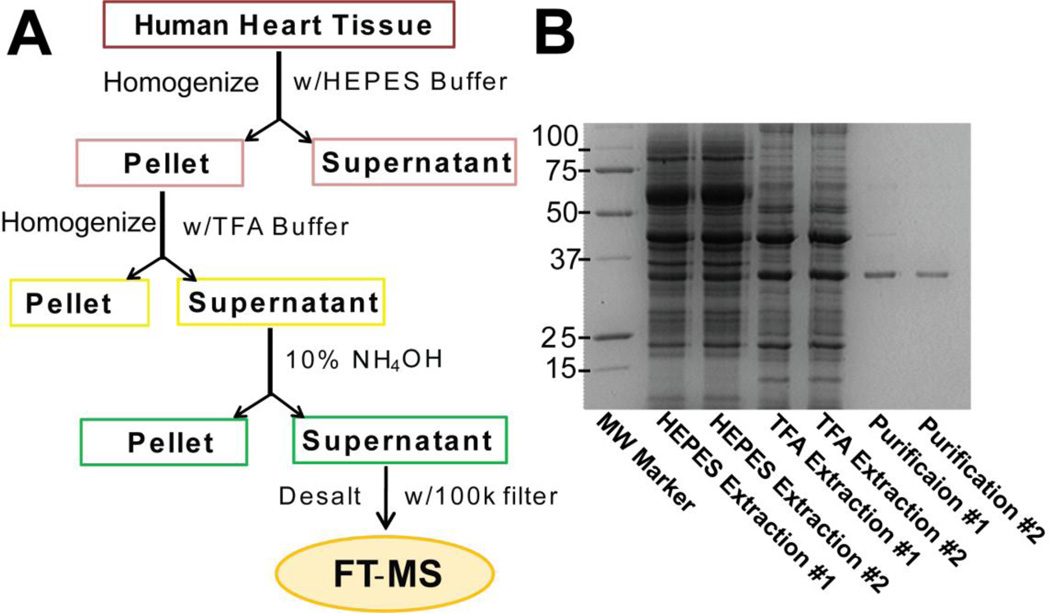
A rapid purification method was used to purify Tm from human hearts as described previously (Peng et al., 2013) with improvements (Figure 1A). In this study, a much smaller amount of tissue (~5 mg) was used for Tm extraction and purification compared to that of swine Tm (100–400 mg). Briefly, approximately 5 mg of heart tissue was homogenized in 50 µL of HEPES extraction buffer (Neverova and Van Eyk, 2002) (HEPES, 25 mM, pH 7.5, NaF, 50 mM, Na3VO4 0.25 mM, PMSF (in isopropanol), 0.25 mM, EDTA, 2.5 mM) using a teflon pestle (1.5 mL tube Rounded tip, Cienceware, Pequannock, NJ, USA). The homogenate was centrifuged at 16,100 g at 4 °C for 15 min (Centrifuge 5415R, Eppendorf, Hamburg, Germany) and the supernatant was discarded. The pellet was then homogenized in 50 µL of TFA extraction buffer (TFA 1%, TCEP, 1 mM) using the same teflon pestle. The homogenate was centrifuged (16,100 g, 4 °C, 15 min) to collect the supernatant (~45 µL). 10% NH4OH was added to the collected supernatant drop-by-drop until the supernatant was neutralized at pH ~ 7.0.Precipitation occurred during the neutralization process and the mixture was then centrifuged at 16,100 g, 4 °C for 2 min to remove the precipitate. Finally, the supernatant was desalted using the Amicon 100 K molecular weight cutoff (MWCO) filter and 0.1% formic acid solution. The entire purification process of Tm took less than 3 h. Protein content in fractions was characterized by SDS-PAGE on 12.5% polyacrylamide gels stained with Coomassie Blue (Figure 1B).
Figure 1.
(A) Flow chart and (B) SDS-PAGE analysis of human Tm extraction and purification procedures. Representative SDS-PAGE analysis of two extraction replicates (#1 and #2) from one human heart tissue.
Top-down MS
Purified human Tm (Tm ~20 ug/mL, methanol: water: acetic acid = 47:47:6) was analyzed using a 7 T linear ion trap/Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer (LTQ/FT Ultra, Thermo Scientific Inc. Bremen, Germany) equipped with an automated chip-based nano ESI source (Triversa NanoMate; Advion Bioscience, Ithaca, NY, USA) as described previously (Peng et al., 2013). Tm sample was introduced into the mass spectrometer using a spray voltage of 1.3–1.5 kV versus the inlet of the mass spectrometer, generating a flow rate of 50–200nL/min. The resolving power of FT-ICR full MS analysis was typically set at 400,000. For CID and ECD, the protein molecular ions of individual charge states were first isolated and then fragmented using 12–20% normalized collision energy (CID) or 2.2–3.0% electron energy (ECD, corresponding to 1.0–18 eV) with a 70 ms duration and no additional delay. In this study, typically, 1,000–4,000 scans were averaged to ensure high-quality CID and ECD spectra.
All MS and MS/MS spectra were analyzed by in-house developed MASH Suite software (version 1.0) using the THRASH algorithm with S/N threshold of 3 and a minimum fit of 60% and then manually validated. The fragment ions in MS/MS spectra were assigned based on the protein sequence of human Tm isoforms obtained from Swiss-Prot database (Unit-ProtKB/Swiss-Prot). Allowance was made for possible PTMs such as acetylation of the N-terminus and phosphorylation using 10 ppm tolerance for the precursor and fragment ions, respectively. For the fragment ions containing possible phosphorylation sites, a mass shift ~ 80 Da was manually validated to confirm or exclude the existence of phosphorylation. All the reported molecular weight (MW) of intact proteins were most abundant MW (the most intense peak in the isotopic distribution) and those of fragment ions were monoisotopic MWs.
Quantitative analysis of major human Tm isoforms and their phosphorylation present in four different chambers of human heart samples were performed as described previously (Dong et al., 2012; Peng et al., 2013; Zhang and Ge, 2011). For each detected protein form, the sum of the peak heights of the top five isotopomers was used to calculate the relative abundance. The percentages of α-, β-, κ-Tm, mono-phosphorylated α-Tm (pα-Tm) were defined as the abundances of α-, β-, κ-, and pα-Tm over the summed abundances of the entire human Tm populations, respectively. The phosphorylation occupancy was calculated as the abundance of the phosphorylated α-Tm over the summed abundance of the total α-Tm population. Minor degradation products and non-covalent phosphate adducts (+H3PO4) were also taken into account in the quantification.
Statistical analysis
Six donor hearts and four different chambers from three of those heart samples were investigated in this study (Supplementary Table 1). Specifically, the de-identified case numbers for the three hearts with tissue from four chambers are 9762, 10108, 9759, and three hearts with tissue from only one chamber are 9773, 9757 and 9774, respectively. Data were represented as means ± S.D. Student’s t-tests were performed between group comparisons to evaluate statistical significance of variance. Differences among means were considered significant at p < 0.05.
Results
SDS-PAGE and high-resolution MS of human Tm
Tm was extracted and purified from human heart tissue and analyzed by SDS-PAGE (Figure 1B). The purified Tm collected after desalting with 100 kDa MWCO (“purification”) was used for further MS analysis (Figure 1A). Although numerous bands were observed in the HEPES and TFA extractions, only one major band was detected in the purification with a MW ~32 kDa (Figure 1B) , indicating a highly efficient purification of Tm from human heart tissue. SDS-PAGE analysis of two parallel extractions from the same tissue samples (“extraction replicates”) demonstrated the reproducibility of this purification method. FT-ICR MS analysis with high-resolution and high mass accuracy provides an overview of all detectable protein forms present in human Tm (Figure 2 and Supplementary Figure 1 and 2). High-resolution MS spectra of purified human Tm from different donor hearts exhibited comparable profiles and the protein form with MW of 32749.92 was the predominant peak in those spectra, corresponding to the major human Tm isoform, α-Tm (P09493, TPM1_HUMAN, UniProtKB/Swiss-Prot) with the consideration of one acetylation (+42 Da) (Supplementary Figure 3). Since N-terminal acetylation is prevalent in eukaryotic proteins, it was reasonable to assume this modification occurs to human Tm. The peak next to it, with a MW of 32829.88 and a mass discrepancy of 79.96 Da, was assigned as the mono-phosphorylated form of the predominant peak, pα-Tm. We also noticed that a peak with a mass discrepancy of 141.88 (MW, 32891.80) from the predominant Tm isoform was detected in the spectra, which is assigned as human β-Tm based on accurate MW match (3.65 ppm) with N-terminal acetylation (Table 1). In addition, a peak with calculated MW of 32971.75 can be observed in some human hearts, of which the MW matched with that of mono-phosphorylated β-Tm (Calc’d: 32971.58). Interestingly, the experimental MW of a very minor peak (Expt’l: 32690.88) matched the theoretical value (Calc’d: 32690.70) of a novel isoform, κ-Tm, as reported by Rajan et al. (Rajan et al., 2010). Subsequently, we have performed MS/MS to confirm the sequences of α-, β- and κ- Tm isoforms (vide infra)
Figure 2.
Representative high-resolution MS spectra of human Tm (M31+) purified from donor heart tissues (a) 9759LA, (b) 9773LA, (c) 9762RV, (d) 10108RV, (e) 9759RV, (f) 9757RV, (g) 9774RV. Circles represent the theoretical isotopic abundance distribution of the isotopmers corresponding to the assigned molecular weights. Subscript p: mono-phosphorylation; Single and double stars represent noncovalent adducts of phosphoric acid (+H3PO4, and +2 H3PO4), respectively. Expt’l, experimental molecular weight. Calc’d, calculated molecular weight. Clinical information of the transplant heart is summarized in Supplementary Table1.
Table 1.
Identification of human Tm components in high-resolution MS spectra of the donor human heart tissues.
| Identification | Experimental MW (Da) |
Calculated MW (Da) |
Error (ppm) |
|---|---|---|---|
| Human Tm Isoforms | |||
| α-Tm | 32749.85 | 32749.74 | 3.36 |
| β-Tm | 32891.74 | 32891.62 | 3.5 |
| κ-Tm | 32690.81 | 32690.70 | 3.36 |
| Phosphorylation Products | |||
| pα-Tm | 3282.81 | 3282970 | 3.35 |
| pβ-Tm | 3291.7 | 32971.58 | 3.64 |
| Trace Degradation products | |||
| α-Tm[1–280] | 32317.61 | 3217.54 | 2.17 |
| α-Tm[1–281] | 32448.64 | 32448.58 | 1.85 |
| α-Tm[1–282] | 32549.69 | 3549.63 | 1.84 |
| Unassigned Products | |||
| 32861.80 | |||
| 33017.07 | |||
| 33067.70 | |||
| 33078.25 |
In addition, we have observed a trace amount of degradation products in the spectra, of which the MWs matched with the C-terminal degradation products of α-Tm, [1–280], [1–281] and [1–282], respectively (Table 1); however, due to the low abundances of these peaks in the donor heart samples, MS/MS was not performed in this study. We also detected minor peaks with MWs of 32861.80, 33017.07, 33067.70 and 33078.25 in some but not all human heart samples. Nonetheless, these peaks were not present consistently in the spectra so we cannot confidently assign them. Intriguingly, the experimental MW of one of these unassigned peaks, 32861.80, bears a mass discrepancy of 2 Da compared to the calculated MW of human γ-Tm, 32859.79 (P06753, TPM3_HUMAN, UniprotKB/Swiss-Prot) with acetylation at the N-terminus (Supplementary Figure 2–3). γ-Tm encoded by TPM3 is known to be expressed in slow-twitch skeletal muscles (Perry, 2001; Rajan et al., 2010) but not in cardiac muscles. Due to the large mass error (2.01 Da corresponds to 60 ppm) which greatly exceeded the typical error (1–10 ppm) observed from our high-resolution high accuracy mass spectrometer (7T LTQ/FT), we cannot confidently assign this peak as γ-Tm. Further MS/MS experiments will need to be performed to characterize the sequence of this isoform.
Sequencing major human Tm isoform
After considering of a potential acetylation (42.01), the experimental MW value of the predominant peak 32749.92 (Figure 3A) matched well with calculated MW of α-Tm (P09493, TPM1_HUMAN, UniProtKB/Swiss-Prot) (32707.73+42.01 = 32749.74) (5.5 ppm). To confirm the possible modifications and sequence of α-Tm, the precursor ions of specific charge states of human α-Tm were isolated individually and then fragmented by both ECD and CID. We have collected a total of 5 ECD and 4 CID spectra from multiple charge states (M30+–M35+) of human α-Tm, respectively. The MS/MS data unambiguously confirmed the sequence of human α-Tm (P09493) with N-terminal acetylation. As shown in Figure 3B, 104 c and 64 z· ions from ECD spectra as well as 69 b and 12 y ions from CID spectra were observed, which matched the predicted value of the fragment ions from the sequence of human α-Tm (P09493) with N-terminal acetylation. Please note that c and b ions were counting from the N-terminus and y and z· ions from the C-terminus. Extensive sequencing by both ECD and CID data rendered 185/283 cleavage bonds of α-Tm (Figure 3BC). Thus, top-down MS-based proteomics provided highly accurate mass measurement and effective sequencing of human α-Tm.
Figure 3.
(A) High-resolution MS, (B) MS/MS product ion map, and (C) representative fragment ions from both ECD and CID spectra of unphosphorylated human α-Tm. Fragment assignments were made based on the DNA-predicated sequence of human α-Tm (P09493, TPM1_HUMAN, UniProtKB/Swiss-Prot) with acetylation at the N-terminus (42.01). Expt’l, experimental molecular weight. Calc’d, calculated molecular weight.
Localizing the phosphorylation site of human α-Tm
As shown in Figure 2, the peak with a mass discrepancy of 79.96 (MW: 32829.88) from the predominant peak (MW: 32749.92) was assigned as mono-phosphorylated form of human α-Tm. No bis- or tris-phosphorylated form of human α-Tm was detected in the MS spectra. To map the phosphorylation site of human α-Tm, the precursor ions of a single charge state of mono-phosphorylated α-Tm (pα-Tm) were isolated and fragmented by ECD. 100 c ions and 51 z· ions were detected from four combined ECD spectra of pα-Tm. Figure 4 demonstrated the representative fragment ions and complete ion product map of mono-phosphorylated α-Tm. As shown in Figure 4, no phosphorylated c ions were observed whereas all the z· ions were phosphorylated, suggesting that phosphorylation occurred near the C-terminus of the protein. Since the smallest phosphorylated fragment ion obtained was z·11, the phosphorylation site can be localized to one of the first 11 amino acid at the C-terminus. According to the C-terminal sequence (DHALNADTSI), there were only one serine (Ser283) and one threonine (Thr282) which are likely to be phosphorylated. Indeed, Ser283 is known as the phosphorylation site as reported in the previous studies of Tm (Houle et al., 2007; Mak et al., 1978).
Figure 4.
ECD for localizing phosphorylation sites of human α-Tm. (A–D), representative MS/MS spectra of c and z· ions from ECD data. (E), ECD product ion map of monophosphorylated α-Tm. Ac-, acetylation of the first amino acid at the N-terminus. p, mono-phosphorylation site of human α-Tm at Ser283. Expt’l, experimental molecular weight. Calc’d, calculated molecular weight.
Confirmation of human β-Tm by top-down MS
As discussed above, we detected a Tm isoform (32891.86, Supplementary Figure 4A) with a mass discrepancy of 141.88 from the predominant α-Tm (32749.92), which is comparable to the same mass discrepancy exhibited between swine α-Tm and β-Tm (Peng et al., 2013). Thus, we hypothesized this isoform as human β-Tm and then characterized it using MS/MS method. MS/MS of this isoform confirmed that the sequence of this isoform matched exactly with that of the DNA-predicted amino acid sequence of human β-Tm (P07951, TPM2_HUMAN, UniprotKB/Swiss-Prot). Product ion map and representative b and y ions of β-Tm from CID experiments are shown in Supplementary Figure 4B–C. Due to the low abundance of this isoform, compared to the predominant α-Tm, only CID experiments were performed to characterize this isoform. Moreover, the calculated MW of the modified human β-Tm with acetylation at the N-terminus (42.01) was 32891.70 (32849.69 + 42.01), which matched exactly with the experimental MW (32891.86) of this human Tm isoform (5.2 ppm). Therefore, our top-down MS experiments confirmed the presence of β-Tm in human donor hearts and further characterized its sequence and modification.
Sequence characterization of human κ-Tm
We screened six donor heart samples from Wisconsin Donor Network (Supplementary Table 1). An interesting peak with MW of 32690.88 was detected in the human Tm spectra (Figure 5A). This peak had a mass discrepancy of −59.04 Da from the predominant α-Tm, suggesting that it may be another isoform of human Tm instead of modification or degradation of α-Tm.
Figure 5.
MS/MS comprehensive characterization of human κ-Tm. (A) High-accuracy mass measurement of κ-Tm (M30+). (B) Fragmentation ion map of κ-Tm from CID data. Fragment assignments were made to the DNA-predicted sequence of human κ-Tm (P09493-6, TPM1_HUMAN, UniProtKB/Swiss-Prot) with acetylation at the N-terminus (42.01). (C) Representative b ions from CID data. Expt’l, experimental molecular weight. Calc’d, calculated molecular weight.
To fully characterize this interesting isoform, the precursor ions of a single charge state was isolated and fragmented by CID experiments. 36 b ions and 16 y ions were detected from two combined CID spectra (Figure 5B, supplementary Figure 5) and representative fragment ions are shown in Figure 5C, which unambiguously confirmed the sequence of human κ-Tm. Meanwhile, highly accurate mass measurement of this isoform demonstrated that its experimental MW (32690.88) matched well with the calculated value of human κ-Tm from Swiss-Prot database (P09493-6, TPM1_HUMAN, UniProtKB/Swiss-Prot) with acetylation at the N-terminus (32648.69+42.01=32690.70).
Relative quantification of Tm isoforms in human heart four chambers
In our sample screening process, we noticed that the protein level of κ-Tm in LA was much higher than that expressed in RV of donor heart samples (Figure 2, 9759LA, 9773LA, 9762RV, 10108RV, 9759RV, 9757RV, 9774RV.). This observation led us to hypothesize that heterogeneity of human Tm expression exists across the chambers of hearts. Indeed, we did find that there were regional differences in the expression of Tm as shown in the representative full MS spectra of human Tm protein species (isoforms/phosphorylated forms) from the right atrium (RA), LA, RV, and left ventricle (LV) of one human donor heart (Figure 6A). To quantify the relative expression levels of human Tm isoforms/phosphorylated species in different chambers of human heart, the percentages of human α-Tm, pα-Tm, β-Tm and κ-Tm were calculated based on the molecular ion abundance of Tm purified from three donor hearts with all four chamber tissue samples available (Supplementary Figure 6). The percentages of human α-Tm, pα-Tm, β-Tm and κ-Tm in different heart chambers are shown in Figure 6B–E. Human α-Tm was the predominant form in four chambers with percentage of 66.0 ± 4.0% (RA), 61.5 ± 5.9% (LA), 71.4 ± 2.7% (RV) and 68.9 ± 4.4% (LV), respectively (Figure 6B). The percentages of phosphorylated α-Tm (pα-Tm) over the entire Tm population was 23.4 ± 4.7% (RA), 21.7 ± 3.0% (LA), 12.3 ± 0.7% (RV) and 11.7 ± 2.2% (LV), respectively (Figure 6C). Moreover, the percentages of human β-Tm isoform over the entire Tm population were 5.5 ± 0.6% (RA), 6.0 ± 2.1% (LA), 5.1 ± 0.9% (RV) and 6.7 ± 0.7% (LV), respectively (Figure 6C). Notably, the percentages of κ-Tm isoform were 1.4 ± 1.4% (RA), 3.0 ± 0.6% (LA) and 0.5 ± 0.5% (RV), whereas no κ-Tm was detected in LV of all the human donor hearts (Figure 6E). The relatively large standard errors of κ-Tm percentage in RA and RV were due to the fact that κ-Tm was only detected in 1 out of 3 samples. The phosphorylation occupancy of α-Tm (pα-Tm/ α-Tm) in four chambers was 35.9 ± 7.9% (RA), 36.8 ± 7.7% (LA), 17.3 ± 0.8% (RV) and 17.1 ± 2.9% (LV), respectively (data not shown).
Figure 6.
Quantification of human Tm isoforms present in four chambers of donor human hearts. (A) MS spectra of human Tm isoforms in four chambers. (a)–(d) RA, LA, RV and LV of the donor heart 9759. Relative quantification of Tm isoforms (B) α-Tm, (C) pα-Tm, (D) β-Tm, and (E) κ-Tm in four heart chambers, n=3, from three donor hearts (9759, 10108, 9762). Single and double stars represent non-covalent adducts of phosphoric acid (+H3PO4, and +2 H3PO4), respectively. The data were expressed as mean ± S. D. *, p < 0.05.
Discussion
In this study, we have confirmed the presence of multiple Tm isoforms including α-Tm, β-Tm and κ-Tm in the human heart, with α-Tm being the predominant isoform, followed by minor isoforms of β-Tm and κ-Tm. Moreover, we have investigated the regional variations of Tm isoforms and PTMs in different chambers of the human heart. We found that the expression level of κ-Tm was highest in LA followed by RA and RV, but nearly undetectable in LV. Furthermore, we found that the phosphorylation level of α-Tm (pα-Tm) was significantly higher in the atria than it was in the ventricles. The sequences of all Tm isoforms and the PTM sites were unambiguously characterized by top-down MS.
Tms are a family of actin binding proteins that are encoded by four genes (TPM1, TPM2, TPM3, and TPM4), which can give rise to a variety of tissue specific isoforms through alternative splicing events. By comparison, the human TPM1 gene is far more versatile than swine TPM1 in that a greater number of distinct isoforms can be produced via alterative splicing of the human TPM1 transcript. As shown in Supplementary Figure 7A, 7 different isoforms of human Tm, which are expressed in different muscle types, are encoded by TPM1. Of these isoforms, only α-Tm and κ-Tm have been identified in human cardiac muscle (Rajan et al., 2010). κ-Tm was reported to contain exon 2a among 15 exons of TPM1 instead of 2b in α-Tm expressed in human cardiac tissues (Denz et al., 2004). In this paper, we confirmed that both α-Tm and κ-Tm are expressed in human cardiac tissues and comprehensively characterized their sequences (Figures 3–5, Supplementary Figure 7B). The difference between TPM1 encoded κ-mRNA and TPM1 encoded α-mRNA was a stretch of 40 amino acids encoded by exon 2a/b (Rajan et al., 2010). The top-down MS-based targeted proteomic analysis confirmed that the sequences of the α- and κ- isoforms were highly similar with the only amino acid difference between these isoforms located in the N-terminus (Supplementary Figure 7B).
Previous studies have demonstrated that cardiac structure and function can be dramatically altered via changes in the expression levels of different Tm isoforms. In the adult healthy mouse heart the protein expression level of β-Tm is less than 2%. When there was 55–60% of β-Tm present in the myocardium of transgenic mouse heart, the time of relaxation, maximum rate of relaxation and sensitivity to calcium changed significantly (Muthuchamy et al., 1998; Muthuchamy et al., 1995; Palmiter et al., 1996; Wieczorek et al., 1988). Further altering of β-Tm to an even higher lever (β-Tm/α-Tm=80/20) in the heart of transgenic mice will cause postnatal death between 10–14 days (Muthuchamy et al., 1998; Wieczorek, 2008). In contrast, high expression level of γ-Tm (40–60%) can lead to hyperdynamic effect on systolic and diastolic function and decreased calcium sensitivity (Jagatheesan, 2010a). Recently, a novel isoform, κ-Tm encoded by TPM1 was reported to be expressed in human cardiac muscle (Denz et al., 2004) using a κ-Tm specific antibody, κ-Tm was shown to be expressed in healthy human hearts (~3 - 5%) and up-regulated in human LV free walls of dilated cardiomyopathy and heart failure patients (Rajan et al., 2010). The ratio of β-Tm to α-Tm in healthy pig heart was much higher than that reported in healthy mouse heart, which means that isoform expression can also differ across species (Peng et al., 2013). Here, we have observed a small amount of β-Tm and κ-Tm in the human donor hearts (Figures 2, 6).
In this paper, we investigated for the first time on regional variations of human Tm isoforms in four chambers of normal human heart. Our MS data demonstrated that α-Tm (non-phosphorylated and mono-phosphorylated α-Tm) was the predominant isoform, constituting 84.2% of the total Tm population. Interestingly, the phosphorylation occupancy of human α-Tm in the four heart chambers was significantly different. As shown in Figure 6C, the expression level of phosphorylated α-Tm (pα-Tm) present in the atria (RA and LA) was similar, but was two-fold greater than it was in the ventricles (RV and LV), which means more α-Tm was phosphorylated in atrium compared to that in ventricle of human donor heart (p < 0.05). Meanwhile, β-Tm occupies ~ 3–8% of whole Tm population in human cardiac tissues with no significant difference observed in different chambers (Figure 3D). Interestingly, we found κ-Tm was not equally distributed in the four heart chambers. Notably, κ-Tm has the highest expression level in LA than that in other heart chambers (Figure 6E). Hence, to compare the expression level of human cardiac isoforms, it is necessary to take chamber heterogeneity into consideration.
The heterogeneity of Tm isoforms as well as diverse PTMs occurred to those isoforms makes it difficult to conduct a comprehensive study of Tm molecular forms by traditional methods such as Western blot. To distinguish one Tm isoform from the other, isoform-specific antibodies need to be developed for quantitative analysis. For example, to quantify κ-Tm, Rajan et al. developed an isoform-specific antibody which has an epitope that recognizes residues within the exon 2a sequence of κ-Tm (Rajan et al., 2010). However, antibody development is not only time-consuming but also of variable success because some antibodies are highly specific whereas others are not, especially for a large family of isoforms encoded by different genes with variant sequences, distinct conformations and diverse PTMs.
In contrast to antibody-based Western blot analysis, top-down MS is a universal detection method which can provide an overview of all types of isoforms of the protein of interests from different encoded genes, alternative splicing and PTMs in one spectrum without prior knowledge (Ayaz-Guner et al., 2009; Dong et al., 2012; Ge et al., 2009; Peng et al., 2012; Peng et al., 2013; Roth et al., 2005; Ryan et al., 2010; Siuti and Kelleher, 2007; Xu et al., 2011; Zabrouskov et al., 2008; Zhang and Ge, 2011; Zhang et al., 2011a). Thus, top-down MS is an attractive and unique tool for the analysis of protein isoforms and PTMs (Roth et al., 2005; Sancho Solis et al., 2008; Zhang and Ge, 2011; Zhang et al., 2011a). Moreover, top-down MS combined with ECD and CID offers comprehensive sequencing of proteins to nail down the sequence variations and PTM sites to a single amino acid (Ayaz-Guner et al., 2009; Peng et al., 2013; Xu et al., 2011). In this study, we have shown that top-down MS can identify various human Tm isoforms and their modifications in one MS spectrum (Figure 2, supplementary Figure 2). MS/MS sequencing of the isoforms could unambiguously confirm the sequences and map the modification sites. This top-down MS-based proteomic approach required only 5 mg of heart tissue and the entire purification process takes less than 3 h, which significantly reduced the amount of tissue demanded and greatly increased the throughput. Moreover, it eliminates the need for specific antibodies. Thus, top-down MS is an attractive method for in-depth characterization of Tm isoforms and PTMs with high reproducibility and throughput.
Supplementary Material
Acknowledgement
We would like to thank Wenxuan Cai for her help in the preparation of human Tm, Huseyin Guner for assistance with data analysis and Wei Guo for helpful discussion. Financial support was kindly provided by NIH R01HL096971 (to YG) and T32GM008688. We also would like to thank the Wisconsin Partnership Program for the establishment of UW Human Proteomics Program Mass Spectrometry Facility and the Wisconsin Donor Network Organ Procurement Organization and Blood Center of Wisconsin for acquisition of tissues.
ABBREVIATIONS
- Tm
Tropomyosin
- MS
Mass spectrometry
- DCM
Dilated cardiomyopathy
- CID
Collision-induced dissociation
- ECD
Electron capture dissociation
- PTM
Post-translational modification
- MW
Molecular weight
- MWCO
Molecular weight cutoff
- FT-ICR
Fourier transform ion cyclotron resonance
- SDS–PAGE
Sodium dodecyl sulfate – polyacrylamide gel electrophoresis
- ESI
Electrospray ionization
- RA
Right atrium
- LA
Left atrium
- RV
Right ventricle
- LV
Left ventricle
References
- Ayaz-Guner S, Zhang J, Li L, Walker JW, Ge Y. In Vivo Phosphorylation Site Mapping in Mouse Cardiac Troponin I by High Resolution Top-Down Electron Capture Dissociation Mass Spectrometry: Ser22/23 Are the Only Sites Basally Phosphorylated. Biochemistry. 2009;48:8161–8170. doi: 10.1021/bi900739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denz CR, Narshi A, Zajdel RW, Dube DK. Expression of a novel cardiac-specific tropomyosin isoform in humans. Biochemical and Biophysical Research Communications. 2004;320:1291–1297. doi: 10.1016/j.bbrc.2004.06.084. [DOI] [PubMed] [Google Scholar]
- Dong X, Sumandea CA, Chen Y-C, Garcia-Cazarin ML, Zhang J, Balke CW, Sumandea MP, Ge Y. Augmented Phosphorylation of Cardiac Troponin I in Hypertensive Heart Failure. Journal of Biological Chemistry. 2012;287:848–857. doi: 10.1074/jbc.M111.293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Rybakova IN, Xu Q, Moss RL. Top-down high-resolution mass spectrometry of cardiac myosin binding protein C revealed that truncation alters protein phosphorylation state. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12658–12663. doi: 10.1073/pnas.0813369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P. In: Tropomyosin: Introduction and Historical Perspective. Tropomyosin P, Gunning, editors. New York: Springer; 2008. pp. 1–4. [Google Scholar]
- Gunning P, O'Neill G, Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiological Reviews. 2008;88:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- Gunning PW, Schevzov G, Kee AJ, Hardeman EC. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends in Cell Biology. 2005;15:333–341. doi: 10.1016/j.tcb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Houle F, Poirier A, Dumaresq J, Huot J. DAP kinase mediates the phosphorylation of tropomyosin-1 downstream of the ERK pathway, which regulates the formation of stress fibers in response to oxidative stress. Journal of Cell Science. 2007;120:3666–3677. doi: 10.1242/jcs.003251. [DOI] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Ahmed RPH, Petrashevskaya N, Boivin G, Arteaga GM, Tae HJ, Liggett SB, Solaro RJ, Wieczorek DF. Striated muscle tropomyosin isoforms differentially regulate cardiac performance and myofilament calcium sensitivity. Journal of Muscle Research and Cell Motility. 2010a;31:227–239. doi: 10.1007/s10974-010-9228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Wieczorek DF. Investigations into tropomyosin function using mouse models. Journal of Molecular and Cellular Cardiology. 2010b;48:893–898. doi: 10.1016/j.yjmcc.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak A, Smillie LB, Barany M. Specific Phosphorylation at Serine-283 of Alpha- Tropomyosin from from Skeletal and Rabbit Skeletal and Cardiac-Muscle. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:3588–3592. doi: 10.1073/pnas.75.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston SB, Redwood CS. Modulation of thin filament activation by breakdown or isoform switching of thin filament proteins - Physiological and pathological implications. Circulation Research. 2003;93:1170–1178. doi: 10.1161/01.RES.0000105088.06696.17. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Boivin GP, Grupp IL, Wieczorek DF. beta-tropomyosin overexpression induces severe cardiac abnormalities. Journal of Molecular and Cellular Cardiology. 1998;30:1545–1557. doi: 10.1006/jmcc.1998.0720. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Grupp IL, Grupp G, OToole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF. Molecular and physiological effects of overexpressing striated muscle beta-tropomyosin in the adult murine heart. Journal of Biological Chemistry. 1995;270:30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- Neverova I, Van Eyk JE. Application of reversed phase high performance liquid chromatography for subproteomic analysis of cardiac muscle. Proteomics. 2002;2:22–31. [PubMed] [Google Scholar]
- Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ. Exchange of beta- for alpha-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+ strong cross-bridge binding, and protein phosphorylation. Journal of Biological Chemistry. 1996;271:11611–11614. doi: 10.1074/jbc.271.20.11611. [DOI] [PubMed] [Google Scholar]
- Peng Y, Chen X, Sato T, Rankin SA, Tsuji RF, Ge Y. Purification and High-Resolution Top-Down Mass Spectrometric Characterization of Human Salivary alpha-Amylase. Analytical Chemistry. 2012;84:3339–3346. doi: 10.1021/ac300083y. [DOI] [PubMed] [Google Scholar]
- Peng Y, Chen X, Zhang H, Xu Q, Hacker TA, Ge Y. Top-down Targeted Proteomics for Deep Sequencing of Tropomyosin Isoforms. Journal of Proteome Research. 2013;12:187–198. doi: 10.1021/pr301054n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SV. Vertebrate tropomyosin: distribution, properties and function. Journal of Muscle Research and Cell Motility. 2001;22:5–49. doi: 10.1023/a:1010303732441. [DOI] [PubMed] [Google Scholar]
- Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D'Souza KM, Akhter SA, Boivin GP, et al. Molecular and Functional Characterization of a Novel Cardiac-Specific Human Tropomyosin Isoform. Circulation. 2010;121:410-U116. doi: 10.1161/CIRCULATIONAHA.109.889725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MJ, Forbes AJ, Boyne MT, Kim YB, Robinson DE, Kelleher NL. Precise and parallel characterization of coding polymorphisms, alternative splicing, and modifications in human proteins by mass spectrometry. Molecular & Cellular Proteomics. 2005;4:1002–1008. doi: 10.1074/mcp.M500064-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CM, Souda P, Bassilian S, Ujwal R, Zhang J, Abramson J, Ping P, Durazo A, Bowie JU, Hasan SS, et al. Post-translational Modifications of Integral Membrane Proteins Resolved by Top-down Fourier Transform Mass Spectrometry with Collisionally Activated Dissociation. Molecular & Cellular Proteomics. 2010;9:791–803. doi: 10.1074/mcp.M900516-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho Solis R, Ge Y, Walker JW. Single amino acid sequence polymorphisms in rat cardiac troponin revealed by top-down tandem mass spectrometry. Journal of Muscle Research and Cell Motility. 2008;29:203–212. doi: 10.1007/s10974-009-9168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senko MW, Speir JP, Mclafferty FW. Collisional Activation of Large Multiply-Charged Ions Using Fourier-Transform Mass-Spectrometry. Analytical Chemistry. 1994;66:2801–2808. doi: 10.1021/ac00090a003. [DOI] [PubMed] [Google Scholar]
- Siuti N, Kelleher NL. Decoding protein modifications using top-down mass spectrometry. Nature Methods. 2007;4:817–821. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahebi S, Ota A, Li MX, Warren CM, de Tombe PP, Wang YB, Solaro RJ. p38-MAPK induced dephosphorylation of alpha-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circulation Research. 2007;100:408–415. doi: 10.1161/01.RES.0000258116.60404.ad. [DOI] [PubMed] [Google Scholar]
- Warren CM, Arteaga GM, Rajan S, Ahmed RPH, Wieczorek DF, Solaro RJ. Use of 2-D DIGE analysis reveals altered phosphorylation in a tropomyosin mutant (Glu54Lys) linked to dilated cardiomyopathy. Proteomics. 2008;8:100–105. doi: 10.1002/pmic.200700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek DF. Regulation of Alternatively Spliced Alpha-Tropomyosin Gene-Expression by Nerve Extract. Journal of Biological Chemistry. 1988;263:10456–10463. [PubMed] [Google Scholar]
- Wieczorek DF, Jagatheesan G, Rajan S. Tropomyosin G, Peter, editors. The Role of Tropomyosin in Heart Disease. 2008 doi: 10.1007/978-0-387-85766-4_11. (Spring Science). [DOI] [PubMed] [Google Scholar]
- Wieczorek DF, Smith CWJ, Nadalginard B. The Rat Alpha-Tropomyosin Gene Generates a Minimum of 6 Different Messenger-Rnas Coding for Striated, Smooth, and Nonmuscle Isoforms by Alternative Splicing. Molecular and Cellular Biology. 1988;8:679–694. doi: 10.1128/mcb.8.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Xu Q, Dong X, Guy M, Guner H, Hacker TA, Ge Y. Top-down high-resolution electron capture dissociation mass spectrometry for comprehensive characterization of post-translational modifications in Rhesus monkey cardiac troponin I. International Journal of Mass Spectrometry. 2011;305:95–102. [Google Scholar]
- Zabrouskov V, Ge Y, Schwartz J, Walker JW. Unraveling Molecular Complexity of Phosphorylated Human Cardiac Troponin I by Top Down Electron Capture Dissociation/Electron Transfer Dissociation Mass Spectrometry. Molecular & Cellular Proteomics. 2008;7:1838–1849. doi: 10.1074/mcp.M700524-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ge Y. Comprehensive Analysis of Protein Modifications by Top-Down Mass Spectrometry. Circulation-Cardiovascular Genetics. 2011;4:711. doi: 10.1161/CIRCGENETICS.110.957829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guy MJ, Norman HS, Chen YC, Xu QG, Dong XT, Guner H, Wang SJ, Kohmoto T, Young KH, et al. Top-Down Quantitative Proteomics Identified Phosphorylation of Cardiac Troponin I as a Candidate Biomarker for Chronic Heart Failure. Journal of Proteome Research. 2011a;10:4054–4065. doi: 10.1021/pr200258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Ayaz-Guner S, Chen YC, Dong XT, Xu QG, Ge Y. Phosphorylation, but Not Alternative Splicing or Proteolytic Degradation, Is Conserved in Human and Mouse Cardiac Troponin T. Biochemistry. 2011b;50:6081–6092. doi: 10.1021/bi2006256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Electron capture dissociation for structural characterization of multiply charged protein cations. Analytical Chemistry. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.