Abstract
Background
Recent studies of Toxoplasma gondii isolates from animals in different regions of China have shown a limited genetic diversity and type China 1 was the dominant genotype of T. gondii prevalent in Chinese animals. However, little has been known concerning the isolation and genotyping of T. gondii circulating in chickens, pigs and rodents in China. The aim of the study was to characterize samples of T. gondii isolates obtained from naturally infected cats, pigs and free-range chickens slaughtered for human consumption in China.
Methods
In the present study, brain tissues of 77 animals collected from different areas of China, including 24 free-range chickens (Gallus domesticus) , 13 voles (Rattus flavipectus), 23 pigs and 17 cats, were bioassayed in mice and viable T. gondii were isolated from the brains of eleven. These eleven T. gondii isolates were maintained in Kunming (KM) outbred mice and DNA isolated from tissues of infected mice was characterized using 11 PCR-restriction fragment length polymorphism (PCR-RFLP) markers: SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, Apico, and CS3. Moreover, to determine mouse virulence of China 1 lineage of parasites, a TgCtgy5 genotype isolate was selected randomly and assessed in KM mice with different inoculation doses.
Results
Results of genotyping revealed that ten isolates were type China 1 (ToxoDB PCR-RFLP genotype #9), and TgCksz1 was a new genotype that was reported for the first time designated here as ToxoDB PCR-RFLP #225. No clonal types I, II and III lineages were found. DNA sequencing of four introns (EF1, HP2, UPRT1 and UPRT7) and two genes (GRA6 and GRA7) from representative isolates confirmed the results of PCR-RFLP genotyping. The TgCtgy5 isolate was highly virulent in KM mice; all infected mice died of acute toxoplasmosis, irrespective of the inoculation dose. The results indicate that mouse virulent isolates of T. gondii are predominantly circulating in cats in China.
Conclusions
T. gondii isolated from chickens, pigs, cats and rodents in different locations in China were genotyped and the results reconfirmed the limited diversity of T. gondii in China and showed that type China 1 lineage was dominant in this country.
Keywords: Toxoplasma gondii, Genotyping, Virulence, PCR-RFLP, Type China 1
Background
Toxoplasma gondii is an important water- and food-borne obligate intracellular parasite, which infects almost all homoeothermic animals including humans, birds and domestic animals [1]. It has been estimated that up to 30% of the human population worldwide and 8% of population in China is chronically infected [2-4]. Felids are considered as the key in the transmission of T. gondii to humans and other animals because they are the only definitive hosts that excrete the environmentally resistant oocysts through defecation. Humans become infected postnatally by ingesting tissue cysts from raw or undercooked infected meat, or by consuming food or drink contaminated with T. gondii oocysts. However, only a small percentage of exposed adult humans or animals develop clinical disease. It is not clear why some hosts become ill with toxoplasmosis whereas most remain asymptomatic. Recently, attention has been focused on the genetic diversity among T. gondii isolates from apparently healthy and sick hosts. Severe cases of toxoplasmosis that have been reported in immunocompetent individuals are considered to be due to infection with atypical T. gondii isolates [5-7]. Type II strains are the most prevalent in humans and animals in Europe [8].
Globally, genetic diversity of T. gondii consists of six major clades originating from a small number of distinct ancestral lineages [8]. The clonal population structure with three major types I, II and III is more frequently observed in North America and Europe [9]. These three genotypes differ in their virulence and/or pathogenicity to mice. Type I strains are highly virulent, whereas type II and III strains are intermediately or non virulent. Moreover, infections with the atypical types A and X were also observed in wildlife in North America and have recently been described as members of a fourth clonal lineage, designated Haplogroup 12 [10]. However, subsequent studies using multilocus markers have revealed a greater genetic diversity of T. gondii, particularly isolates from humans and animals in South America [11,12]. These isolates have been historically considered as atypical in order to differentiate them from the dominantly described archetypes. Recent studies of T. gondii in humans and animals in Africa suggested the dominance of type II, III, Africa 1 and Africa 3 [13,14]. Some genotypes of T. gondii isolates from humans and cats in China have been previously reported, and the type China 1 (also known as ToxoDB#9) is widespread and likely the major lineage in mainland China [15-18].
However, the data of T. gondii isolates from food animals, such as chickens and pigs in China are limited. Food animals are the main meat source for human consumption in China, and risk factor analysis indicated that 30 to 63% of human infections can be attributed to the consumption of undercooked meat [19]. Serological and parasitological surveys in China indicate a high prevalence of infection of these animals [20,21], and a viable T. gondii isolate was isolated from tissues of pigs [22]. T. gondii infection in food animals continues to be a significant food safety problem, and has become to a major concern for Chinese consumers over the last decade. Additionally, toxoplasmosis also causes considerable economical loss to the animal husbandry industry worldwide due to increased mortality, abortion, and medical costs [23].
In the present study, we report the isolation and genotyping of T. gondii isolates from naturally infected cats, pigs, voles and free-range chickens in central and southwestern China using 11 polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) markers. Additionally, the virulence of the isolates was determined in a mouse model. These results provide a new insight on the distribution of T. gondii genotypes and on T. gondii diversity in this country.
Methods
Ethics statement
All animals were treated in strict accordance to the guidelines for the Laboratory Animal Use and Care from Chinese CDC and the Rules for Medical laboratory Animals (1998) from Ministry of Health, China. The protocols were approved by the Institutional Review Board (IRB) of the Institute of Biomedicine at Anhui Medical University. All efforts were made to minimize animal suffering during the course of these studies.
Animal samples and bioassay in mice
Experimental samples (brain tissues) were obtained from 24 free-range chickens (from Anhui province), 13 voles (from Hubei province), 23 pigs and 17 cats (from Guizhou province). To avoid any chance of cross-contamination, each sample was first separated from the body and placed into a labeled sterile plastic bag, and then the bag was placed in a cooler with ice packs to maintain the temperature at 4°C and taken to the laboratory. Samples were transported by train within 24 h of collection to the laboratory for T. gondii examination and isolation. All animals were anesthetized before being sacrificed.
The isolates were obtained by means of bioassays in 5-week-old Kunming (KM) female mice (specific pathogen free, SPF) following the previously described protocol [16,24]. Tissue homogenate was inoculated intraperitoneally into each of 5 mice. The mice were then monitored daily for illness. Peritoneal exudates were examined from mice for viable T. gondii isolates as soon as obvious clinical manifestations were observed. Survivors were killed on day 45 postinoculation and brains of all mice were microscopically examined for tissue cysts as a squash preparation as described [25]. The inoculated mice were considered infected with T. gondii when stages of the parasite (tachyzoites and/or tissue cysts) were demonstrated in their tissues. T. gondii isolates were cryopreserved in liquid nitrogen for future studies using homogenates of brains from infected mice, or viable tachyzoites from intraperitoneal fluids.
DNA extraction and genotyping
DNA was extracted from ascitic fluids or tissues of all infected mice using QIAamp DNA Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The purified DNA was dissolved in 20 μl of double-distilled water and stored at −20°C for multilocus genotyping studies. A negative control for DNA extraction was included for each group of five samples.
Genotyping of T. gondii isolates was performed using multilocus PCR-RFLP with 11 genetic markers as previously described: SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, Apico [26,27] and marker CS3 [28]. Reference strains of T. gondii were also used in genotyping, including type I (GT1), type II (PTG), type III (CTG) and other strains (TgCgCa1, MAS, TgCatBr5, TgCatBr64, TgRsCr1). Briefly, the target DNA sequences were first amplified using a set of mixed external primers in a single reaction. Then multiplex products were 1:1 diluted in water and served as template DNA for nested PCR with internal primers for each marker separately. A known purified genomic DNA of T. gondii isolate (RH) was used as a positive control and DNA from non-infected mice were included as negative controls. To confirm successful amplification, 5 μl of the nested PCR products was run on a 1% agarose gel containing ethidium bromide prior to purification and digestion. The remaining products were purified using AxyPrepTM PCR Cleanup Kit (Axygen, Union, USA) and digested with appropriate restriction endonucleases [29]. The restriction fragments were run by electrophoresis through a 2.5% ethidium bromide stained agarose gel for all markers except Apico, which required a 3% gel, and then visualized under UV light. A low DNA (25–700 bp) ladder (Ferments, Vilnius, Lithuania) was employed as size standard. The data were analyzed using ToxoDB (http://www.toxodb.org) database and compared with the reference strain profiles [30].
SAG3 marker sequencing and phylogenetic analysis of T. gondii isolates
The nested PCR products for the marker SAG3 from T. gondii isolate TgCksz1 were sequenced in both directions using the internal T. gondii primers on an automated sequencer (Applied Biosystems, USA), as described previously [31]. Nucleotide sequences were analyzed and aligned using BioEdit Sequence Align-ment Editor [16]. For additional comparison, sequences from T. gondii RH strain (GenBank: JX218225), T. gondii PTG strain (GenBank: JX218226), and T. gondii CTG strain (GenBank: JX218227) available in the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were also inputted.
To further characterize these isolates, three isolates (TgCtgy2, TgCtgy5 and TgPggy1) were selected randomly and sequences were generated at four introns (EF1, HP2, UPRT1 and UPRT7) and two dense granule proteins (GRA6 and GRA7) as described previously method [10]. The evolutionary history was inferred using a reticulated network by SplitsTree4 [32], combining the RFLP genotype results from this paper and predominant genotypes described to date in South America, Europe and North America.
T. gondii isolate virulence in mice
To determine the pathogenicity of T. gondii isolates with type China 1 widespread in China, TgCtgy5 was selected randomly to study its virulence phenotype in mice. For this, tachyzoites were collected from peritoneal cavities of infected mice and purified as described [16]. Subsequently, parasites were diluted in phosphate buffered saline (PBS) and groups of ten five-week-old KM female mice were inoculated intraperitoneally with 101, 102, 103, and 104 tachyzoites, in a final volume of 200 μl PBS (per injection), as defined previously [33]. Five animals inoculated intraperitoneally with PBS were maintained as negative controls. The amount of tachyzoites was determined in a haemocytometer counting chamber. Mice mortality and morbidity was recorded daily for 45 d after infection, and survivors were killed under anesthesia and brains were examined for tissue cysts described as above. Meanwhile, the rest of the brains were tested for 529 bp repetitive fragment of T. gondii DNA by PCR using the primer pairs TOX4/TOX5 [34].
Results
Multilocus PCR-RFLP genotyping of T. gondii isolates
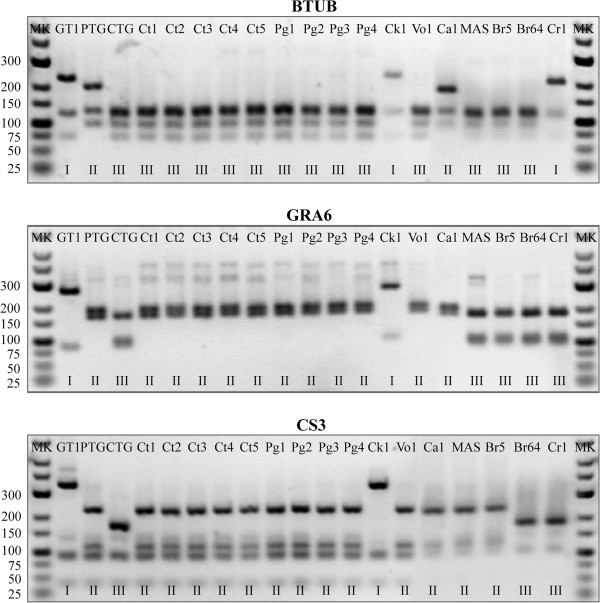
For the present study, 11 isolates of T. gondii isolated from 3 locations were genotyped. Five isolates were obtained from cats, four from pigs, one from chickens and one from voles by following essentially the procedures described previously [24]. To identify the genotype of the T. gondii isolates and improve the sensitivity of detection, a Mn-PCR-RFLP was employed and genotyping results for the 11 T. gondii isolates for all markers are shown in Figure 1 and Table 1. Two PCR-RFLP genotypes were detected and none of the isolates displayed a clonal genotype of types I, II or III.
Figure 1.
Representative gel image of RFLP genotyping (markers BTUB, GRA6 and CS3). Samples IDs are at the top of the gel images, genotype results are at the bottom. GT1, PTG, CTG, Ca1 (TgCgCa1), MAS, Br5 (TgCatBr5) and Br64 (TgCatBr64) are reference strains. Ct1: TgCtgy1; Ct2: TgCtgy2; Ct3: TgCtgy3; Ct4: TgCtgy4; Ct5: TgCtgy5; Pg1: TgPggy1; Pg2: TgPggy2; Pg3: TgPggy3; Pg4: TgPggy4; Ck1: TgCksz1; Vo1: TgVowh1. MK: molecular markers.
Table 1.
Multilocus genotyping of Toxoplasma gondii isolates from different areas and hosts from China
| Isolates | Host | Origin | SAG1 | SAG2 | SAG3 | BTUB | GRA6 | c22-8 | c29-2 | L358 | PK1 | Apico | CS3 | ToxoDB PCR-RFLP # | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GT1, reference |
Goat |
USA |
I |
I |
I |
I |
I |
I |
I |
I |
I |
I |
I |
#10 |
Type I |
| PTG, reference |
Sheep |
USA |
II/III |
II |
II |
II |
II |
II |
II |
II |
II |
II |
II |
#1 |
Type II |
| CTG, reference |
Cat |
USA |
II/III |
III |
III |
III |
III |
III |
III |
III |
III |
III |
III |
#2 |
Type III |
| TgCgCa1, reference |
Cougar |
Canada |
I |
II |
III |
II |
II |
II |
μ-1a |
I |
μ-2 a |
I |
II |
#66 |
Atypical |
| MAS, reference |
Human |
France |
μ-1 a |
II |
III |
III |
III |
μ-1 a |
I |
I |
III |
I |
II |
#17 |
Atypical |
| TgCatBr5, reference |
Cat |
Brazil |
I |
III |
III |
III |
III |
I |
I |
I |
μ-1 a |
I |
II |
#19 |
Atypical |
| TgCatBr64, reference |
Cat |
Brazil |
I |
μ-1 a |
III |
III |
III |
μ-1 a |
I |
III |
III |
I |
III |
#111 |
Atypical |
| TgRsCr1, reference |
Toucan |
Costa Rica |
μ-1 a |
II |
III |
I |
III |
μ-2 a |
I |
I |
III |
I |
III |
#52 |
Atypical |
| TgCtgy1,2,3,4,5 |
Cat |
Guiyang, Guizhou |
μ-1 a |
II |
III |
III |
II |
II |
III |
II |
II |
I |
II |
#9 |
China 1 |
| TgPggy1,2,3,4 |
Pig |
Guiyang, Guizhou |
μ-1 a |
II |
III |
III |
II |
II |
III |
II |
II |
I |
II |
#9 |
China 1 |
| TgCksz1 |
Chicken |
Suzhou, Anhui |
I |
I |
III |
I |
I |
I |
I |
I |
I |
I |
I |
#225 |
|
| TgVowh1 | Vole | Wuhan, Hubei | μ-1 a | II | III | III | II | II | III | II | II | I | II | #9 | China 1 |
a u-1 and u-2 are new alleles that are different from the clonal type I, II and III alleles.
The present results showed that 10 isolates distributed in two provinces were grouped into one genotype (type China 1) based on the 11 markers analyzed. The genotype presented type II patterns at SAG2, GRA6, L358, PK1, c22-8 and CS3, but c29-2, SAG3, BTUB loci displayed a type III pattern and type I at the Apico locus. This genotype has already been found in three other hosts (cats, pigs and humans) but in voles for the first time from China. Type China 1 has been described not only in isolates from eastern China (Shandong, Jiangsu, Anhui and Zhejiang) but also in isolates from southern China (Guangdong), Central China (Henan and Hubei), northwest (Gansu) and southwest (Yunnan) of China. Therefore, the genotype is most likely a common genotype circulating in mainland China.
The remaining isolate (TgCksz1) was a variant of the archetypal type I, as all alleles observed were of type I except for the SAG3 locus where an allele of type III was observed. The SAG3 fragment from GT1 strain (type I) contained two restriction sites for the enzyme NciI, whereas the homologous PCR products from the CTG strain (type III) consisted of one restriction site. The nested PCR product for the marker SAG3 from PTG (type II) was not cleaved by NciI. The RFLP analysis of the SAG3 fragment from TgCksz1 revealed an identical pattern with the type III lineages (Table 1). The data were designated here as genotype #225. Furthermore, we determined the genotype by sequencing of a SAG3 gene of T. gondii TgCksz1, reconfirmed that the isolate contained a type III sequence without evidence of any additional mutations (Figure 2). The genotype was new and reported for the first time. This is the first report of genetic typing of T. gondii isolates from chickens and voles in China.
Figure 2.
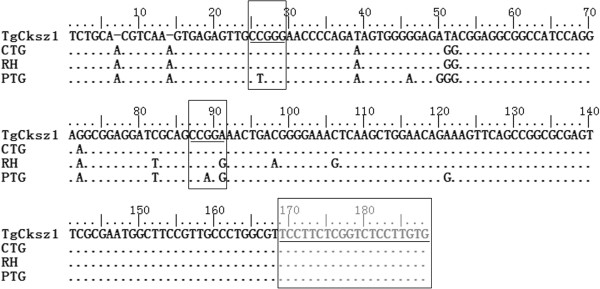
Alignment of sequences of SAG3 fragment from T. gondii types I, II, and III and genotype #225. Shaded box at the end of the sequences corresponds to hybridization sites of primer. Unshaded boxes correspond to the restriction sites for the enzyme NciI.
The phylogenetic network of the representative isolates of T. gondii obtained from chickens, pigs and cats, together with previously published data were compared using SplitsTree4 software [32], with taxa positioned in the typical star-like network (Figure 3). A cluster analysis of the data showed that isolates TgCtgy2, 5 and TgPggy1 formed a single group, and a few major clonal lineages of T. gondii dominant in different geographical regions.
Figure 3.
Phylogenetic network analysis with representatives of T. gondii genotypes available in ToxoDB database. Genotype number (#) and the representative strains are listed for each taxonomic branch.
Virulence of T. gondii isolates with type China 1
The in vivo virulence of the T. gondii isolate TgCtgy5 in KM mice was determined using different tachyzoite doses. Mortality data after inoculation with tachyzoites are shown in Figure 4. All mice inoculated with tachyzoites died of toxoplasmosis between 5 d and 15 d post-infection, irrespective of the inoculation dose, indicating that the isolate was virulent in mice. The tachyzoites were found in peritoneal fluid of all dead mice, and the day of death was correlated with the inoculum dose. This result indicates that highly virulent T. gondii isolates are circulating in cats and they may cause more severe toxoplasmosis when spreading into human population.
Figure 4.
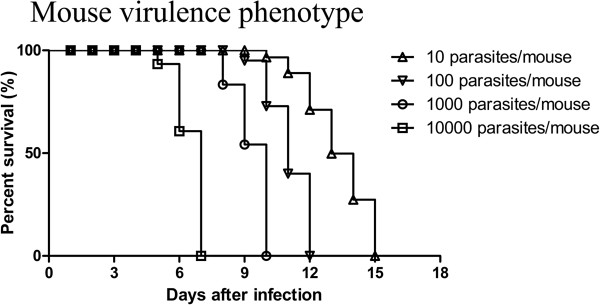
Mouse survival rates with T. gondii genotype China 1. Virulence of T. gondii type China 1 isolate TgCtgy5 was monitored in mice. Survival of KM mice was monitored for 45 d following intraperitoneal inoculation with different doses of tachyzoites indicated. Ten parasites of TgCtgy5 isolate killed 100% mice within 15 d.
Discussion
We investigated the genetic diversity of T. gondii isolates from chickens, pigs, cats and voles from central and southwestern China. Among food animals, chickens and pigs are considered the most important reservoirs for T. gondii transmission and the isolation of T. gondii from chickens and pigs is a public health concern as human consumption of these meats continues to increase. The detection and identification of T. gondii from chickens, pigs and rodents facilitate our understanding of the epidemiology, population structure and virulence of T. gondii. Worldwide seroprevalence of T. gondii infection in chickens and pigs are summarized by Dubey [35,36], depending on the source of chickens and pigs and the sensitivity of diagnostic tests. In China, the seroprevalence of T. gondii infection in free-range chickens from 13 provinces/municipalities was 6.7%-47.3% [20], and that of pigs in most studies ranged between 12.0% and 53.4% [19,21,37], no genotyping data have been presented so far. These survey results indicated that infection with T. gondii in chickens and pigs is widespread in China, and that chicken and pork may be an important source for human infection with T. gondii.
Genetic diversity of T. gondii isolates has been an interesting and important research topic. In the present study, we isolated and completely genotyped 11 T. gondii isolates from chickens, pigs, cats and voles in eastern, southwest and central China, respectively. Analysis of the multilocus PCR-RFLP revealed 2 genotypes, one was identified in ten isolates (type China 1), while the other is represented by only one isolate and was described for the first time in the literature (Table 1). Our results indicated a limited diversity of T. gondii isolates from the animal population in China, a similar result as reported by other authors in studies of parasites isolated from different regions and host species [16,17,38,39]. All previous RFLP genotyping data and the present experiments of T. gondii isolates (totally 119) from China are summarized in Table 2. The compiled results indicated that type II and type III are rare in China. However, the type China 1 and type I accounted for 73.9 and 10.9%, respectively. The genotype China 1 has already been described in several hosts such as pigs, cats and humans, but this is the first report of this genotype in voles. Among intermediate hosts, infected rodents are considered the most important sources of T. gondii infection for cats. The type China 1 lineage was found for vole isolates in the present study, reconfirming that this lineage is the most common and widespread in different hosts and geographical locations in China. Overall, our current study is in agreement with previous report that T. gondii type China 1 is the dominating genotype in animals as well as humans within China [16]. The limited data on T. gondii isolates detected in food animals and wildlife, like pigs or voles, seem to reflect the dominant T. gondii type China 1 which is commonly found in livestock, poultry and humans. Interestingly, this genotype has also been identified in T. gondii isolates of dogs from Sri Lanka and Colombia [40,41], chickens from Brazil [27] and of sheep from the United States [42], suggesting that such a genotype might be widespread from Asia to North and South America. Further analysis of these T. gondii isolates at the DNA sequence level may help us understand the origin and migration of this parasite among different continents.
Table 2.
Genetic PCR-RFLP types of T. gondii in animals and humans in China
| Host | No. of isolates |
ToxoDB PCR-RFLP genotypes |
References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #10 (Type I) | #1 (Type II) | #2 (Type III) | #3 (Type II-variant) | #9 (China 1) | #18 | #204 | #205 | #213 | #225 | |||
| Cats |
72 |
1 |
0 |
0 |
0 |
65 |
2 |
0 |
4 |
0 |
0 |
[15,16,38] and this study |
| Pigs |
33 |
12 |
0 |
0 |
0 |
19 |
0 |
0 |
0 |
2 |
0 |
[22,39,43] and this study |
| Sheep |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
[17] |
| Rabbits |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
[44] |
| Wild birds |
3 |
1 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
[45] |
| Chickens |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
This study |
| Voles |
1 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
This study |
| Humans |
7 |
2 |
1 |
0 |
0 |
3 |
0 |
1 |
0 |
0 |
0 |
[16,17] |
| Total | 119 | 13 (10.9 %) | 1 (0.8 %) | 1 (0.8 %) | 3 (2.5%) | 88 (73.9 %) | 2 (1.7 %) | 1 (0.8 %) | 4 (3.4 %) | 2 (1.7 %) | 1 (0.8 %) | |
There is scarce information concerning the isolation and genotyping of T. gondii isolates from chickens in China. Because free-range chickens become infected mainly by feeding from ground/soil contaminated with oocysts, the prevalence of T. gondii in this host has been widely used as an indicator of the strains prevalent in the environment. In addition, tissues of infected chickens are considered a good source of infection for cats, humans and other animals. In the present investigation, the isolate TgCksz1 (genotype ToxoDB#225) isolated from a chicken in Suzhou city is similar to the genotype of isolate TgCkBr136 (genotype ToxoDB#41), which was collected from chickens in the state of Rondönia in Brazil and identified by Dubey [46]. The genotype ToxoDB#225 was a variant of the type I with a type III genotype at the SAG3 loci. Meanwhile, the genotype ToxoDB#41 showed type II pattern at GRA6 and the remaining 9 loci types were identical to type #225, suggesting that they are phylogenetically related. In addition, the genotype #41 was also identified from newborns in the Minas Gerais state in Brazil [47]. These results suggest that whether this isolate TgCksz1 has been imported into China or is endemic remains unknown based on the genetic data. However, as indicated above, genetically similar isolates were observed in distant regions like these should be investigated in future studies. To our knowledge this is the first report of genetic typing of T. gondii isolates from chickens from China. None of the isolates displayed a clonal genotype (type I, II and III).
Before the recognition of the three T. gondii genotypes, isolates were phenotypically classified as mouse virulent or avirulent. Phenotypically, type I strains are uniformly lethal to outbred mice and type II and III strains are significantly less virulent [11]. In this study, we observed variability in the mouse virulence of the T. gondii isolates with the genotype China 1, including virulent isolates and intermediately isolates. The TgCtgy5 isolate was lethal to outbred KM mice; all infected mice died of acute toxoplasmosis, regardless of the dose used. However, all mice inoculated with 1 × 103 tachyzoites of the isolate TgCtgy2 (type China 1) died but two survived acute infection and cysts were found in the brain tissues of the survivals. The differences of biological features among the isolates sharing the common genotype must not be neglected and it may be possible that the genetic markers used in this study are incapable of reflecting possible phenotypic differences between the isolates under question [48]. Also, in the previous report, we showed that mouse virulence and genetic type are not strictly correlated because two T. gondii isolates (TgCtwh3 and TgCtwh6) share the common genotype but have markedly different mouse virulence, including CS3 locus (type II). Similar phenomenon could also be found in other studies on type China 1 isolates [38]. The marker CS3, located on chromosome VIIa of T. gondii, was previously reported to be linked with the acute virulence in mice of T. gondii and was also used to determine its association to parasite virulence in mice [49]. Several studies indicated that isolates with the alleles I and II at the CS3 locus are strongly associated with virulence in mice [28,31]. The Chinese isolate of T. gondii TgCtwh6, however, has a low virulence to mice but shares the allele II at the CS3 locus. These results suggest that better genetic markers are needed to correlate pathogenicity of T. gondii in animals and humans.
Conclusions
The present study is the first report of T. gondii isolates collection and genotyping obtained from chickens and voles, enriching the limited T. gondii genotype database in China. The genetic structure here found in the isolates from chickens, pigs, cats and rodents corroborates the findings of previous studies that T. gondii has a limited diversity in China. In addition, the results provided preliminary data for further approaches of epidemiology of T. gondii in food animals and wildlife and analysis of population genetics of T. gondii organisms.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JLS, LJ, LW and HWC conceived and designed the study, and critically revised the manuscript. LW, HWC, KQH, YHX and YNL performed the experiments, analyzed the data and drafted the manuscript. JD, LY, QLL and WW participated in the analysis and interpretation of data. All authors have read and approved the final manuscript.
Contributor Information
Lin Wang, Email: linzi6688@126.com.
Hua-Wei Cheng, Email: chenghuawei878@126.com.
Kai-Quan Huang, Email: hkq1510@163.com.
Yuan-Hong Xu, Email: xyhong1964@163.com.
Yong-Nian Li, Email: lyn@gmc.edu.cn.
Jian Du, Email: dujane@163.com.
Li Yu, Email: lilyyu33@126.com.
Qing-Li Luo, Email: ahyslql@126.com.
Wei Wei, Email: wwei@ahmu.edu.cn.
Ling Jiang, Email: ahslyyjl@126.com.
Ji-Long Shen, Email: shenjilong53@126.com.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program, No. 2010CB530001) and National Natural Science Foundation of China (No. 81271864, 30801329). The authors would like to thank the technical and support staff of the Key Laboratory of Zoonoses Anhui for their assistance during sample collection. We also thank Associate Professor Chunlei Su at the Department of Microbiology, the University of Tennessee, Knoxville, Tennessee, for kindly providing with the reference strains (type I (GT1), type II (PTG), type III (CTG) and other strains (TgCgCa1, MAS, TgCatBr5, TgCatBr64, and TgRsCr1).
References
- Dubey JP. Toxoplasmosis of animals and humans. Boca Raton, Florida: CRC Press; 2010. p. 313. [Google Scholar]
- Zhou DH, Zhao FR, Huang SY, Xu MJ, Song HQ, Su C, Zhu XQ. Changes in the proteomic profiles of mouse brain after infection with cyst-forming Toxoplasma gondii. Parasit Vectors. 2013;6:96. doi: 10.1186/1756-3305-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Chen N, Zhang RL, Lin RQ, Zhu XQ. Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. 2008;24:190–196. doi: 10.1016/j.pt.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Zhou P, Chen Z, Li HL, Zheng H, He S, Lin RQ, Zhu XQ. Toxoplasma gondii infection in humans in China. Parasit Vectors. 2011;4:165. doi: 10.1186/1756-3305-4-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbez-Rubinstein A, Ajzenberg D, Darde ML, Cohen R, Dumetre A, Yera H, Gondon E, Janaud JC, Thulliez P. Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J Infect Dis. 2009;199:280–285. doi: 10.1086/595793. [DOI] [PubMed] [Google Scholar]
- Wendte JM, Miller MA, Lambourn DM, Magargal SL, Jessup DA, Grigg ME. Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites Sarcocystis neurona and Toxoplasma gondii. PLoS Genet. 2010;6:e1001261. doi: 10.1371/journal.pgen.1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demar M, Hommel D, Djossou F, Peneau C, Boukhari R, Louvel D, Bourbigot AM, Nasser V, Ajzenberg D, Darde ML, Carme B. Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin Microbiol Infect. 2012;18:E221–E231. doi: 10.1111/j.1469-0691.2011.03648.x. [DOI] [PubMed] [Google Scholar]
- Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Darde ML, Zhu XQ, Ajioka JW, Rosenthal BM, Dubey JP, Sibley LD. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A. 2012;109:5844–5849. doi: 10.1073/pnas.1203190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM, Sibley LD. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol. 2011;41:645–655. doi: 10.1016/j.ijpara.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran C, Su C, Dubey JP. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect Genet Evol. 2012;12:359–368. doi: 10.1016/j.meegid.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Minot S, Melo MB, Li F, Lu D, Niedelman W, Levine SS, Saeij JP. Admixture and recombination among Toxoplasma gondii lineages explain global genome diversity. Proc Natl Acad Sci U S A. 2012;109:13458–13463. doi: 10.1073/pnas.1117047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier A, Devillard S, Ngoubangoye B, Bonnabau H, Banuls AL, Durand P, Salle B, Ajzenberg D, Darde ML. Additional haplogroups of Toxoplasma gondii out of Africa: population structure and mouse-virulence of strains from Gabon. PLoS Negl Trop Dis. 2010;4:e876. doi: 10.1371/journal.pntd.0000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kappany YM, Rajendran C, Abu-Elwafa SA, Hilali M, Su C, Dubey JP. Genetic diversity of Toxoplasma gondii isolates in Egyptian feral cats reveals new genotypes. J Parasitol. 2010;96:1112–1114. doi: 10.1645/GE-2608.1. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Gao JM, Huo XX, Wang L, Yu L, Halm-Lai F, Xu YH, Song WJ, Hide G, Shen JL, Lun ZR. Genotyping of Toxoplasma gondii isolates from cats in different geographic regions of China. Vet Parasitol. 2011;183:166–170. doi: 10.1016/j.vetpar.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen H, Liu D, Huo X, Gao J, Song X, Xu X, Huang K, Liu W, Wang Y. et al. Genotypes and mouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS One. 2013;8:e53483. doi: 10.1371/journal.pone.0053483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Zhang H, Lin RQ, Zhang DL, Song HQ, Su C, Zhu XQ. Genetic characterization of Toxoplasma gondii isolates from China. Parasitol Int. 2009;58:193–195. doi: 10.1016/j.parint.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Chen J, Li ZY, Zhou DH, Liu GH, Zhu XQ. Genetic diversity among Toxoplasma gondii strains from different hosts and geographical regions revealed by sequence analysis of GRA5 gene. Parasit Vectors. 2012;5:279. doi: 10.1186/1756-3305-5-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Wang Z, Feng H, Fang R, Nie H, Hu M, Zhou Y, Zhao J. Seroprevalence and risk factors for Toxoplasma gondii infection on pig farms in central China. J Parasitol. 2011;97:262–264. doi: 10.1645/GE-2646.1. [DOI] [PubMed] [Google Scholar]
- Zhao G, Shen B, Xie Q, Xu LX, Yan RF, Song XK, Hassan IA, Li XR. Detection of Toxoplasma gondii in free-range chickens in China based on circulating antigens and antibodies. Vet Parasitol. 2012;185:72–77. doi: 10.1016/j.vetpar.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu C, Liu Y, Jin H, Zhao Y, Chen J, Yang M, Liu Q. Seroprevalence of Toxoplasma gondii infection in slaughtered pigs and cattle in Liaoning Province, northeastern China. J Parasitol. 2012;98:440–441. doi: 10.1645/GE-2989.1. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang T, Luo Q, Huo X, Wang L, Liu T, Xu X, Wang Y, Lu F, Lun Z. et al. Prevalence and genotypes of Toxoplasma gondii in pork from retail meat stores in Eastern China. Int J Food Microbiol. 2012;157:393–397. doi: 10.1016/j.ijfoodmicro.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Buxton D, Maley SW, Wright SE, Rodger S, Bartley P, Innes EA. Toxoplasma gondii and ovine toxoplasmosis: new aspects of an old story. Vet Parasitol. 2007;149:25–28. doi: 10.1016/j.vetpar.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet Parasitol. 1998;74:75–77. doi: 10.1016/S0304-4017(97)00135-0. [DOI] [PubMed] [Google Scholar]
- El Behairy AM, Choudhary S, Ferreira LR, Kwok OC, Hilali M, Su C, Dubey JP. Genetic characterization of viable Toxoplasma gondii isolates from stray dogs from Giza, Egypt. Vet Parasitol. 2013;193:25–29. doi: 10.1016/j.vetpar.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Su C, Zhang X, Dubey JP. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int J Parasitol. 2006;36:841–848. doi: 10.1016/j.ijpara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Gennari SM, Minervino AH, Farias NA, Ruas JL, dos Santos TR, Cavalcante GT, Kwok OC, Su C. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Para state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Vet Parasitol. 2007;143:182–188. doi: 10.1016/j.vetpar.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Pena HF, Gennari SM, Dubey JP, Su C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int J Parasitol. 2008;38:561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Khan A, Su C, German M, Storch GA, Clifford DB, Sibley LD. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J Clin Microbiol. 2005;43:5881–5887. doi: 10.1128/JCM.43.12.5881-5887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- Pena HF, Vitaliano SN, Beltrame MA, Pereira FE, Gennari SM, Soares RM. PCR-RFLP genotyping of Toxoplasma gondii from chickens from Espirito Santo state, Southeast region, Brazil: new genotypes and a new SAG3 marker allele. Vet Parasitol. 2013;192:111–117. doi: 10.1016/j.vetpar.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS. et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- Homan WL, Vercammen M, De Braekeleer J, Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol. 2000;30:69–75. doi: 10.1016/S0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health. 2010;57:60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis in pigs–the last 20 years. Vet Parasitol. 2009;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Zhang Z, Liu Z, Qu DF, Zhang DF, Zhang HL, Zhou QJ, Du AF. Seroprevalence of Toxoplasma gondii infection in pigs, in Zhejiang Province, China. J Parasitol. 2011;97:748–749. doi: 10.1645/GE-2713.1. [DOI] [PubMed] [Google Scholar]
- Qian W, Wang H, Su C, Shan D, Cui X, Yang N, Lv C, Liu Q. Isolation and characterization of Toxoplasma gondii strains from stray cats revealed a single genotype in Beijing, China. Vet Parasitol. 2012;187:408–413. doi: 10.1016/j.vetpar.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Zhou P, Sun XT, Yin CC, Yang JF, Yuan ZG, Yan HK, Zhu XQ, Zou FC. Genetic characterization of Toxoplasma gondii isolates from pigs in southwestern China. J Parasitol. 2011;97:1193–1195. doi: 10.1645/GE-2851.1. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Rajapakse RP, Wijesundera RR, Sundar N, Velmurugan GV, Kwok OC, Su C. Prevalence of Toxoplasma gondii in dogs from Sri Lanka and genetic characterization of the parasite isolates. Vet Parasitol. 2007;146:341–346. doi: 10.1016/j.vetpar.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Huong LT, Sundar N, Su C. Genetic characterization of Toxoplasma gondii isolates in dogs from Vietnam suggests their South American origin. Vet Parasitol. 2007;146:347–351. doi: 10.1016/j.vetpar.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Hill D, Velmurugan GV, Bandini LA, Kwok OC, Majumdar D, Su C. High prevalence and abundant atypical genotypes of Toxoplasma gondii isolated from lambs destined for human consumption in the USA. Int J Parasitol. 2008;38:999–1006. doi: 10.1016/j.ijpara.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Zhou P, Nie H, Zhang LX, Wang HY, Yin CC, Su C, Zhu XQ, Zhao JL. Genetic characterization of Toxoplasma gondii isolates from pigs in China. J Parasitol. 2010;96:1027–1029. doi: 10.1645/GE-2465.1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang H, Cao J, Gong H, Zhou J. Isolation and genotyping of Toxoplasma gondii from domestic rabbits in China to reveal the prevalence of type III strains. Vet Parasitol. 2013;193:270–276. doi: 10.1016/j.vetpar.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Huang SY, Cong W, Zhou P, Zhou DH, Wu SM, Xu MJ, Zou FC, Song HQ, Zhu XQ. First report of genotyping of Toxoplasma gondii isolates from wild birds in China. J Parasitol. 2012;98:681–682. doi: 10.1645/GE-3038.1. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Velmurugan GV, Chockalingam A, Pena HF, de Oliveira LN, Leifer CA, Gennari SM, Bahia Oliveira LM, Su C. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet Parasitol. 2008;157:299–305. doi: 10.1016/j.vetpar.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AC, Andrade GM, Costa JG, Pinheiro BV, Vasconcelos-Santos DV, Ferreira AM, Su C, Januario JN, Vitor RW. Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in southeastern Brazil. J Clin Microbiol. 2013;51:901–907. doi: 10.1128/JCM.02502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares RM, Silveira LH, da Silva AV, Ragozo A, Galli S, Lopes EG, Gennari SM, de Jesus Pena HF. Genotyping of Toxoplasma gondii isolates from free range chickens in the Pantanal area of Brazil. Vet Parasitol. 2011;178:29–34. doi: 10.1016/j.vetpar.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Khan A, Taylor S, Su C, Mackey AJ, Boyle J, Cole R, Glover D, Tang K, Paulsen IT, Berriman M. et al. Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii. Nucleic Acids Res. 2005;33:2980–2992. doi: 10.1093/nar/gki604. [DOI] [PMC free article] [PubMed] [Google Scholar]