Abstract
Macrophages play a critical role in mediating not only normal tissue healing, but also the host reaction against biomaterial scaffolds. There is increasing interest in regenerative medicine to combine mesenchymal stromal/stem cells (MSCs) with biomaterial scaffolds to modulate inflammatory response while restoring tissue architecture. The objective of the current study was to investigate the interaction between MSCs, encapsulated in hyaluronan–based hydrogel, and differentiating macrophages as measured by extracellular matrix (ECM) gene expression and cytokine, chemokine and growth factors concentrations. Gene expression was analyzed using real-time PCR from MSCs embedded in Carbylan-GSX after 7 days of co-culture with or without CD14+ cells. Protein concentrations were measured using a Bio-plex assay from cell culture supernatants on days 3 and 7 of all conditions. Following seven days, we identified upregulation of collagen-I, collagen-III, pro-collagen, and matrix metalloproteinase-9 genes compared to control conditions. We demonstrate increased concentrations of immunoregulatory cytokines (IL-1β, TNF-α, MIP-1α, IFN-γ, IL-12, IL-10) and remodeling growth factors (VEGF, HGF) in MSC-3D constructs co-cultured with macrophages compared to control conditions, with some temporal variations. Our results indicate an alteration of expressions of ECM proteins important to tissue regeneration and cytokines critical to the inflammatory cascade when 3D constructs were cultured with differentiating macrophages.
Keywords: mesenchymal stromal/stem cells, immunomodulation, biomaterials, tissue engineering, hyaluronic acid
Introduction
A major challenge for cell based therapy has been finding a suitable cell source that can fulfill the function of replacing or remodeling injured tissue and further predicting how that cell will interact within the complex milieu of that wounded tissue bed. Mesenchymal stromal/stem cells (MSCs) are multipotent progenitor cells that as defined by criteria established by the International Society for Cellular Therapy can be derived from several tissue sources such as bone marrow (BM), adipose tissue (AT), and vocal folds (VF)1, 2. MSCs hold promise for clinical applications due to their unique immunomodulatory properties, ability to differentiate into multiple tissue lineages and ease of culture expansion. These cells are also thought to be “immune privileged” and therefore can be rapidly expanded in vitro for “off the shelf” allogeneic transplantation 3–5. In addition, MSCs secrete a spectrum of bioactive molecules that can alter the tissue microenvironment by regulating angiogenesis, inflammation and native cell growth 3, 6.
MSCs together with biomaterial scaffolds have the potential to enhance the regeneration of injured tissues by modulating both the inflammatory response and reparative phases of wound healing. Biocompatible polymers, such as hyaluronan (HA) hydrogel scaffolds are of interest for tissue engineering approaches because they can be modified to mimic the unique properties of the extracellular matrix (ECM) 7 and support cellular attachment, proliferation and engraftment of an injured tissue 8–10. In addition, HA has been demonstrated to participate in the inflammatory cascade by recruiting immune cells to the site of wounded tissue leading to repair and remodeling 8. The dual role of HA in tissue healing could compliment the many functional mechanisms of MSCs. Thus, the use of MSCs together with injectable HA hydrogel scaffolds may provide synergistic effects needed to restore normal tissue function in patients with chronic debilitating soft tissue injuries.
Macrophages play a pivotal role in the host reaction not only to wounded tissue but also against methods to address such defects, including biological scaffolds, dressings, or cell-based therapies. Macrophages exhibit functional plasticity, which allows them to adapt their response to a changing microenvironment 11 such as the implantation of a material or device, and differentiate into distinct immunophenotypes that can either drive or resolve inflammation 12. These polarizations are referred to as “classically activated” macrophages (M1) that upregulate pro-inflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor (TNF), and IL-6, or “alternatively activated” macrophages (M2) that produce anti-inflammatory cytokines, such as IL-1013. As with any physiological response, these broad characterizations are not static and there is overlap among many of the cells, cytokines and growth factors involved in wound healing, inflammation, and foreign body reaction.
Given the known immunomodulatory effects of MSCs, combining these cells with biomaterials may provide an approach for restoring injured tissues and suppressing inflammation. Our research group has previously developed a unique co-culture system in which differentiating macrophages were directly cultured on three dimensional constructs of MSCs encapsulated in an HA based hydrogel. We have shown that following seven days of co-culture, macrophages displayed a more anti-inflammatory phenotype including decreased expression of CD16 and human leukocyte antigen (HLA) – DR and increased expression of CD 20614. In this study we further investigated interactions between macrophages and MSCs derived from different tissue sources while embedded in the hydrogel constructs by analyzing ECM gene expression and cytokine and growth factor concentrations in this unique co-culture system.
Materials and Methods
Human mesenchymal stromal cell isolation
Human MSCs were isolated from BM, AT, and VF of healthy donors based on protocols approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board (IRB) and fully characterized as previously described 2, 15. Two MSC tissue donors were used per source. Cells were cultured at 37° C in a humidified 5% CO2 incubator and media was changed every three days [alpha minimum essential media (α-MEM) supplemented with 10% fetal bovine serum (FBS-Hyclone, Logan, UT, USA), 1X non-essential amino acids (NEAA, Sigma Aldrich, St. Louis, MO, USA), and 4 mM L-glutamine (Sigma Inc.), 100U/mL penicillin, and 0.01 mg/mL streptomycin sulfate (Sigma Inc.)]. VF-MSCs were cultured in Dulbecco’s modified essential medium (DMEM) with 10% FBS, 100U/mL penicillin, 0.01 mg/mL streptomycin sulfate and 1X NEAA (all from Sigma Inc). Cells were expanded until passages four to seven, at which time they were used for this study.
Human monocyte isolation
Human peripheral blood mononuclear cells were isolated from buffy coat samples obtained from three normal healthy donors (Interstate Blood Bank, TN) and isolated by density grade centrifugation as previously described 14, 16. An AutoMACS Pro Separator (Miltenyi Biotech) was used to derive a pure population of CD14+ cells according to manufacturer’s instructions. This isolation method can yield >95% cell purity, as previously shown through flow cytometry analysis 16. Cells were stored at −80°C.
Hyaluronic Acid Hydrogel preparation
A chemically modified HA – gelatin hydrogel (Carbylan – GSX) was developed in collaboration with the Center for Therapeutic Biomaterials at the University of Utah 10, 17, 18. The Carbylan-GSX consists of 8.2% polyethylene glycol diacrylate (PEG-DA, MW 3.4kDa), 1.4% crosslinked thiol-modified HA (HA-DTPH) and 1% thiol-modified gelatin (Gtn-DTPH) and was prepared as previously described 9, 10.
Cell Culture
To analyze the immunomodulatory properties of MSC hydrogel constructs, we embedded MSCs derived from BM, AT, or VF in Carbylan – GSX at a final concentration of 2 × 106 cells/mL as previously described 14. For 3D conditions, 500μL of the mixture was seeded on a transwell permeable support (3.0 μm pore size, Corning, Lowell, MA). The hydrogels were incubated for fifteen minutes at 37° C with 5% CO2 to allow for gel formation and then cell culture medium (phenol red free RPMI – 1640 supplemented with 10% FBS, 2 mM L-glutamine-alanine, 1% sodium pyruvate and 1% NEAA) was added. Isolated CD14+ cells (1 × 106 cells) were thawed, washed, added to each well and cultured for seven days at 37° C in 5% CO2 humidified atmosphere. Three control conditions were included in this study: CD14+ cells cultured on tissue culture polystyrene (TCPS), CD14+ cells cultured on Carbylan-GSX without MSC and MSC encapsulated in Carbylan – GSX without CD14+ cells. Media was changed on day four. After 7 days, macrophages were harvested from the hydrogels and analyzed with flow cytometry for surface marker activation including CD14, CD16, CD206, and HLA-DR as previously described 14.
Gene Expression
To directly compare extracellular matrix gene expression of BM, AT and VF MSC hydrogel constructs co-cultured with CD14+ monocyte derived macrophages (MQ), we measured genes involved in the synthesis and degradation of tissue remodeling. Following seven days of culture, media was aspirated from each well and adherent MQ were harvested from the surface of the hydrogel using previously described methods 14. Total cellular RNA was isolated from MSC-hydrogel constructs cultured with and without MQ using Rneasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. First strand cDNA was synthesized from 1μg of total RNA by using an Omniscript Reverse Transcription Kit (Qiagen). mRNA levels were derived using fold change methods by real-time polymerase chain reaction (RT-PCR) with the LightCycler System and FastStart DNA Master SYBR Green I kit (Roche), as described previously 10, 15. mRNA from the cDNA samples were amplified with specific primer pairs for collagen I α-2 (Col I), pro-collagen (pCol), collagen III (Col III), fibronectin (FN), hyaluronic acid synthase 2 (HAS2), hyaluronidase 2 (HYAL2), elastin, matrix metalloproteinase (MMP) -2 and -9, and vascular endothelial growth factor (VEGF). Amplification of β-actin was used as an internal control. The specificity of each primer pair was confirmed by melting curves and PCR reactions, which showed a single peak and the appropriate sized DNA band for each gene product. The primer sequences, gene bank access number, and expected PCR product sizes are listed in Table 1 for HAS2, HYAL2 and MMP-9. All others have been previously described 10, 15. Exact amplification efficiencies of target and reference genes were assessed by LightCycler software and specificity of each pair of primers was confirmed by melting curves. To normalize the gene expression of each source of MSC-hydrogel construct, ΔCt values were derived for each sample by calculating the difference between gene of interest and the housekeeping gene. The log scale of the difference in ΔΔCt values of experimental and control samples derived the fold change in gene expression. A diluted PCR product was used to assess the PCR replication efficiency for all genes.
Table 1.
Primer Sequence and Products of Real-Time Polymerase chain reaction (RT-PCR)
| Gene | GenBank # | Forward Primer | Reverse Primer | Size of PCR |
|---|---|---|---|---|
| MMP9 | NM_004994 | 5′-ATTTCTGCCAGGACCGCTTCTACT-3′ | 5′-CAGTTTGTATCCGGCAAACTGGCT-3′ | 195bp |
| HAS2 | NM_005328 | 5′-CCCTTTGCATCGCTGCCTATCAAG-3′ | 5′-GGCTGATTTGTCTCTGCCCATGAC-3′ | 176bp |
| HYAL2 | NM_003773 | 5′-AACGTGTGGAGCACTACATTCGGA-3′ | 5′-ACCGGCGATACACATCTTTGTCCT-3′ | 117bp |
Protein Expression
To determine differences in cytokine, chemokine and growth factor protein expression from BM, AT and VF MSC-hydrogel constructs co-cultured with CD14+ cells, a Bio-Plex assay was performed according to the manufacturer’s protocol (Bio-rad Laboratories, Inc., CA). The following human analytes were measured: Interluekin (IL) -1β, IL-6, IL-8, IL-10, IL-12, basic fibroblast growth factor (FGF), granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, macrophage inflammatory protein (MIP)-1α, TNF-α, monocyte chemotactic protein (MCP)-1, VEGF, hepatocyte growth factor (HGF), macrophage colony stimulating factor (M-CSF), SDF-1α, vascular cell adhesion protein (VCAM), and inter-cellular adhesion molecule (ICAM). Briefly, 50μL of cell-free culture supernatants from days 3 and 7 were incubated with 50 μL of coupled magnetic beads in a 96-well microtitre plate for 30 minutes. All incubations were done at room temperature on a shaker at 300 rpm. The filter plate was then washed three times using a MultiScreen Vacuum Manifold (Millipore) and 25μL of detection antibodies was added. After 30 minutes of incubation, the plate was washed three times and 50μL streptavidin-phycoerythrin (PE) added for 10 minutes. After three washes, the beads were re-suspended in each well with 125 μL of assay buffer and plates were read on the Bio-Plex Suspension Array System. Data were then analyzed with Bio-plex Manager software version 5.0.
Statistical Analysis
Independent experiments were performed for each condition in duplicate, using three separate CD14+ cell donors. For each MSC source, two individual donors were used. Data is expressed as the mean ± standard deviation (SD). Statistical comparisons between gene productions of BM, AT, and VF MSC-hydrogel constructs was performed by one-way analysis of variance (ANOVA). A repeated measure ANOVA was used to analyze differences in protein expression between group, day and group by day interaction. If the F-test revealed significant differences at the 0.05 level, pairwise comparisons were used to determine statistical differences between samples (least squares means). A Spearman rank correlation coefficient was used to analyze the relationship between mean CD206 expression and the seventeen secreted proteins. Mean CD206 expression was obtained from previous study 14. A p-value of <0.05 was considered significant. Mean and SD values represent differences across biologic replicates. Statistical interpretations were made using SAS version 9.1.3 (SAS Institute, Cary, N.C.).
Results
Following seven days of co-culture in which CD14+ monocytes were plated in direct contact with HA hydrogel constructs with or without encapsulated MSCs, supernatant was removed and analyzed for protein concentration via Bio-plex assay, macrophages were studied with flow cytometry for surface marker expression (previously reported 14), and MSCs were extracted from the gels and analyzed via RT-PCR. Our interest was in comparing expression of mRNA encoding for ECM proteins and proteolytic enzymes important in tissue remodeling, pCol, Col I, Col III, FN, HAS2, HYAL2, elastin, MMP -2 and -9, and VEGF. Additionally, to see if the inflammatory profile demonstrated during CD14+ monocyte to macrophage differentiation was associated with a functional difference, we looked at cytokines and growth factors important in the balance of inflammation and tissue regeneration, including IL-1β, IL-6, IL-8, IL-10, IL-12, FGF, GM-CSF, IFN-γ, MIP-1α, TNF-α, MCP-1, VEGF, HGF, M-CSF, SDF-1α, VCAM and ICAM, from supernatant collected at days three and seven. In the following results, growth factors, proteins and cytokines are categorized based on their predominant physiological role in wound healing and inflammation, though there is certainly overlap among these subgroups, as follows: tissue remodeling, early phase inflammatory cytokines, chemotactic cytokines, immunoregulatory cytokines, growth factors, and adhesion molecules.
The results include studies containing 3D MSCs Carbylan-GSX constructs co-cultured with CD14+ macrophages (MSC-3D+ MQ), MSC-Carbylan – GSX controls without macrophages (MSC-3D), CD14+ macrophages cultured on Carbylan-GSX without MSCs as the macrophage – hydrogel control (MQ-2D), and CD14+ macrophages cultured on polystyrene (MQ-TCPS) as the unstimulated macrophage control. Co-cultured MSC-macrophage control experiments on polystyrene dishes were performed in these studies; however, these groups could not be adequately standardized to account for MSC proliferation, overcrowding, and other factors that would affect cell viability, paracrine or autocrine signaling, and misrepresent these conditions as a true control.
ECM remodeling is upregulated in MSCs encapsulated in Carbylan – GSX co-cultured with macrophages
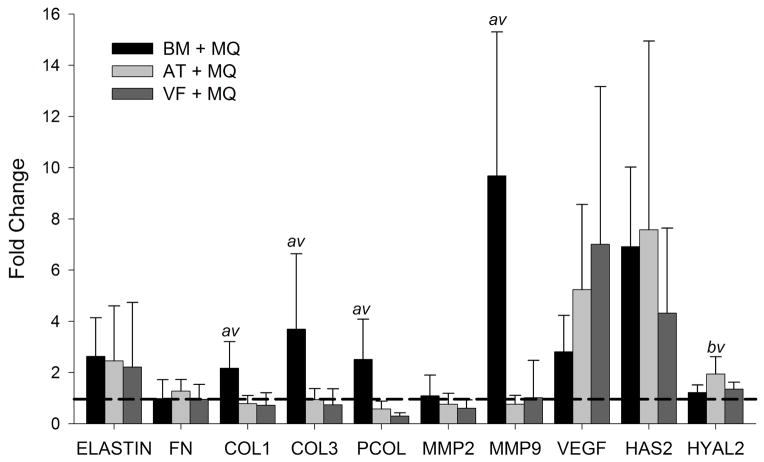
Gene expression was analyzed using RT-PCR in MSCs extracted from construct digests following seven days of co-culture. Results from mRNA expression of BM, AT and VF MSCs are shown in Figure 1. Data are shown as the fold change, comparing the ratio of the gene expression levels in the control group MSCs Carbylan – GSX hydrogel (MSC-3D) and co-culture conditions of MSC – Carbylan – GSX hydrogels with CD14+ (MSC-3D+MQ). Collagen homeostasis is a hallmark of the remodeling phase of wound healing and characterized by variable expression of pCol, Col I and Col III, as well as MMP-9 responsible for protein degradation. Co-cultures containing BM –MSCs demonstrate significantly higher expression of pCol, (fold change 2.504, p<0.0025, p<0.001), Col I (2.168, p< 0.004, p<0.003), Col III (3.691, p <0.015, p<0.01), and MMP-9 (9.684; p<0.001, p<0.001) compared to AT – (0.757) and VF – (1.012) MSC groups, respectively. Additionally, AT-3D+MQ conditions had significant upregulation of HYAL 2 (1.9442) compared to BM (p<0.02) and VF (p<0.04) MSC-3D+MQ conditions. Of the remaining genes investigated, all of the 3D co-culture conditions showed increased expression of VEGF (BM 2.805, AT 5.234, VF 7.009), elastin (BM 2.628, AT 2.453, VF 2.210) and HAS2 (BM 6.912, AT 7.574, VF 4.313) compared to MSC-3D controls (p<0.05); there were no significant differences in expression between MSC subtypes.
Figure 1.
BM, AT, and VF MSC in Carbylan-GSX produce unique gene profiles after 7 days co-culture with macrophages (MSC-3D+ MQ). Y-axis is the fold change of the target gene normalized by the housekeeping gene, β-Actin, relative to the untreated control (MSC-2D). The dashed line represents controls. Letters represents a statistical significance of p<0.05 when compared to b = bone marrow, a= adipose, or v = vocal fold MSC-3D+MQ.
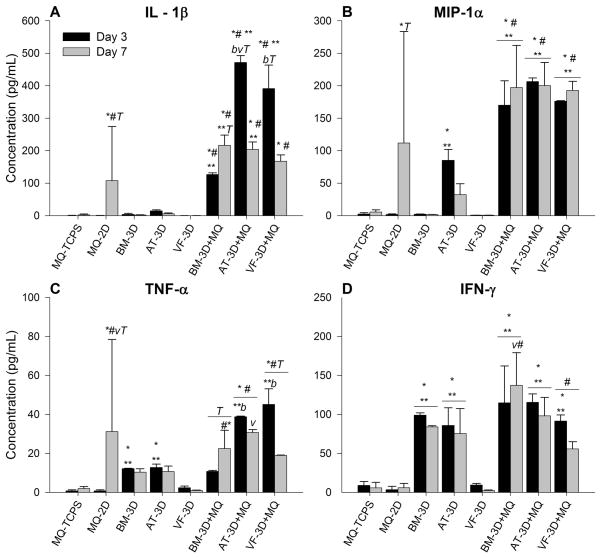
Early phase inflammatory cytokines demonstrate temporal variation in co-culture studies
Inflammation is initiated by early expression of several cytokines, leading to a complex, coordinated signaling cascade with downstream regulation and multiple feedback loops, both positive and negative. Predominantly pro-inflammatory cytokines include: IL-1β, TNF-α, IFN – γ and MIP-1α. These are early phase proteins released by activated macrophages. Mean concentrations of these cytokines (± standard deviation) from supernatant taken at days three and seven are shown in Table 2 and graphically in Figure 2. There are differentially expressed concentrations of all four predominantly pro-inflammatory cytokines at days three and seven. IL-1β concentrations (Figure 2A) increased early (day 3) in the MSC-3D+MQ culture groups compared to macrophage 2D controls (BM p<0.01, AT p<0.001, VF p<0.001), TCPS controls (BM p<0.01, AT p<0.001, VF p<0.001) and MSC-3D controls (BM p<0.001, AT p<0.01, VF p<0.001). These concentrations significantly decreased with time in MSC containing groups, MSC-3D+MQ (BM p<0.01, AT p<0.001, VF p<0.001) and MSC-3D (BM p<0.001, AT p<0.001, VF p<0.001) and there was a similar significant increase in the macrophage 2D hydrogel control (p<0.03). By day 7 there was no difference between BM, AT and VF – MSC-3D+MQ groups though BM (p<0.01) and AT (p<0.02) conditions remained slightly higher than MQ-2D controls. Concentrations of MIP-1α (Figure 2B) were significantly elevated at day 3 in all MSC-3D+MQ groups compared to macrophage 2D controls (BM p< 0.002, AT p< 0.001, VF p< 0.001), TCPS controls (BM p< 0.002, AT p< 0.001, VF p< 0.001) and MSC controls (BM p<0.001, AT p<0.002, VF p<0.001) and remained significantly higher at day 7 (BM p< 0.03, p<0.001, p<0.001; AT p< 0.03, p<0.001 p<0.001; VF p< 0.03, p<0.001 p<0.001). There was no statistical difference between BM, AT and VF MSC subgroups. There was a significant increase in concentrations observed in the MQ-2D controls at day 7 (p<0.02) that approached concentrations of the MSC-3D+MQ groups though this remained significantly less.
Table 2.
Pro-inflammatory Cytokines. Expression levels of IL-1β, IFN-γ, TNF-α, and MIP-1α at days 3 and 7 in the following conditions: BM, AT, and VF MSC embedded in Carbylan-GSX co-cultured with CD14+ cells (MSC-3D+ MQ), MSC embedded in Carbylan-GSX alone (MSC-3D), CD14+ cells cultured on Carbylan-GSX alone (MQ-2D), and MQ-TCPS. Data reflects the mean ± SD in pg/ml. Each symbol, number, letter and day (Δ) represents p<0.05 of the differences between the means.
| IL-1β | IFN-γ | TNF-α | MIP-1α | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Days | 3 | 7 | 3 | 7 | 3 | 7 | 3 | 7 |
| MQ-TCPS (*) | 0.50 ± 0.33 | 2.3 ± 2.96 | 8.9 ± 4.90 | 5.8 ± 7.00 | 0.80 ± 0.45 | 2.0 ± 1.00 | 2.4 ± 2.12 | 5.6 ± 3.20 |
| MQ-2D (#) | 0.42 ± 0.16 | 107.9 ±166.51*12? | 3.4 ± 4.17 | 6.0 ± 5.34 | 0.73 ± 0.58 | 31.2 ± 47.26*23v ? | 1.8 ± 0.91 | 111.7 ± 171.50*123? |
| BM - 3D (1) | 3.89 ± 2.97 | 1.7 ± 0.98 | 99.0 ± 2.85*#3 | 83.9 ± 1.54*#3 | 12.2 ± 0.18* #3 | 10.4 ± 1.74#3b | 2.1 ± 0.32 | 1.8 ± 0.18 |
| AT - 3D (2) | 14.6 ± 2.99 | 5.6 ± 2.34 | 85.7 ± 22.69*#3 | 75.4 ± 32.11*#3 | 12.8 ± 1.74* #3 | 10.7 ± 2.82#3 | 85.1 ± 16.80*#13 | 32.3 ± 16.85 |
| VF - 3D (3) | 0.32 ± 0.03 | 0.27 ± 0.02 | 9.4 ± 1.96 | 2.5 ± 0.61 | 2.3 ± 0.89 | 0.96 ± 0.20 | 0.64 ± 0.10 | 0.57 ± 0.08 |
| BM-3D+MQ(b) | 126.7 ± 5.23*#13v ? | 216.4 ± 31.32*#2 | 114.7 ± 47.38*# | 137.1 ± 41.90*#123v | 10.8 ± 0.46 | 22.4 ± 9.38*123? | 169.9 ± 37.61*#123 | 197.2 ± 64.81*#123 |
| AT-3D+MQ(a) | 471.6 ± 20.94*#123bv ? | 204.3 ± 22.38*#123 | 115.5 ± 10.78*#3 | 98.1 ± 23.70*#3 | 38.8 ± 0.33* #123b | 30.7 ± 1.47*123v | 206.4 ± 5.50* #123 | 200.2 ± 35.49*#123 |
| VF-3D+MQ(v) | 391.2 ± 72.32*#13ab? | 167.5 ± 19.67* | 91.5 ± 7.84*#23 | 55.7 ± 9.193 | 45.1 ±8.04*#123b? | 19.0 ± 0.16*3 | 176.2 ± 0.73* #123 | 192.6 ± 14.15*#123 |
Figure 2.
BM, AT or VF MSC embedded in Carbylan-GSX co-cultured with macrophages (MSC-3D+ MQ) secrete more pro-inflammatory cytokines compared to hydrogel only conditions. Data from days 3 and 7 are presented as the mean ± SD of the following analytes: (A) IL-1β, (B) MIP-1α, (C) TNF-α, (D) IFN-γ. Symbols represents a statistical significance of p<0.05 when compared to * = MQ-TCPS, ** = MQ-2D, and # = MSC-3D (no CD14+ cells). Letters represents a statistical significance of p<0.05 when compared to b = bone marrow, a= adipose, or v = vocal fold MSC-3D+MQ and T = days 3 and 7 are compared within treatment groups.
There was temporal variation in concentration of TNF-α, with a significant increase at day 3, primarily in the AT (p< 0.001) and VF (p< 0.001) MSC-3D+MQ groups compared to controls and BM MSC-3D+MQ groups (Figure 2C). By day seven, however, these values normalize and there was no statistical difference between MSC-3D+MQ subgroups and MQ-2D hydrogel controls. Finally, IFN – γ concentrations remained low in the MQ-2D hydrogel and VF MSC-3D controls at days 3 and 7, similar to unstimulated macrophage controls (MQ-TCPS) (Figure 2D). Interestingly, BM and AT MSC-containing groups (i.e. MSC-3D controls as well as the MSC-3D+MQ co-culture groups) demonstrated expression patterns independent of macrophages with no significant differences found across culture conditions whereas VF MSC-3D controls had significantly lower expression than their MSC-3D+MQ cultures (day 3: p<0.002; day 7: p<0.01). Statistical differences were also found between BM and VF MSC-3D+ MQ at day 7 with BM co-culture conditions having high expression levels (p<0.01)
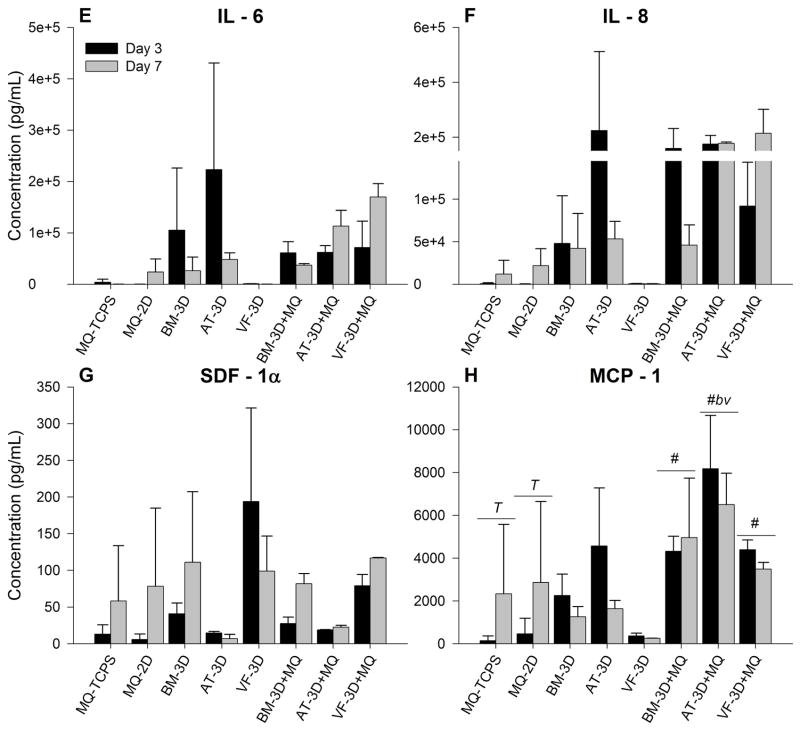
Chemotactic cytokines are equivalent among co-culture studies
The initiation of an inflammatory response, through IL-1β and TNF-α among others, is followed by chemotaxis and activation of both circulating and resident tissue cells. This is led in part by IL – 6, IL – 8, SDF – 1 and MCP – 1. In addition to recruiting neutrophils and macrophages to the wounded tissue, these cytokines signal endothelial progenitor cells, fibroblasts and keratinocytes as a wound bed transitions from acute inflammation to granulation and remodeling 19. Mean concentrations of these cytokines (± standard deviation) from supernatant taken at days 3 and 7 are shown in Table 3 and graphically in Figure 3. While there were increased expression of IL – 6, IL – 8, and SDF – 1a in the MSC-3D+MQ and MSC-3D control conditions these did not achieve statistical significance with any group. Concentrations of MCP – 1 were significantly different between MSC subtypes at day 3, with AT MSC-3D+MQ conditions having the highest expression compared to similar conditions with BM- (p<0.04) and VF MSC (p<0.04). Significant elevation was also found at day 7 in the MSC-3D+MQ conditions compared to MSC-3D controls (BM p<0.03, AT p<0.01, VF p<0.05), however these were comparable to both macrophage control groups (MQ-2D and MQ-TCPS) (Figure 3H).
Table 3.
Chemokines. Expression levels of IL-6, IL-8, SDF-1, and MCP-1 at days 3 and 7 in the following conditions: BM, AT, and VF MSC embedded in Carbylan-GSX co-cultured with CD14+ cells (MSC-3D+ MQ), MSC embedded in Carbylan-GSX alone (MSC-3D), CD14+ cells cultured on Carbylan-GSX alone (MQ-2D), and MQ-TCPS. Data reflects the mean ± SD in pg/ml. Each symbol, number, letter and day (Δ) represents p<0.05 of the differences between the means.
| IL-6 | IL-8 | SDF-1 | MCP-1 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Days | 3 | 7 | 3 | 7 | 3 | 7 | 3 | 7 |
| MQ-TCPS (*) | 3,805 ± 5,991 | 83 ± 128.35 | 660 ± 1,023 | 11,877 ± 16,249 | 13 ± 12.87 | 58.4 ± 75.18 | 145 ± 225 | 2,335 ± 3,242 |
| MQ-2D (#) | 6 ± 8.37 | 23,986 ± 25,370 | 206 ± 319 | 21,867 ± 19,890 | 6 ± 7.28 | 78.5 ± 106.25 | 468 ± 727 | 2,864 ± 3,785 |
| BM - 3D (1) | 105,365 ± 120,962 | 26,003 ± 26,903 | 47,979 ± 56,163 | 42,178 ± 41,061 | 41 ± 14.65 | 111 ± 96.13 | 2,260 ± 995 | 1,266 ± 472 |
| AT - 3D (2) | 223,243 ± 207,343 | 48,285 ± 12,781 | 224,320 ± 287,640 | 53,291 ± 20,722 | 15 ± 2.14 | 7.01 ± 5.97 | 4,572 ± 2,706*3 | 1,641 ± 387 |
| VF - 3D (3) | 1,133 ± 164.25 | 159 ± 55.08 | 776 ± 96.49 | 407 ± 347.09 | 194 ± 127.39 | 98.8 ± 47.97 | 365 ± 133 | 255 ± 5.36 |
| BM-3D+MQ(b) | 61,170 ± 21,829 | 37,116 ± 3,047 | 159,479 ± 72,205 | 45,968 ± 23,837 | 28 ± 8.91 | 81.9 ± 13.83 | 4,323 ± 7023 | 4,959 ± 2,77513 |
| AT-3D+MQ(a) | 62,118 ± 12,997 | 113,131 ± 30,675 | 174,618 ± 31,734 | 177,838 ± 4,414 | 19 ± 0.64 | 22.8 ± 2.40 | 8,180 ± 2,481 *#123bv | 6,508 ± 1,460123 |
| VF-3D+MQ(v) | 71,407 ± 51,229 | 169,789 ± 25,912 | 92,075 ± 51,447 | 214,370 ± 87,668 | 79 ± 15.18 | 117 ± 0.81 | 4,388 ± 4623 | 3,491 ± 3103 |
Figure 3.
BM, AT or VF MSC embedded in Carbylan-GSX co-cultured with macrophages (MSC-3D+ MQ) secrete more hematopoietic growth factors and adhesions molecules compared to hydrogel only conditions. Data from days 3 and 7 are presented as the mean ± SD of the following analytes: (E) IL-6, (F) IL-8, (G) SDF-1α, (H) MCP-1. Symbols represents a statistical significance of p<0.05 when compared to * = MQ-TCPS, ** = MQ-2D, and # = MSC-3D (no CD14+ cells). Letters represents a statistical significance of p<0.05 when compared to b = bone marrow, a= adipose, or v = vocal fold MSC-3D+MQ and T = days 3 and 7 are compared within treatment groups.
Regulatory cytokines are elevated in co-culture studies
IL – 10 is thought to be the primary signal along an anti-inflammatory pathway. While traditionally considered a pro-inflammatory cytokine, IL – 12 has been shown in vitro to influence stromal cell interactions and matrix remodeling as well as angiogenesis. This has largely been demonstrated in anti-tumor immunity and the exact interactions in tissue healing are less well-known 6. The mean concentrations of these cytokines (± standard deviation) from supernatant taken at days 3 and 7 are shown in Table 4 and graphically in Figure 4. Both macrophage control groups, MQ-2D and MQ-TCPS, had very low concentrations of IL – 10 and IL – 12 over seven day cultures. There were significantly higher concentrations of IL – 10 at day 3 and 7 in only the AT-3D+MQ conditions compared to macrophage 2D controls (p< 0.01, p< 0.03) and TCPS controls (p< 0.01, p< 0.02), while IL – 12 was significantly elevated in AT- and VF- MSC-3D+MQ containing groups compared to macrophage 2D control (AT p<0.005, p<0.01; VF p<0.01, p<0.03) and TCPS controls (AT p<0.005, p<0.01; VF p<0.01, p<0.03) (Figure 4J). Significantly elevated expression of IL -10 and -12 was seen at day 3 with AT MSC-3D+MQ conditions compared to similar conditions with BM (p<0.02; p<0.003) and their MSC-3D controls (p<0.04).
Table 4.
Regulatory Cytokines. Expression levels of IL-10 and IL-12 at days 3 and 7 in the following conditions: BM, AT, and VF MSC embedded in Carbylan-GSX co-cultured with CD14+ cells (MSC-3D+ MQ), MSC embedded in Carbylan-GSX alone (MSC-3D), CD14+ cells cultured on Carbylan-GSX alone (MQ-2D), and MQ-TCPS. Data reflects the mean ± SD in pg/ml. Each symbol, number, letter and day (Δ) represents p<0.05 of the differences between the means.
| IL-10 | IL-12 | |||
|---|---|---|---|---|
|
| ||||
| Days | 3 | 7 | 3 | 7 |
| MQ-TCPS (*) | 0.1 ± 0.13 | 0.46 ± 0.37 | 0.03 ± 0.07 | 0.1 ± 0.13 |
| MQ-2D (#) | 0.03 ± 0.04 | 1.83 ± 2.22 | 0 ± 0.01 | 0.33 ± 0.53 |
| BM - 3D (1) | 5.51 ± 0.35 | 5.15 ± 0.26 | 30.1 ± 1.50* #3b | 24.68 ± 2.43*#3b |
| AT - 3D (2) | 7.03 ± 0.69 | 24.18 ± 14.83*#23bv | 27.03 ± 7.30* #3b | 22.21 ± 3.33*#3 |
| VF - 3D (3) | 1.67 ± 0.69 | 1.64 ± 0.88 | 12 ± 6.23 | 7.11 ± 3.56 |
| BM-3D+MQ (b) | 5.64 ± 1.59 | 11.48 ± 0.22 | 3.12 ± 1.37 | 12.88 ± 11.87 |
| AT-3D+MQ (a) | 23.1 ± 6.18*#123b | 19.84 ± 7.32*#23 | 27.45 ± 0.40*#3b | 23.29 ± 0.54*#3 |
| VF-3D+MQ (v) | 15.25 ± 0.58 | 8.31 ± 0.91 | 22.06 ± 2.84*#b | 17.68 ± 5.82*#3 |
Figure 4.
BM, AT or VF MSC embedded in Carbylan-GSX co-cultured with macrophages (MSC-3D+ MQ) secrete more regulatory cytokine and stromal growth factors compared to hydrogel only conditions. Data from days 3 and 7 are presented as the mean ± SD of the following analytes: (I) IL-10, (J) IL-12, (K) VEGF, (L) HGF. Symbols represents a statistical significance of p<0.05 when compared to * = MQ-TCPS, ** = MQ-2D, and # = MSC-3D (no CD14+ cells Letters represents a statistical significance of p<0.05 when compared to b = bone marrow, a= adipose, or v = vocal fold MSC-3D+MQ and T = days 3 and 7 are compared within treatment groups.
Stromal growth factors are elevated in co-culture studies
In addition to inflammatory markers, we analyzed stromal and hematopoietic growth factors. VEGF concentrations were significantly elevated in BM and AT MSC– 3D controls (p<0.001, p<0.01) and their co-culture groups (MSC-3D+MQ) (p<0.001, p<0.02) compared to macrophage 2D and TCPS controls (Figure 4K). Of note, the BM MSC-3D+MQ condition was significantly higher than similar conditions with adipose or vocal fold MSC (p<0.02, p<0.002). There were no statistically significant temporal differences found with VEGF. HGF was increased early (day 3) in all MSC-groups, however, only in the vocal fold cultures, both VF MSC – 3D and VF-3D+MQ conditions, was this statistically significant at days 3 and 7 compared to other MSC groups (all p<0.001) and macrophage controls (all p<0.002) (Figure 4L). Minimal amounts of basic FGF were detected across all conditions and no significant differences were found (Table 5).
Table 5.
Stromal Growth Factors. Expression levels of VEGF, HGF, and Basic FGF at days 3 and 7 in the following conditions: BM, AT, and VF MSC embedded in Carbylan-GSX co-cultured with CD14+ cells (MSC-3D+ MQ), MSC embedded in Carbylan-GSX alone (MSC-3D), CD14+ cells cultured on Carbylan-GSX alone (MQ-2D), and MQ-TCPS. Data reflects the mean ± SD in pg/ml. Each symbol, number, letter and day (Δ) represents p<0.05 of the difference between the largest sample mean and the condition.
| VEGF | HGF | Basic FGF | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Days | 3 | 7 | 3 | 7 | 3 | 7 |
| MQ-TCPS (*) | 26.7 ± 18.03 | 33.1 ± 46.15 | 2.6 ± 2.86 | 61.0 ± 86.21 | 0.41 ± 1.00 | 0.07 ± 0.17 |
| MQ-2D (#) | 1,106 ± 1,675 | 914.6 ± 1,346 | 26.5 ± 40.94 | 16.8 ± 7.71 | 0.07 ± 0.17 | 0.07 ± 0.17 |
| BM - 3D (1) | 20,860 ± 5,208*#3v | 17,604 ± 1,475*#3v | 1,827 ± 312 | 895 ± 5.40 | 4.45 ± 0.34 | 2.87 ± 0.41 |
| AT - 3D (2) | 15,373 ± 5,475*#3 | 12,700 ± 4,915*#3 | 1,814 ± 954 | 397 ± 168 | 36.05 ± 40.35 | 5.56 ± 3.96 |
| VF - 3D (3) | 2,007 ± 587.28 | 1,453 ± 319.84 | 17,168 ± 389*#12abvΔ | 8,081 ± 257*#12ab | 0.05 ± 0.07 | 0 ± 0 |
| BM-3D+MQ (b) | 17,747 ± 6,870*#3av | 24,516 ± 12,947*#3av | 1,366 ± 697 | 708 ± 522 | 5.5 ± 1.90 | 6.8 ± 1.65 |
| AT-3D+MQ (a) | 10,785 ± 1,252*#3 | 13,237 ± 1,837*#3 | 502 ± 361 | 189 ± 72.04 | 27.11 ± 22.35 | 8.41 ± 2.41 |
| VF-3D+MQ (v) | 7,242 ± 2,648 | 8,185 ± 1,279 | 11,368 ± 3,361* #12ab | 9,864 ±1,941*#12ab | 4.72 ± 0.52 | 2.89 ± 0.19 |
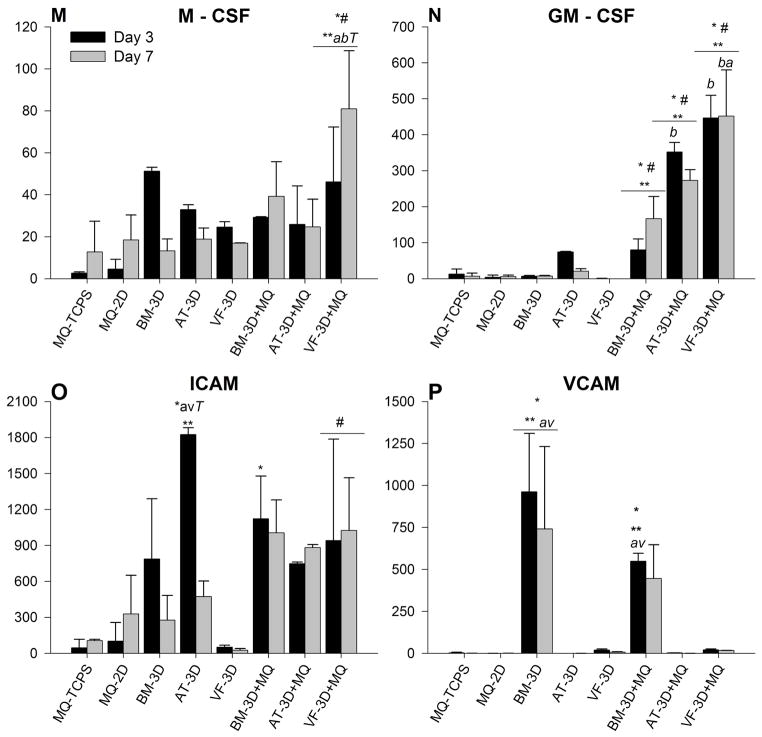
Hematopoietic growth factors are elevated in co-culture studies
As the name implies, M – CSF and GM – CSF are responsible for recruitment and activation of monocytes and granulocytes, a role vital to the inflammatory phase of wound healing. The mean concentrations of these factors (± standard deviation) from supernatant taken at days 3 and 7 are shown in Table 6 and graphically in Figure 5. M – CSF concentrations were variable among all groups with significance increases found in only the VF MSC-3D co-culture conditions compared to similar conditions with other subtypes of MSCs (AT p<0.01, BM p<0.03) and their VF and macrophage controls (all p<0.01)(Figure 5M). No statistically significant temporal differences were found. GM – CSF concentrations in the control groups - MQ-TCPS, MQ-2D and MSC-3D - remained low during the seven day cultures. AT and VF MSC-3D+MQ conditions expressed significantly higher protein concentrations of GM-CSF compared to controls (day 3: all p<0.001; day 7: AT p<0.01, VF p<0.001), again with the VF-3D+MQ group demonstrating the highest levels at day 7 (BM p<0.001; AT p<0.01) (Figure 5N). The BM -MSC-3D+MQ were significantly higher than controls at day 7 (all p< 0.03), though they remained lower than the VF- MSC-3D+MQ values (p<0.001).
Table 6.
Hematopoietic Growth factors and Adhesion Molecules. Expression levels of GM-CSF, M-CSF, ICAM, and VCAM at days 3 and 7 in the following conditions: BM, AT, and VF MSC embedded in Carbylan-GSX co-cultured with CD14+ cells (MSC-3D+ MQ), MSC embedded in Carbylan-GSX alone (MSC-3D), CD14+ cells cultured on Carbylan-GSX alone (MQ-2D), and MQ-TCPS. Data reflects the mean ± SD in pg/ml. No day or group differences were found in M-CSF data. Each symbol, number, letter and day (Δ) represents p<0.05 of the differences between the means.
| GM-CSF | M-CSF | ICAM | VCAM | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Days | 3 | 7 | 3 | 7 | 3 | 7 | 3 | 7 |
| MQ-TCPS (*) | 13.09 ± 13.83 | 6.71 ± 9.17 | 2.68 ± 0.57 | 12.7 ± 14.64 | 47.0 ± 69.93 | 107 ± 11.02 | 2.9 ± 4.45 | 0.26 ± 0.65 |
| MQ-2D (#) | 3.92 ± 5.93 | 5.64 ± 4.62 | 4.64 ± 4.58 | 18.4 ± 11.94 | 102 ± 156.64 | 330 ± 321.79 | 0.16 ± 0.17 | 0.26 ± .65 |
| BM - 3D (1) | 6.79 ± 2.33 | 7.47 ± 1.74 | 51.2 ± 1.84 | 13.2 ± 5.68 | 788 ± 502.40 | 277 ± 206 | 963 ± 348*#23av | 741.4 ± 490*#23av |
| AT - 3D (2) | 74.59 ± 1.33 | 20.8 ± 7.18 | 33 ± 2.25 | 18.8 ± 5.30 | 1824 ± 58*#13avΔ | 473 ± 130.01 | 0 ± 0 | 0.03 ± 0.04 |
| VF - 3D (3) | 0.46 ± 0.56 | 0 ± 0 | 24.6 ± 2.55 | 16.9 ± 0.12 | 51.7 ± 16.53 | 27.6 ± 13.11 | 20.19 ± 5.76 | 6.71 ± 3.09 |
| BM-3D+MQ (b) | 80.29 ± 30.18 | 166.9 ± 61123 | 29.2 ± 0.31 | 39.2 ± 16.47 | 1123 ± 356*3 | 1006 ± 2743 | 548.5 ± 4823av | 446.1 ± 201.07 |
| AT-3D+MQ (a) | 352.1 ± 27*#123b | 273.1 ± 30*#123 | 25.8 ± 18.36 | 24.7 ± 13.25 | 747 ± 14.32 | 883 ± 24.93 | 2.41 ± 0.91 | 0.19 ± 0.27 |
| VF-3D+MQ (v) | 447 ± 63*#123b | 452.1 ± 128*#23ab | 46.2 ± 26*#123ab | 80.9 ± 28*#123ab | 941 ± 8453 | 1025 ± 439.73 | 20.99 ± 5.33 | 18.16 ± 0.19 |
Figure 5.
BM, AT or VF MSC embedded in Carbylan-GSX co-cultured with macrophages (MSC-3D+ MQ) secrete more chemokines compared to hydrogel only conditions. Data from days 3 and 7 are presented as the mean ± SD of the following analytes: (M) M-CSF, (N) GM-CSF, (O) ICAM, (P) VCAM. Symbols represents a statistical significance of p<0.05 when compared to * = MQ-TCPS, ** = MQ-2D, and # = MSC-3D (no CD14+ cells). Letters represents a statistical significance of p<0.05 when compared to b = bone marrow, a= adipose, or v = vocal fold MSC-3D+MQ and T = days 3 and 7 are compared within treatment groups.
Adhesion molecules varied in co-culture studies
Adhesion molecules ICAM and VCAM are involved in cell-to-cell interaction and transmigration throughout the phases of wound healing and tissue remodeling. Mean concentrations of these factors (± standard deviation) from supernatant taken at days 3 and 7 are shown in Table 6 and graphically in Figure 5. While there was elevated expression levels of ICAM observed in all conditions, AT MSC-3D had significantly higher expression at day 3 compared to macrophage controls (p< 0.01) and AT MSC-3D+MQ condition (p< 0.02). Conversely, VF MSC-3D+MQ had significantly higher expression on day 3 (p< 0.03) and day 7 (p< 0.02) compared to their MSC-3D control. VCAM concentrations were only statistically elevated in the BM – MSC containing conditions, at day three BM MSC-3D+MQ co-cultures were significantly higher than similar conditions with AT and VF- MSCs (p< 0.03), while BM MSC-3D controls were statistically higher at day 3 (p<0.01) and day 7 (p<0.02) compared to all other groups (Figure 5P).
Relationship between CD206 expression and protein secretion levels
We have previously described our model co-culturing CD14+ monocytes on MSCs embedded in Carbylan–GSX over a 7 day period, demonstrating an anti-inflammatory macrophage immunophenotype with increased expression of CD 206 and decreased expression of CD 16 and HLA-DR. To further characterize this interaction, we compared gene expression profiles of MSCs encapsulated in HA hydrogel after seven days of co-culture with macrophages and analyzed soluble factors secreted in the culture supernatant at days 3 and 7. In comparing the mean macrophage surface marker expression of CD206 at day 714{{}} to the mean protein concentrations found in our current cell culture supernatant, we found significant positive correlation between expression of CD206 and secretion levels in IL-10 (0.810, p<0.015) and IL-1β (0.762, p<0.03).
Discussion
Macrophages play a pivotal role in mediation of both engraftment and foreign body reaction to biomaterials. The balance of inflammatory and regenerative molecules secreted by macrophages after biomaterial implantation is of significance in these physiological processes. Because of this, there is great interest in developing material that influence macrophage differentiation, tissue construct engraftment, and tissue remodeling 12, 20. MSCs have independently been shown to modulate macrophages to an alternative activated phenotype and are an ideal cell type for allogeneic cell therapies 14, 16, 21. In a recent study, we have shown that encapsulation of MSCs in an injectable modified hyaluronan based hydrogel, Carbylan – GSX can reduce macrophages inflammatory profile resulting in low CD16, low HLA-DR and high CD206, which is compatible to an alternatively activated phenotype 14. While much of the work regarding MSCs immunoregulation of macrophage activation and inflammatory response refers to dichotomies of classic and alternative or pro- and anti-inflammatory macrophages, the outcome of inflammation and remodeling with biomaterials is unique and further encapsulation of MSCs only adds to the complexity of signals being coordinated in the local environment. In the current study, we investigated the effect of interactions between macrophages and MSCs derived from different tissue sources while embedded in the hydrogel constructs on secreted protein expression and MSCs gene profiles. We found differences in protein expression profiles across all conditions, as well as modulation in MSCs gene profiles.
Recent evidence suggests that MSCs can accelerate wound healing by secreting a rich source of growth factors and ECM molecules, thereby promoting angiogenesis, cell migration and ECM synthesis and remodeling 19, 22, 23. Encapsulating BM -, AT -, or VF - MSCs in chemically modified HA hydrogels can potentially influence their immunoregulation, differentiation, proliferation and motility during contact with macrophages at the site of implantation. Similarities were found in gene expression profiles of MSC hydrogel constructs co-cultured with macrophages promoting genes involved in ECM production (elastin), cell proliferation and migration (hyaluronan) and angiogenesis (VEGF) while BM-MSCs promoting collagen homeostasis (pCol, Col I, Col III and MMP-9) and AT- MSCs stimulating greater breakdown of HA (hyaluronidase). Further investigation is needed to determine the functional implications of these changes induced in the gene expression profile of MSCs 24.
Macrophages’ rapid response to implanted biomaterials promotes the production IL – 1β, TNF – α, IFN – γ, and MIP – 1α pro-inflammatory cytokines, which are also involved in host defense, biomaterial degradation, tissue regeneration, and scar formation 25–27. We found elevated concentrations of these markers in our in vitro co-cultures. While initially higher concentrations of IL – 1β and TNF – α inflammatory cytokines were observed in the AT- and VF- MSC-3D hydrogel – macrophage co-cultures, by day 7 these and MIP – 1α levels were similar to conditions with BM-MSC and only slightly higher than the macrophage 2D hydrogel controls, indicating perhaps that MSCs encapsulated in the constructs do not further promote inflammation. The time dependent increase in expression found with these cytokines is likely associated with MSCs interaction with macrophages 28 or monocyte to macrophage differentiation rather than the result of a foreign body response 3. Our results demonstrate that BM- MSC hydrogel constructs co-cultured with macrophages expressed the lowest concentrations of pro-inflammatory cytokines (i.e. IL – 1β, TNF – α, IL-12) at day 3 compared to similar conditions with other MSC subtypes, while no statistical significant differences were found with inflammatory cytokines IFN-γ and IL-6. This could suggest BM-MSCs could have a more favorable profile as a cell therapy product for certain clinical indications.
Chemokines and adhesion molecules secreted by MSCs and macrophages via paracrine or autocrine signaling patterns can promote re-epithelialization and modulate inflammation by stimulating the infiltration of monocytes, neutrophils and T cells to the implant site 6. Our results demonstrated fluctuations in the expression of monocyte chemoattractants (MCP-1) with higher concentrations being found in AT-MSC hydrogel co-culture conditions. Close proximity with immune cells is also thought to be one of the primary mechanisms of MSC mediated immune suppression 29, where high concentrations of bioactive molecules produced by MSCs can modulate their phenotype. In our study, BM-MSC hydrogel constructs demonstrated constitutively high expression of vascular adhesion molecules whereas conditions with AT- and VF-MSCs had minimal measureable quantities. Fluctuations in expression of intracellular adhesion molecules were found within MSC groups between their 3D control and co-culture condition. Induced expression was found with VF-MSC-3D co-cultures compared to mono-cultures whereas the opposite effect occurred with AT-MSC-3D co-cultures expressing lower levels compared to their controls. MSCs are known to express high amounts of surface adhesion molecules and chemokines in response to inflammatory cytokines, suggesting that these differences in patterns across mono- and co-culture conditions may be a result of macrophages regulating MSCs function.
The restoration of the tissue architecture after an injury requires a number of growth factors to stimulate neovascularization, remove granulation tissue and synthesize new ECM components. Our findings demonstrated that MSCs embedded in hydrogel construct constitutively expressed angiogenesis and morphogenesis factors, however individual differences in their rate of expression were apparent such as high expression of HGF by VF-MSCs. Further investigation into the long-term effects of high constitutive expression of HGF with VF-MSCs is necessary prior to their clinical use.
Recent evidence has suggested that macrophages activated by biomaterials exhibit unique phenotypes not inclusive to classical or alternative activated states 30. The present study demonstrates increased concentrations of pro-inflammatory cytokines by day 7 in macrophages cultured on the hydrogel alone, including TNF-α, IL-1β, MIP-1α, and MCP-1, while IL-10, IL-12, IFN-γ, VCAM, HGF and VEGF remained low. When macrophages were cultured on HA hydrogel containing MSC, we found increased expression with these inflammatory cytokines and chemokines, however we also found increased concentrations of several immunosuppressive and growth factors as well. Individual findings during co-culture conditions with hydrogel constructs included BM-MSC + MQ producing greater collagen homeostasis (pCol, Col I & III, MMP-9), cell adhesion (VCAM), and angiogenic (VEGF) molecules, AT-MSC + MQ producing greater tissue repair (HYAL), increased anti-inflammatory molecules (IL-10) and chemokines (MCP-1) and VF-MSC +MQ producing more growth factors (HGF, M- CSF, GM-CSF). These differences may be of clinical importance in distinguishing a subtype of MSC to use within a hydrogel construct during inflammatory stages of wound healing as a potential therapeutic agent for soft tissue augmentation and tissue regeneration. Given the complexity of the inflammatory response to injectable or implantable biomaterials, the in vivo interaction of these MSC hydrogel constructs merits investigation to further elucidate the role of such novel cell-based therapies in regenerative medicine.
There are significant limitations to the current study that warrant further discussion. First, the experimental design is based upon previously published work, demonstrating that MSC mediated immunosuppression requires direct cell-cell contact to induce various autocrine and paracrine signaling loops 16. Previous studies have analyzed the effects of a foreign body response to cell-biomaterial constructs using different exogenous agents (i.e. lipopolysaccharide, inflammatory cytokine) alone or in combination with macrophages, which researchers have shown to be overly simplified and inaccurate models 31. We, on the other hand, studied the effects of macrophage interaction with MSC hydrogel constructs undergoing un-stimulated monocyte differentiation to macrophages, as this is more applicable to determining the direct effects of cell-to-cell and cell-to-biomaterial interactions that would occur in vivo. In doing such, cells and constructs were plated in direct contact limiting our ability to clearly identify the cell source of protein production. Additionally, for many of the cytokines and growth factors studied, both MSCs and macrophages could be responsible for their secretion. While we recognize that this limits our conclusions, we were interested in using the isolated cells for characterization specific to that cell type (i.e. macrophages immunophenotype, MSCs ECM production). Macrophage-HA and MSC-HA controls were included, allowing us to extrapolate data that are related to the presence or absence of MSCs, hydrogel, or macrophages for the cytokines that are produced by both. Second, donor dependent differences can influence the functional characteristics of the cells. Attempts were made to control for this variation by using biologic replicates for each MSC tissue source (two donors each) and CD14+ cell (three donors). However, it stands to reason that differences still may exist. Further studies are needed, with a greater number of MSC donors per tissue source to discern between small differences in protein and gene expression.
Conclusions
In summary, we offer evidence of unique cytokine, chemokine and growth factor expression across different MSC tissue sources embedded in HA hydrogel constructs co-cultured with macrophages. Of particular interest was MSCs stimulation of bioactive molecules involved in chemotaxis, cell adhesion, and immunosuppression, which could promote local accumulation of immune cells near areas of high concentrations of anti-inflammatory and growth factor molecules. Our previous study established that MSC hydrogel constructs can modulate macrophages to a more anti-inflammatory like phenotypes, however MSCs immunosuppressive and regenerative abilities across different tissue sources were unclear 14. The present study lends support to distinctive differences in the preserved regenerative capacity of MSCs derived from multiple tissue sources while embedded in the hydrogel.
Acknowledgments
The authors thank Glen Leverson, Ph.D., Department of Surgery University of Wisconsin-Madison, for statistical assistance, and Stephanie Bartley, BS, Department of Surgery University of Wisconsin- Madison for technical assistance. We also thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its facilities to complete this research. This project was funded by the following sources: NIDCD - NIH grants R01 DC4336, R01 DC4336S1 (S.L. Thibeault), T32 DC009401 (S.N. King); NHLBI – NIH grant K08 HL081076 (P. Hematti).
Footnotes
Conflict of Interest: No benefit of any kind will be received either directly or indirectly by the author(s).
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Hanson SE, Kim J, Johnson BH, Bradley B, Breunig MJ, Hematti P, Thibeault SL. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope. 2010;120:546–551. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 5.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 7.Thibeault SL, Klemuk SA, Chen X, Quinchia Johnson BH. In Vivo engineering of the vocal fold ECM with injectable HA hydrogels-late effects on tissue repair and biomechanics in a rabbit model. J Voice. 2011;25:249–253. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12:3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 9.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Thibeault SL. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010;6:2940–2948. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson SE, King SN, Kim J, Chen X, Thibeault SL, Hematti P. The effect of mesenchymal stromal cell-hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A. 2011;17:2463–2471. doi: 10.1089/ten.tea.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Thibeault SL. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Eng Part C Methods. 2009;15:201–212. doi: 10.1089/ten.tec.2008.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 18.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 19.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantu DA, Hematti P, Kao WJ. Cell encapsulating biomaterial regulates mesenchymal stromal/stem cell differentiation and macrophage immunophenotype. Stem Cells Transl Med. 2012;1:740–749. doi: 10.5966/sctm.2012-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, Hocking AM. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316:48–54. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurila JP, Laatikainen L, Castellone MD, Trivedi P, Heikkila J, Hinkkanen A, Hematti P, Laukkanen MO. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy. 2009;11:726–737. doi: 10.3109/14653240903067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomsen P, Gretzer C. Macrophage interactions with modified material surfaces. Current Opinion in Solid State and Materials Science. 2001;5:163–176. [Google Scholar]
- 26.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 28.Hattori H, Amano Y, Habu-Ogawa Y, Ando T, Takase B, Ishihara M. Angiogenesis following cell injection is induced by an excess inflammatory response coordinated by bone marrow cells. Cell Transplant. 2012 doi: 10.3727/096368912X658863. [DOI] [PubMed] [Google Scholar]
- 29.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83:585–596. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 31.Holt DJ, Chamberlain LM, Grainger DW. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 2010;31:9382–9394. doi: 10.1016/j.biomaterials.2010.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]