Abstract
Although joint pain is common, its mechanism(s) remain undefined, with little known about the spinal neuronal responses that contribute to this type of pain. Afferent activity and sustained spinal neuronal hyperexcitability correlate to facet joint loading and the extent of behavioral sensitivity induced after painful facet injury, suggesting spinal neuronal plasticity is induced in association with facet-mediated pain. This study used a rat model of painful C6/C7 facet joint stretch, together with intrathecal administration of gabapentin, to investigate the effects of one aspect of spinal neuronal function on joint pain. Gabapentin or saline vehicle was given via lumbar puncture prior to and at 1 day after painful joint distraction. Mechanical hyperalgesia was measured in the forepaw for 7 days. Extracellular recordings of neuronal activity and astrocytic and microglial activation in the cervical spinal cord were evaluated at day 7. Gabapentin significantly (p=0.0001) attenuated mechanical hyperalgesia and the frequency of evoked neuronal firing also significantly decreased (p<0.047) with gabapentin treatment. Gabapentin also decreased (p<0.04) spinal GFAP expression. Although spinal Iba1 expression was doubled over sham, gabapentin did not reduce it. Facet joint-mediated pain appears to be sustained through spinal neuronal modifications that are also associated with astrocytic activation.
Keywords: neuronal hyperexcitability, facet joint, astrocyte, spinal cord, gabapentin
Introduction
The facet joint and its capsule have been reported to contribute to persistent pain.3,4,29 Excessive stretching of the facet capsule beyond its physiologic range of motion can induce retraction balls and swelling in axons of the nerve fibers that innervate the facet capsule.22 Those same joint loading conditions that produce excessive capsular stretch have been shown also to induce increased firing in the dorsal rootlets in the goat,30 as well as sustained neuronal sensitivity in the spinal cord in the rat in association with behavioral sensitivity.34 Painful transient facet joint loading also produces a sustained increase in the glutamate receptor and a reduction in the neuronal glutamate transporter in the spinal cord,16 suggesting altered neuronal signaling in the spinal cord.55,58 Despite growing speculation suggesting the involvement of spinal neuronal plasticity in facet-mediated pain, this hypothesis has not been tested.
Although gabapentin’s specific mechanism of action is still unknown, it has been shown to be anti-hyperalgesic.37,40 Studies have proposed that gabapentin acts on the α2δ-1 subunit of voltage-dependent Ca2+ channels and prevents entry of extracellular Ca2+, thereby reducing overall neuronal activity.18,31 Anti-hyperalgesic effects of gabapentin have been demonstrated both clinically and in animal models.8,26,41,42,45,51 For example, surgical studies suggest that pre-operative administration of gabapentin decreases postoperative pain scores and opioid analgesic requirements after mastectomy, spinal and otolaryngologic surgeries.14,17,49 Further, spinal application of gabapentin before the induction of knee joint inflammation in the rat prevents the development of heat hyperalgesia.29 Gabapentin also attenuates mechanical allodynia in the diabetic rat.57 Collectively, these studies demonstrate the effectiveness of gabapentin in attenuating or abolishing pain in several different diverse models of pain, but no study has investigated whether gabapentin can attenuate facet-mediated pain from joint trauma.
These studies tested the hypothesis that spinal administration of gabapentin can mitigate facet-induced pain via reducing the hyperexcitability of the dorsal horn neurons in the spinal cord that is normally induced. As such, painful facet joint distraction was imposed using previously published methods23–25,34 and gabapentin was administered intrathecally. Behavioral hypersensitivity in the forepaw was measured every other day until day 7 to evaluate the effects on pain symptoms. On day 7, electrophysiological recordings in the spinal dorsal horn were acquired after either gabapentin or vehicle treatment. Since painful facet joint loading has been shown to activate spinal glia23,54,56 and those cells contribute to both the development and maintenance of neuropathic and inflammatory pain,13,33,36,39,43,52,53 spinal glial activation was also evaluated at day 7.
Methods
Experiments were performed using male Holtzman rats (Harlan Sprague-Dawley; Indianapolis, ID) weighing 394±23 grams at the start of the study. Rats were housed under USDA- and AAALAC-compliant conditions with a 12-12 hour light-dark cycle and free access to food and water. All experimental procedures were IACUC-approved, and followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain.61 Separate studies were performed to determine the effects of gabapentin on behavioral hypersensitivity and on cellular responses such as neuronal and glial activity in the spinal cord.
Joint Distraction
Surgical methods to impose painful facet joint distraction in the rat were similar to those described in previous studies.23–25,35 Briefly, under inhalation anesthesia (4% isoflurane for induction, 2.5% for maintenance), the C6/C7 facet joints were exposed and isolated bilaterally by surgical procedures. The C6 and C7 posterior spinous processes were each isolated and rigidly attached to a customized loading device using microforceps. The joint was manually distracted using a micrometer to displace the C6 facet 0.7 mm while the C7 facet remained stationary.23 Prior to distraction, polystyrene markers (diameter=0.17±0.01 mm) (Spherotech Inc.; Libertyville, IL) were placed at the center of each of the C6 and C7 laminae and were used to track the bony motions as a measurement of the magnitude of applied joint distraction.23,24 Sham surgeries were also performed as surgical controls in which there was attachment to the device but no distraction. After loading, wounds were closed using 3-0 polyester suture and surgical staples. Surgical procedures lasted for no more than 60 minutes from induction of anesthesia until wound closure. After which, rats were recovered in room air and monitored throughout the recovery period.
Gabapentin Treatment & Administration
Both injury and sham groups were separately administered either intrathecal gabapentin or vehicle via lumbar puncture injection. Pilot dose-response studies were performed to identify the optimal dosing paradigm for gabapentin, based on studies in the literature in other models of knee osteoarthritis and spinal nerve injury.2,6,32,60 Accordingly, gabapentin (4.2 μmol in 30 μL of sterile saline) was delivered via lumbar puncture at 90 minutes prior to, and then again 1 day after, either injury (injury-GBP; n=12) or sham (sham-GBP; n=4) surgery. Separate groups of rats received vehicle treatment (30 μL of sterile saline) after injury (injury-vehicle; n=12) or sham (sham-vehicle; n=4) under the same dosing paradigms. All intrathecal treatments were performed in the space between L4 and L5 using a 25G needle under inhalation isoflurane. A Student’s t-test was used to compare the magnitudes of vertebral distraction between injury-GBP and injury-vehicle to test that the same injury severity was imposed in both of the injury groups.
Behavioral Assessment
A subset of rats was evaluated for behavioral hypersensitivity (injury-GBP; n=6; injury-vehicle; n=6; sham-GBP; n=4; sham-vehicle; n=4). Rats were evaluated for bilateral mechanical hyperalgesia in the forepaws on postoperative days 1, 3, 5, and 7. Following behavioral testing on day 1, lumbar puncture was performed to administer either gabapentin or vehicle treatment. Mechanical hyperalgesia was also measured prior to surgery, as baseline values and to serve as each rat’s own control. The testing procedures are customary and have been detailed in previous reports,16,24 using a series of logarithmically-increasing von Frey filaments. Briefly, each filament was applied five times before progressing to the next higher filament having a greater strength, and the response threshold was taken as the first filament to elicit a positive response. A positive response was identified by emphatic lifting of the paw. Each testing session consisted of three rounds, and was performed on each forepaw separately. Because the injury is applied to the bilateral facet joints simultaneously, with little-to-no motion off the midline, and the resulting behavioral and spinal responses do not show a sidedness,10,15,16,23–25,34,54 behavioral responses were averaged across both sides. Mechanical hyperalgesia was compared across all groups (injury-GBP, injury-vehicle, sham-GBP, sham-vehicle) using a repeated-measures ANOVA following by a one-way ANOVA with Bonferroni correction comparing groups on each day.
For the electrophysiological study, a subset of rats (injury-GBP n=6; injury-vehicle n=6, sham n=6) was used to evaluate neuronal excitability in the spinal cord. For those groups, mechanical hyperalgesia was measured only at baseline and on days 1 and 7, in order to confirm the onset and persistence of sensitivity. A repeated-measures ANOVA was used to compare the differences in hyperalgesia at days 0 (baseline), 1, and 7 between this study and the responses from the rats in the behavioral study above, for each group (injury-GBP, injury-vehicle, sham).
Electrophysiological Recordings in the Spinal Cord
In order to determine the effects of gabapentin treatment on spinal neuronal excitability, extracellular electrophysiological recordings were acquired in the deeper laminae (IV-VI) of the C6-C7 spinal dorsal horn at day 7 after facet joint distraction, for the injury-GBP (n=6), injury-vehicle (n=6), and sham (n=6) groups, using previously published methods.35 Measurements were made in those laminae, since that region of the spinal cord contains multi-receptive, wide dynamic range neurons that modulate central sensitization in many chronic pain states and exhibits increased neuronal firing after joint distraction in this same painful facet injury model.9,20,35,50 At day 7 after the initial injury or sham procedures, anesthesia was induced using sodium pentobarbital (45 mg/kg, i.p.) and supplementary doses were given as needed (5–10 mg/kg, i.p.). A bilateral dorsal laminectomy and dural resection at the C6 and C7 spinal levels were performed to expose the spinal cord. The spinal cord was bathed in 37°C mineral oil for the duration of the recordings. Following the surgical preparation, the rat was immobilized in a stereotaxic frame using ear bars and a vertebral clamp at T2 to stabilize the cervical spine. The forepaw was inverted and secured to the platform to expose the plantar surface for mechanical stimulation during recording. Core temperature was monitored and maintained at 35–37°C using a heating plate with a temperature controller and isolated rectal probe (Physitemp Instruments, Inc.; Clifton, NJ).
Sensory afferents were identified by lowering the electrode (400–1000 μm) below the pial surface of the spinal cord using a micropositioner (Narishige; Tokyo, JP), while lightly brushing the plantar surface of the forepaw with a cotton swab.20,34 A neuron was identified if spikes were distinguishable from the background activity during the brushing.50 Once an evoked potential was identified, the receptive field of the neuron was marked at the forepaw location that evoked the response, and a stimulation protocol was performed that included light brushing and a series of non-noxious and noxious von Frey filaments.34 Prior to performing the stimulation protocol, 30 seconds of baseline activity was recorded at each probe location and taken as the unstimulated response. Following that baseline period, stimulation with 10 consecutive light brush strokes was applied at the targeted location on the forepaw using a cotton swab. Four logarithmically-spaced filament strengths that included the non-noxious (1.4 and 4 g) and noxious (10 and 26 g) filaments that are used in behavioral assessment were applied. For each of the four filament strengths, five stimulations were applied for 1 second each, at approximately 1 second apart. At least 30 seconds were allowed between stimuli to prevent windup of mechanically sensitive neurons. All von Frey filaments were mounted to a load cell (SMT S-Type Model; Interface Inc.; Scottsdale, AZ) to synchronize the application of the mechanical stimulus with the acquisition of the extracellular recordings.
Extracellular voltage potentials were continuously recorded using a carbon fiber electrode (<5 μm tip; Kation Scientific, Inc.; Minneapolis, MN). Signals were amplified with a gain of 1000 and a passband filter between 300 Hz and 3000 Hz. The amplified signal was processed with a 60 Hz noise eliminator (Hum Bug; Quest Scientific; North Vancouver, BC), digitally stored at 25 kHz (CED; Cambridge, UK), and monitored with a speaker for audio feedback (A-M Systems; Calsborg, WA). Voltage recordings during the stimulation protocol of each neuron were spike-sorted using Spike 2 software (CED; Cambridge, UK) to ensure that only the firing of a single unit was measured from each recording. The total number of spikes during the period of light brushing was counted for each neuron. For von Frey filament stimuli, spikes were counted if action potentials were recorded either during the stimulation period or the rest period immediately following stimulation. Each filament had a total of five sets of spike counts because each filament was applied five times. The baseline firing rate measured in the 30-second period prior to stimulation was subtracted from the spike counts for the brush and each von Frey stimulus to yield only the evoked spikes;34 the spike counts were log-transformed due to a positive skew. Residuals from the statistical models were plotted after the transformation to confirm a normal distribution. Neurons were classified as spontaneously firing if spikes were recorded during a 2-second period immediately before the application of the stimulation protocol.34 Neurons were classified as wide dynamic range or low threshold neurons based on their response to a noxious pinch administered after the completion of the stimulation protocol.34 Neurons with evoked spikes during the noxious stimulus were considered wide dynamic range, while those that did not respond to pinch were classified as low threshold neurons.
All statistical analyses were performed using JMP8 (SAS Institute Inc.; Cary, NC). Evoked activity in response to the brush was tested between groups using a mixed-effect ANOVA with neurons nested within rats and rats nested within groups,34 because both fixed (treatment group) and random (rats) effects were present in the model. Post-hoc Tukey HSD analysis tested the differences between groups. A mixed-effect ANOVA with the same levels of nesting was used to analyze differences between groups, von Frey stimulation magnitudes, stimulus order, and their interactions. The number of spontaneously firing neurons and the number of wide dynamic range neurons in each group were compared using Pearson’s chi-square tests. All statistical tests were performed with α=0.05, and all values are expressed as mean ± standard error (S.E.).
GFAP and Iba1 Expression in the Spinal Cord
Expression of glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba1) in the spinal dorsal horn were quantified by immunohistochemistry at day 7. As such, rats were perfused with 250 ml of PBS followed by 250 ml of 4% paraformaldehyde in PBS (pH 7.4). After perfusion, the cervical spinal cord was exposed by laminectomy, the C6 segment of the cervical spinal cord was harvested, and tissue was post-fixed in 4% paraformaldehyde at 4°C for 14–18 hours. Tissue was transferred to 30% sucrose/PBS and stored for 5–7 days at 4°C. Samples were freeze-mounted with OCT medium (Fisher Scientific; Waltham, MA) for cryosectioning.
Thin (16 μm) axial C6 spinal cord sections were mounted onto APES-coded slides for immunohistochemical labeling. For each rat, 5–6 axial sections spanning the rostral and caudal regions of the C6 cord were collected to ensure unbiased sampling. Polyclonal antibodies to GFAP (Millipore; Billerica, MA) and Iba1 (Wako Chemicals USA; Richmond, VA) were individually used as markers of astrocytic and microglial activation, respectively. Slides were blocked with normal goat serum (Vector Labs; Burlingame, CA) for 2 hours followed by incubation in a primary antibody solution for GFAP (1:1000), or Iba1 (1:1000) overnight. Sections were then treated with the secondary antibody containing an Alexa 546 conjugated anti-mouse antibody (Invitrogen; Carlsbad, CA) to label GFAP (1:1000) and Alexa 488 goat anti-rabbit secondary antibody (1:500; Invitrogen; Carlsbad, CA) to label Iba1.
Quantification of GFAP or Iba1 reactivity in the spinal cord was performed using densitometry.21,23 Each sample was imaged at 10X using a Carl Zeiss LSM 510 microscope (Carl Zeiss LLC; Thornwood, NY). Images were cropped to include several regions of interest in the dorsal horn, including the superficial laminae and the deeper laminae.22 Densitometry was performed using a customized MATLAB code to quantify the percentage of pixels above a defined threshold for staining in normal tissue.1,39 Measurement for each protein for each spinal cord region was averaged and compared among injury-GBP, injury-vehicle, sham-GBP, sham-vehicle using an one-way ANOVA.
Results
All groups receiving a facet joint distraction underwent the same degree of joint injury, regardless of whether they received treatment or not. The mean vertebral distraction for the injury-GBP group was 0.66±0.08 mm, and was not different from the mean distraction measured for the injury-vehicle group (0.69±0.07 mm). Similarly, the distractions were closely aligned with a direction along the long-axis of the spine, with only 3.0±3.4° off the midline. Gabapentin treatment reduced behavioral hypersensitivity after distraction and nearly abolished it (Fig. 1). There was no difference in the baseline unoperated withdrawal threshold between any groups. Thresholds for eliciting a paw withdrawal also were not significantly different between the right and left forepaws in any rat; as such, the data from each side were averaged for each rat. Response thresholds for injury-GBP and sham-vehicle were unchanged from their corresponding baseline (uninjured) values. In addition, the withdrawal threshold for the injury-vehicle group was significantly lower (p=0.0001) than each of the injury-GBP, the sham-vehicle and the sham-GBP groups (Fig. 1). On each day, injury-vehicle exhibited a significantly lower response threshold than that for injury-GBP (p<0.001) or for sham-GBP (p<0.01). Of note, there was no difference in response thresholds for injury-GBP, sham-vehicle, and sham-GBP throughout the testing period (Fig. 1). In addition, the behavioral responses produced in the groups (injury-GBP, injury-vehicle, sham) used for the measurement of spinal neuronal excitability were not different from their corresponding groups (data not shown).
Fig. 1.
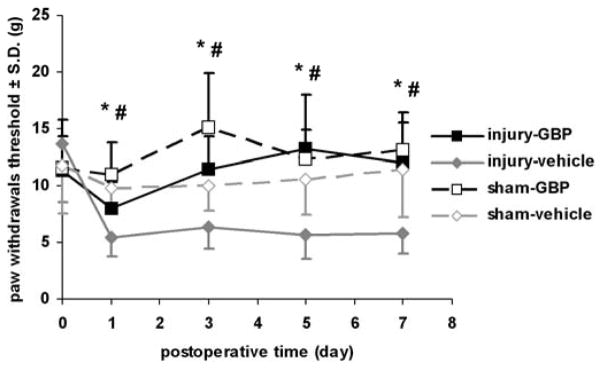
The response threshold to von Frey filament stimulation in the forepaw is significantly (p=0.0001) lower for injury-vehicle compared to each of injury-GBP, sham-GBP and sham-vehicle. On each postoperative day, the response threshold for the injury-vehicle group is significantly lower (p=0.0001) than its corresponding baseline control values, while the response threshold after sham-vehicle is not different from its baseline. Additionally, injury-vehicle exhibits a significantly lower response threshold than that for the injury-GBP (*p<0.001) and for sham-GBP groups (#p<0.01) on days 1, 3, 5, and 7. There is no difference between injury-GBP, sham-GBP, and sham-vehicle throughout the testing period.
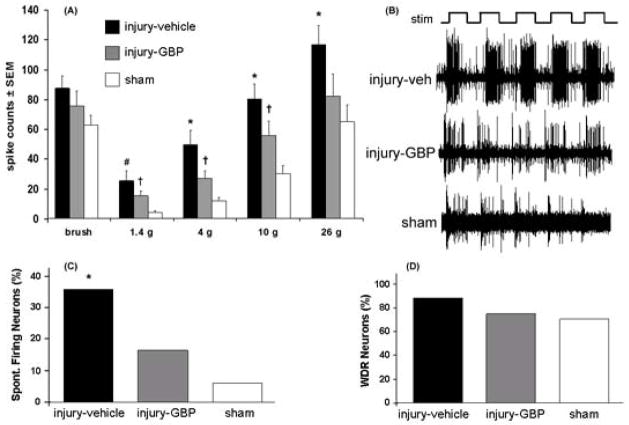
A total of 101 neurons were recorded at an average depth of 627±167 μm from the pial surface of the spinal cord. Twenty-four neurons were recorded at 555±179 μm for the injury-GBP group, 44 neurons were recorded in the injury-vehicle group at a comparable depth of 648±166 μm, and 33 neurons were recorded in the sham group at a depth of 642±169 μm. In general, gabapentin treatment decreased the neuronal firing in the deeper laminae of the spinal cord but firing was not decreased back to sham levels (Fig. 2). Firing was not different between any groups for light brushing. Neuronal firing in response to most of the von Frey stimuli was reduced in the injury-GBP group compared to the injury-vehicle group, but was still greater in the injury-GBP group compared to the sham group (Fig. 2). Specifically, the spike counts evoked by light brushing of the forepaw in the receptive field of each neuron were not different between the injury-vehicle (85±8 spikes/10 brushes), injury-GBP (73±10 spikes/10 brushes) and sham groups (61±6 spikes/10 brushes) at day 7 (Fig. 2). The 26 g filament evoked an average of 112±12 spikes over the five applications in the injury-vehicle group, which was significantly greater (p<0.03) than the number of spikes evoked in the injury-GBP group (79±14 spikes) and the sham group (63±11 spikes) by the same stimulus train (Fig. 2). Evoked firing was significantly different between all three of the groups for stimulation with the 10 g (p<0.04) and 4 g (p<0.04) filaments (Fig. 2). For the 1.4g filament, evoked firing in both the injury-vehicle (25±6 spikes) and injury-GBP (15±3 spikes) groups was significantly greater (p<0.03) than the sham group (4±1 spikes) (Fig. 2).
Fig. 2.
Electrophysiological responses in the spinal dorsal horn. (A) The total number of evoked spikes is not different between groups in response to brush. The injury-vehicle group has significantly greater firing than the sham group across all von Frey stimuli. For the 4, 10, and 26 g von Frey filaments, the number of evoked spikes is significantly reduced for the injury-GBP group compared to the injury-vehicle group. For 1.4, 4, and 10 g von Frey filaments though, the injury-GBP group is still significantly greater than the sham group. The asterisk (*) denotes significant differences (p<0.047) between injury-vehicle and both injury-GBP and sham; the pound sign (#) denotes a significant difference (p=0.0001) between injury-vehicle and sham and the dagger (†) denotes significant differences (p<0.036) between injury-GBP and sham. (B) Representative extracellular recordings for injury-vehicle, injury-GBP, and sham groups during the application of a 26 g von Frey filament (stim). (C) The percentage of spontaneously firing neurons is significantly decreased for injury-GBP (*p=0.048) and sham (*p=0.002) relative to injury-vehicle. (D) The percentage of wide dynamic range (WDR) neurons is lower in the injury-GBP and sham groups than the injury-vehicle.
Spontaneous firing in the spinal cord was altered following the intrathecal gabapentin treatment. The number of spontaneously firing neurons (presented as a percentage of total neurons in each group) was 36% (16 of 44 neurons) in the injury-vehicle group, which was a significantly greater percentage of spontaneously firing neurons than in the injury-GBP group (17%; 4 of 24 neurons; p=0.048), and in the sham group (6%; 2 of 33 neurons; p=0.002) (Fig. 2C). The injury-vehicle group also had more neurons classified as wide dynamic range neurons (84%) than were detected in the injury-GBP (71%) and sham (67%) groups, but these differences were not significant.
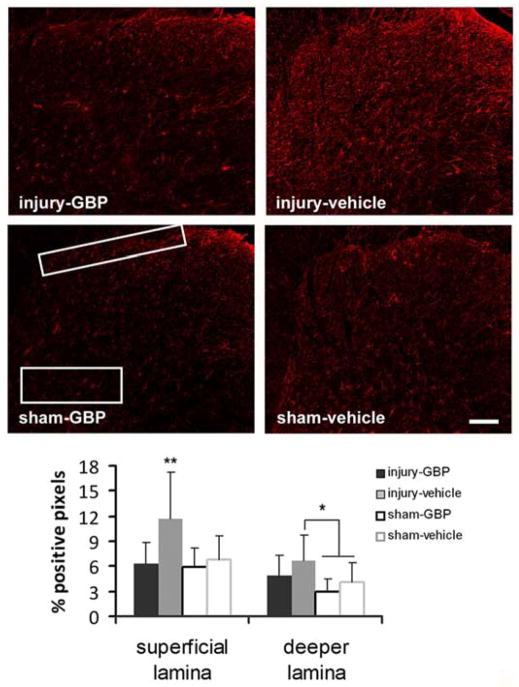
Gabapentin treatment significantly reduced astrocytic activation in the superficial lamina, but not in the deeper lamina (Fig. 3). Specifically, spinal GFAP expression at day 7 after treatment in the injury-vehicle group in both regions of the spinal cord was significantly (p<0.01) elevated above that expressed in both the sham-GBP and sham-vehicle groups (Fig. 3). In addition, injury-vehicle exhibited significantly elevated (p<0.001) GFAP expression in the superficial laminae compared to expression in those regions of injury-GBP; but, this relationship was not observed in the deeper laminae (Fig. 3). There was no difference in GFAP expression between the sham-GBP and the sham-vehicle groups, in either the superficial or the deeper laminae of the spinal cord.
Fig. 3.
Spinal GFAP expression in the superficial and deep laminae of the dorsal horn after injury or sham with either gabapentin (injury-GBP, sham-GBP) or vehicle (injury-vehicle, sham-vehicle) treatment. In the superficial lamina, GFAP is significantly elevated (**p<0.001) in injury-vehicle above all other groups (injury-GBP, sham-GBP, sham-vehicle). In contrast, the increase in injury-vehicle is only significantly higher (*p<0.01) than sham-GBP and sham-vehicle in the deeper lamina. There is no difference between injury-GBP, sham-GBP, and sham-vehicle in either region. The scale bar (100 μm) applies to all panels.
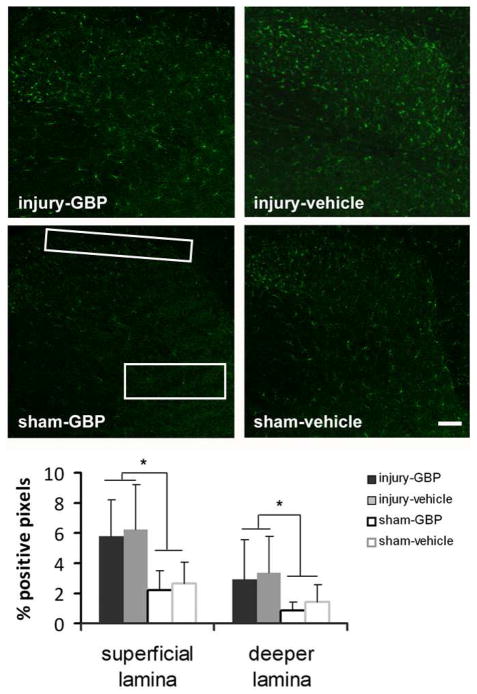
In contrast to the astrocytic activation, microglial activation in the spinal cord at day 7 was not modified in either the superficial or deeper laminae after gabapentin treatment (Fig. 4). Iab1 expression was not different between the injury-GBP and injury-vehicle groups in either region of the spinal cord that was probed (Fig. 4). However, Iba1 expression in both of the injury-GBP and injury-vehicle groups was significantly higher (p<0.01) than the expression levels in the sham-GBP and sham-vehicle groups (Fig. 4). Further, no significant difference was detected between sham-GBP and sham-vehicle in Iba1 expression for either spinal region measured.
Fig. 4.
Spinal Iba1 expression in the superficial and deep laminae of the dorsal horn after injury or sham procedures is unchanged with gabapentin (injury-GBP, sham-GBP) treatment compared to vehicle (injury-vehicle, sham-vehicle). Iba1 expression at day 7 exhibits the same trend in both dorsal horn regions, with no significant difference between injury-GBP and injury-vehicle in either the superficial or the deeper laminae. There is a significant difference (*p<0.01) between those groups and both sham-GBP and sham-vehicle. The scale bar (100 μm) applies to all panels.
Discussion
This study is the first to demonstrate that early intrathecal administration of gabapentin modulates neuronal hyperexcitability and astrocytic activation in the spinal cord in association with a reduction in pain (Figs. 1–3). Specifically, results showed that gabapentin reduced the frequency of neuronal firing in the spinal dorsal horn in association with attenuating behavioral hypersensitivity at day 7 (Figs. 1 and 2). Interestingly, it reduced the firing rates evoked by von Frey stimulations using filaments above 4 g (4–26 g), but did not return the responses to sham levels (Fig. 2). Responses to the 1.4g filament were not reduced by gabapentin treatment; however, that filament strength has previously been shown to be non-noxious for stimulation to the forepaw, whereas the other filaments used in this study elicit noxious responses in normal rats.21,23 Further, since the 1.4 g filament likely activates only low-threshold mechanoreceptors, the electrophysiological findings from our current study suggest that gabapentin may attenuate behavioral hypersensitivity by reducing the firing rate of those high-threshold afferents that are sensitive to noxious stimulation. Consistent with the current findings, another study also demonstrated that a decrease in the amplitude of calcium transients evoked by depolarization of the membrane was only evident in the nociceptive small neurons, but not in the non-nociceptive large diameter neurons of the DRG.38 Although the specific action(s) of gabapentin on neuronal sub-populations requires further work, results from our study demonstrate that gabapentin reduced the percentage of spontaneously firing neurons and wide dynamic range (WDR) neurons after injury to sham levels (Fig. 2). Spontaneous firing and the development of sensitivity to a broader range of non-noxious and noxious stimuli could contribute to hyperexcitability in the dorsal horn.35 Preventing the development of spontaneous firing and a phenotypic shift to WDR neurons in the dorsal horn may contribute to the elimination of behavioral sensitivity that is evident with the gabapentin treatment (Fig. 1). The generation of neuronal after-discharge following mechanical stimuli has been observed after this painful facet joint injury;34 although such an outcome may provide additional insight in to the effects of gabapentin on reducing behavioral sensitivity in this model, the design of the current study does not enable such evaluations.
Gabapentin is believed to act by binding to the α2δ-1 subunit of the voltage-gated calcium channel.11,47,51 Previous work in this same joint injury model showed that hyperexcitability of neurons in the spinal dorsal horn is associated with facet-mediated pain.34 As such, it was hypothesized that by limiting the activity of calcium channels or by binding to one of the calcium channel subunits, the neuronal excitability may also be sufficiently decreased to produce a reduction in hyperalgesia. Indeed, overall the sensitivity after injury was reduced with gabapentin treatment (Fig. 1). However, the small group size of the sham groups and the averaging of the right and left paw responses weakens the strength of the statistical relationships in behavioral outcomes for the current study. Of note, binding of gabapentin to the subunit of calcium channels may be optimized if delivered to the site of injury prior to the onset of the neurons becoming hyperexcitable (for this model, between 6 and 24 hours after injury).10 Since treatment in the current study is given via lumbar puncture and requires time for its travel to the cervical region, the current study administered gabapentin before the facet joint injury. However, this gabapentin treatment timing has limited clinical relevance, since pre-injury treatment is not possible. In addition, neither expression of voltage-gated calcium channels nor calcium transients were specifically evaluated in this study, so the action of gabapentin in reducing spinal and behavioral sensitivity is not known. Future studies assaying the different aspects of neuronal function in the afferents and spinal cord would more specifically define the specific mechanisms of joint pain.
Since the deeper laminae of the spinal dorsal horn contain mostly wide dynamic range neurons5 and our study mostly made measurements in laminae IV-VI, which contains both nociceptive and non-nociceptive neurons, this study is unable to detect whether gabapentin treatment modified the responses of nociceptive (Aδ and C) or mechanoreceptive (Aβ) fibers in the superficial laminae. In carrageenan-induced pain in the rat, gabapentin reduced the C fiber-evoked phase II pain, but not the acute pain evoked by Aβ fiber activity in the spinal dorsal horn.46 Further, gabapentin also reduced Aδ fiber-evoked excitatory postsynaptic currents after carregeenan injection, in isolated neurons prepared from the spinal cord.27 Those findings together with the current data, suggest that gabapentin may mitigate the behavioral hypersensitivity in our painful facet joint model by acting specifically on nociceptive fibers. In a goat model, both Aδ and C fibers were identified in the facet joint capsule and were activated in response to magnitudes of joint distraction analogous to those used in this study.7 In fact, previous work with our model of joint distraction also suggest that those mechanoreceptors and nociceptors may act as ‘sensors’ to transmit pain signals from the periphery to the central nervous system; mechanical allodynia produced by facet distraction was eliminated following transection of the facet capsule and the neuronal stress response in the DRG was activated after a painful distraction.15,56 Nociceptive fibers also project to the superficial laminae of the dorsal horn,5,59 which exhibited a greater decrease in astrocytic activation following gabapentin treatment than in the deeper laminae (Fig. 3). However, this study did not record the neuronal activity by electrophysiology in the superficial laminae. Such work would provide further insight in to the effects of gabapentin specifically on nociceptors and shed light on the mechanism(s) by which nociceptors contribute to the maintenance of facet-mediated pain.
Although gabapentin reduced astrocytic activation in the superficial laminae, it did not modulate spinal microglial activation in either of the spinal regions probed (Figs. 3 and 4). This observation is in contrast to a report of reduced spinal microglial activation in association with reduced allodynia after intrathecal gabapentin in streptozotocin (STZ)-diabetic rats.57 This discrepancy may be due to the putative roles of microglia and astrocytes in the two different pain models. Specifically, spinal microglial activation has been shown to contribute to behavioral hypersensitivity in STZ-diabetic pain,12,48 whereas astrocytic activation is not directly linked to pain symptoms.57 In contrast, after painful facet joint injury, astrocytes have been implicated as having an integral role in the maintenance of pain, while microglia activation has not been evident.23 Collectively, these spinal glial outcomes in mechanically-induced joint pain suggest that the effects of gabapentin on spinal glia may depend on the specific responses of these cells in the different pain models and the timing of the intervention. In fact, we have evidence that spinal neuronal hyperexcitability is established between 6 hours and 1 day after painful joint distraction.10 Repeated administration of intrathecal gabapentin was given in the lumbar region prior to and at 1 day after injury, which may correspond to the time at which these changes could be prevented. Nonetheless, based on current study, it can be hypothesized that gabapentin may reduce behavioral hypersensitivity and neuronal hyperexcitability independent of modulating spinal microglia, but having effects that directly or indirectly prevent astrocytic activation. It is possible that the lack of microglial changes may also be due to the lumbar administration of gabapentin and evaluation of cervical spinal responses. Conversely, the reduced astrocytic activation could itself be due to the decreased neuronal hyperexcitability.
In conclusion, this study demonstrates that early intrathecal administration of gabapentin can eliminate the development of behavioral hypersensitivity that is elicited by painful facet joint distraction, and also reduces neuronal hyperexcitability in the spinal cord at day 7. Spinal astrocytic activation is decreased in the superficial laminae with gabapentin, while microglial activation was not changed. This further suggests that astrocytic responses may be more involved in this painful injury condition. Overall, results from this study provide evidence that spinal neuronal dysfunction, astrocytic activation, and/or their interaction may be responsible for facet joint-induced pain.
Perspective.
Intrathecal gabapentin treatment was used to investigate behavioral, neuronal and glial response in a rat model of painful C6/C7 facet joint stretch. Gabapentin attenuated mechanical hyperalgesia, reduced evoked neuronal firing and decreased spinal astrocytic activation. This study supports facet joint pain being sustained through spinal neuronal andastrocytic activation.
Acknowledgments
This work was supported by grants from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288 & BIRT Supplement) and the Catherine D. Sharpe Foundation.
Footnotes
Disclosures
There are no conflicts of interests for any authors with any aspect of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbadie C, Brown JL, Manthh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–9. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- 2.Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesthe Analg. 1998;87:1360–6. [PubMed] [Google Scholar]
- 3.Aprill C, Bogduk N. The Prevalence of Cervical Zygapophyseal Joint Pain; A First Approximation. Spine. 1992;17:744–7. doi: 10.1097/00007632-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain. 1994;58:283–307. doi: 10.1016/0304-3959(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 5.Basbaum AI, Bautista DM, Scherer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandran P, Pai M, Blomme EA, Hsieh GC, Decker MW, Honore P. Pharmacological modulation of movement-evoked pain in a rat model of osteoarthritis. Eur J Pharmacol. 2009;613:39–45. doi: 10.1016/j.ejphar.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Lu Y, Kallakuri S, Patwardhan A, Cavanaugh JM. Distribution of A-delta and C-fiber receptors in the cervical facet joint capsule and their response to stretch. J Bone Joint Surg Am. 2006;88:1807–16. doi: 10.2106/JBJS.E.00880. [DOI] [PubMed] [Google Scholar]
- 8.Cheng C, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthast-1, and cardiovascular function. J Clin Invest. 2006;116:1391–9. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14:517–37. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- 10.Crosby ND, Weisshaar CL, Winkelstein BA. Spinal neuronal plasticity is evident within 1 day after a painful cervical facet capsule injury. Neurosci Lett. 2013;542:102–6. doi: 10.1016/j.neulet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RS. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci. 2004;20:1566–76. doi: 10.1111/j.1460-9568.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- 12.Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–54. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- 13.DeLeo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Dirks J, Fredensborg BB, Christensen D, Fomsgaard JS, Flyger H, Dahl JB. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology. 2002;97:560–4. doi: 10.1097/00000542-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Dong L, Odeleye AO, Jordan-Sciutto KL, Winkelsten BA. Painful facet injury induces neuronal stress activation in the DRG: implications for cellular mechanisms of pain. Neurosci Lett. 2008;443:90–4. doi: 10.1016/j.neulet.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotrauma. 2010;27:163–74. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fassoulaki A, Patris K, Sarantopoulos C, Hogan Q. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth Analg. 2002;95:985–91. doi: 10.1097/00000539-200210000-00036. [DOI] [PubMed] [Google Scholar]
- 18.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 19.Hains BC, Fullwood SD, Eaton MJ, Hulsebosch CE. Subdural engraftment of serotonergic neurons following spinal hemisection restores spinal serotonin, downregulates serotonin transporter, and increases BDNF tissue content in rat. Brain Res. 2001;913:35–46. doi: 10.1016/s0006-8993(01)02749-4. [DOI] [PubMed] [Google Scholar]
- 20.Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–92. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: Mechanical factors in painful neck injuries. Spine. 2005;301:1924–32. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 22.Kallakuri S, Singh A, Lu Y, Chen C, Patwardhan A, Cavanaugh JM. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17:556–63. doi: 10.1007/s00586-007-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004;48:373–96. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 24.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383–93. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 25.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10:436–45. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–10. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Xu R, Yang K. Inflammation unmasks gabapentin’s effect on A delta-fiber evoked excitatory postsynaptic currents in substantia gelatinosa neurons of rat spinal cord. Chin Med J (Eng) 2003;116:883–7. [PubMed] [Google Scholar]
- 28.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005:779–87. doi: 10.1016/j.orthres.2005.01.002. [DOI] [PubMed]
- 29.Lu Y, Westlund KN. Gabapentin attenuates nociceptive behaviors in an acute arthritis model in rats. J Pharmacol Exp Ther. 1999;290:214–9. [PubMed] [Google Scholar]
- 30.Manchikanti L, Singh V, Rivera J, Pampati V. Prevalence of cervical facet joint pain in chronic neck pain. Pain Physician. 2002;5:243–9. [PubMed] [Google Scholar]
- 31.Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol. 2001;59:1243–8. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- 32.O-Arciniega M, Diaz-Reval MI, Cortes-Arroyo AR, Dominguez-Ramirez AM, Lopez-Munoz FJ. Anti-nociceptive synergism of morphine and gabapentin in neuropathic pain induced by chronic constriction injury. Pharmacol Biochem Behav. 2009;92:457–64. doi: 10.1016/j.pbb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Qin M, Wang JJ, Cao R, Zhang H, Duan L, Gao B, Xiong YF, Chen LW, Rao ZR. The lumbar spinal cord glial cells actively modulate subcutaneous formalin induced hyperalgesia in the rat. Neurosci Res. 2006;55:442–50. doi: 10.1016/j.neures.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Quinn KP, Dong L, Golder FJ, Winkelstein BA. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–21. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn KP, Lee KE, Ahaghotu CC, Winkelstein BA. Structural changes in the cervical facet capsular ligament: potential contributions to pain following subfailure loading. Stapp Car Crash J. 2007;51:169–87. doi: 10.4271/2007-22-0008. [DOI] [PubMed] [Google Scholar]
- 36.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–73. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 37.Rock DM, Kelly KM, Macdonald RL. Gabapentin actions on ligand- and voltage-gated responses in cultured rodent neurons. Epilepsy Res. 1993;16:89–98. doi: 10.1016/0920-1211(93)90023-z. [DOI] [PubMed] [Google Scholar]
- 38.Romanenko SV, Kostyuk PG, Kostyuk EP. Effects of gabapentin on calcium transients in neurons of the rat dorsal root ganglia. Neurophysiol. 2008;40:231–7. [Google Scholar]
- 39.Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo J. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–26. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose MA, Kam PC. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–62. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–8. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Rosner H, Rubin L, Kestenbaum A. Gabapentin adjunctive therapy in neuropathic pain states. Clin J Pain. 1996;12:56–8. doi: 10.1097/00002508-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 44.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–5. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segal AZ, Rordorf G. Gabapentin as a novel treatment for postherpetic neuralgia. Neurology. 1996;46:1175–6. doi: 10.1212/wnl.46.4.1175. [DOI] [PubMed] [Google Scholar]
- 46.Stanfa L, Singh L, Williams R, Dickenson A. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997;8:587–90. doi: 10.1097/00001756-199702100-00002. [DOI] [PubMed] [Google Scholar]
- 47.Stefani A, Spadoni F, Giacomini P, Lavaroni F, Bernardi G. The effect of gabapentin on different ligand- and voltage-gated currents in isolated cortical neurons. Epilepsy Res. 2001;43:239–48. doi: 10.1016/s0920-1211(00)00201-1. [DOI] [PubMed] [Google Scholar]
- 48.Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia. 2008;56:378–86. doi: 10.1002/glia.20623. [DOI] [PubMed] [Google Scholar]
- 49.Turan A, Karamanlioğlu B, Memiş D, Hamamcioglu MK, Tükenmez B, Pamukçu Z, Kurt I. Analgesic effects of gabapentin after spinal surgery. Anesthesiology. 2004;100:935–8. doi: 10.1097/00000542-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 50.Urch CE, Dickenson AH. In vivo single unit extracellular recordings from spinal cord neurones of rats. Brain Res Brain Res Protoc. 2003;12:26–34. doi: 10.1016/s1385-299x(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 51.Vonsy JL, Ghandehari J, Dickenson AH. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain. 2009;13:786–93. doi: 10.1016/j.ejpain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–85. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 53.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in Neurosci. 2001;24:450–5. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 54.Weisshaar CL, Dong L, Bowman AS, Perez FM, Guarino BB, Sweitzer SM, Winkelstein BA. Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J Neurotrauma. 2010;27:2261–71. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- 55.White SR, Duffy P, Kalivas PW. Methylenedioxymethamphetamine depresses glutamate-evoked neuronal firing and increases extracellular levels of dopamine and serotonin in the nucleus accumbens in vivo. Neuroscience. 1994;62:41–50. doi: 10.1016/0306-4522(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 56.Winkelstein BA, Santos DG. An intact facet capsular ligament modulates behavioral sensitivity and spinal glial activation produced by cervical facet joint tension. Spine. 2008;33:856–62. doi: 10.1097/BRS.0b013e31816b4710. [DOI] [PubMed] [Google Scholar]
- 57.Wodarski R, Clark AK, Grist J, Marchand F, Malcangio M. Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain. 2009;13:807–11. doi: 10.1016/j.ejpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 59.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–8. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 60.Zanella JM, Burright EN, Hildebrand K, Hobot C, Cox M, Christoferson L, McKay WF. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine. 2008;33:227–34. doi: 10.1097/BRS.0b013e318162340a. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]