Abstract
Adenosine is an endogenous metabolite that is released from all tissues and cells including liver, pancreas, muscle and fat, particularly under stress, intense exercise, or during cell damage. The role of adenosine in glucose homeostasis has been attributed to its ability to regulate, through its membrane receptors, processes such as insulin secretion, glucose release and clearance, glycogenolysis, and glycogenesis. Additionally, adenosine and its multiple receptors have been connected to lipid metabolism by augmenting insulin-mediated inhibition of lipolysis, and the subsequent increase in free fatty acids and glycerol levels. Furthermore, adenosine was reported to control liver cholesterol synthesis, consequently affecting plasma levels of cholesterol and triglycerides, and the amount of fat tissue. Alterations in the balance of glucose and lipid homeostasis have implications in both cardiovascular disease and diabetes. The ability of different adenosine receptors to activate and inhibit the same signaling cascades has made it challenging to study the influence of adenosine, adenosine analogs and their receptors in health and disease. This review focuses on the role and significance of different adenosine receptors in mediating the effect of adenosine on glucose and lipid homeostasis.
Keywords: Adenosine, glucose, lipids
Introduction
Adenosine is an endogenous purine nucleoside that is released from cells upon injury or inflammation. Adenosine can be generated from adenosine triphosphate (ATP) in the extracellular space through the action of two endonucleotidases: CD39 (ENTPD1; nucleoside triphosphate diphosphorylase 1) and CD73 (NT5E; ecto-5’-nucleotidase) (Hasko et al., 2008; Yegutkin, 2008). In similar fashion, adenosine can be generated intracellularly from ATP and exported to the extracellular space by two transporters called equilibrative nucleoside transporters one and two (ENT1 and ENT2) (Eltzschig et al., 2005). Once in the extracellular space, adenosine acts on four different G-protein coupled receptors that are classified as adenylyl cyclase inhibiting (A1 and A3) or adenylyl cyclase activating (A2a and A2b ) (Tucker and Linden, 1993). Studies have implicated adenosine, signaling through the A2-type adenosine receptors, as an anti-inflammatory autocrine molecule responsible for inhibition of inflammatory cytokine release at baseline (Apasov et al., 2000; Lukashev et al., 2004; Ohta and Sitkovsky, 2001; Ryzhov et al., 2008; Yang et al., 2006b), upon injury (Day et al., 2004; Lukashev et al., 2007; Okusa et al., 2001; Yang et al., 2008), or upon bacterial invasion (Lappas et al., 2005; Yang et al., 2006b). The anti-inflammatory role of adenosine has also been associated with protection of the vasculature in a restenosis like model (Yang et al., 2008) and in improving overall myocardial tone (Guo et al., 2001; Law et al., 1988). It is important to mention that in addition to inflammation, adenosine and adenosine receptors have been associated with regulation of glucose clearance (Han et al., 1998; Johnston-Cox et al., 2012a; Maeda and Koos, 2008), lipolysis (Johansson et al., 2008; Ulrich Schwabe, 1974) and fatty liver prevention post alcohol intake (Peng et al., 2009), or prevention of liver steatosis post high fat diet (Koupenova et al., 2012), regulation of cholesterol synthesis (Koupenova et al., 2012), or fat deposition (Johnston-Cox et al., 2012a).
Glucose homeostasis is achieved by a fine balance among exogenous dietary intake, endogenous release from the liver, and clearance by the muscle and adipose tissues. These processes are precisely regulated by the two major hormones released by the pancreatic alpha and beta cells, glucagon and insulin, respectively (Bansal and Wang, 2008). When blood glucose levels rise after exogenous dietary intake, insulin is released and stimulates glucose uptake by the skeletal muscle and adipose tissue and inhibits glucose production by the liver (Saltiel and Kahn, 2001). At low glucose blood levels, glucagon is released into the circulation. In the liver, glucagon initiates glycogenolysis and gluconeogenesis and the consequent increase in glucose availability, while in adipose tissue it initiates lipolysis and the subsequent release of free fatty acids (FFA) (Bansal and Wang, 2008; Ruan and Lodish, 2003). Lipolysis, in turn, is initiated by Protein kinase A (PKA)-mediated activation of hormone sensitive triacylglycerol lipase, which leads to the increase of triglyceride (TG) break down to glycerol and fatty acids (Allen et al., 1986).
Glucose clearance from the circulation depends on insulin and insulin receptor signaling in adipose and muscle tissue. Under certain physiological conditions such as obesity and/or type II diabetes, insulin is unable to effectively clear blood glucose. The impaired ability of insulin to clear glucose from the circulation is defined as insulin resistance (McGarry, 2002). As a consequence, insulin can no longer inhibit lipolysis in the adipose tissue and levels of free fatty acids and glycerol rise in the plasma. The increase of plasma FFA results in their elevated uptake by the liver which leads to their oxidation and consequent accumulation of acetyl coenzyme A (Acetyl CoA). In turn, the elevated levels of Acetyl CoA in the liver stimulate the rate limiting enzymes for gluconeogenesis (pyruvate carboxylase and phosphoenolpyruvate carboxykinase) and glycogenolysis (glucose-6-phosphatase) to produce more glucose, which in turn results in the production of more insulin by the pancreas (Kovacs and Stumvoll, 2005). On the other hand, accumulation of FFA in the liver gives rise to non-alcoholic fatty liver disease. In that sense, improper glucose homeostasis can cause tissue damage and whole body deterioration (McGarry, 2002).
Under non-pathological conditions, excess blood glucose is cleared by the liver and later stored as glycogen or fatty acids. Fatty acids are stored in the form of triglycerides and esterified cholesterol, which are delivered to the periphery via vesicles called very low density lipoprotein (VLDL). In the circulation VLDL particles ultimately get hydrolyzed to a low density lipoprotein vesicle (LDL) as triglycerides and cholesterol get redistributed (Boullier et al., 2001). Cholesterol from the periphery is delivered back to the liver by high density lipoprotein (HDL) particles, termed the reverse cholesterol transport. Excess cholesterol in the blood is associated with an increased amount of LDL particles which can progress into pathological conditions such as atherosclerosis (Li and Glass, 2002).
Adenosine has been associated with lipolysis and glucose clearance as well. It has been proposed that adenosine signaling through its receptors in adipose tissue increases insulin sensitivity (Dong et al., 2001b; Green et al., 1997; Vannucci et al., 1992; Vannucci et al., 1989). Consequently, glucose tolerance has also been improved in studies using adenosine-based pharmacological reagents (Crist et al., 1998; Xu et al., 1998); even though studies using adenosine agonists and antagonists seem to exhibit contradictory effects (Crist et al., 1998; Schoelch et al., 2004; Xu et al., 1998). Recent work also associates adenosine with pancreatic insulin synthesis, reverse cholesterol transport and fatty liver prevention (Peng et al., 2009), liver steatosis amelioration (Koupenova et al., 2012), improvement of glucose and insulin clearance (Johnston-Cox et al., 2012a; Yang et al., 2012) .This review will outline the role of the different adenosine receptors in the above mentioned processes.
Adenosine and A1 Adenosine Receptors
Adenosine has been associated with the regulation of glucose homeostasis for more than thirty years (Dole, 1962; Ismail et al., 1977; Jain and Logothetopoulos, 1978; Loubatieres-Mariani et al., 1979; Schütz et al., 1978; Schütz et al., 1978; Ulrich Schwabe, 1974; Van Calker, 1979). Studies with non-selective adenosine antagonists have shown that adenosine can improve insulin secretion and lower glucose production (Table I) (Arias et al., 2001; Corssmit et al., 1994; Rüsing et al., 2006). Furthermore, adenosine can decrease insulin (Bertrand et al., 1989; Hillaire-Buys et al., 1987) and stimulate glucagon (Chapal et al., 1985; Chapal et al., 1984; Schütz et al., 1978; Schütz et al., 1978 ) secretion. Adenosine can also increase hepatic glycogenolysis (González-Benítez et al., 2002; Hoffer and Lowenstein, 1986; Oetjen et al., 1990) by activation of A1 adenosine receptor (A1AR) in Ca2+-dependent manner (González-Benítez et al., 2002).
Table I.
Adenosine in glucose and lipid metabolism
| Treatment | Effect on ARs | Model system | Physiological effect | References |
|---|---|---|---|---|
|
2-chloroadenosine or N6-phenylisopropyladenosine |
Stimulation | Muscle soleus | reduced insulin sensitivity by increasing the half maximum dose of insulin required to stimulate glycolysis | (Budohoski et al., 1984) |
| 8-phenyltheophylline | Inhibition | Muscle soleus | Increased insulin sensitivity by counteracting the effect of 2-chloroadenosine | (Budohoski et al., 1984) |
|
Adenosine Micromolar concentration |
Stimulation | Rat islets | Decrease of insulin release | (Bertrand et al., 1989a; Bertrand et al., 1989b; Campbell and Taylor, 1982; Hillaire-Buys et al., 1987; Ismail et al., 1977) |
|
Adenosine Milimolar concentrations |
Stimulation | Mouse islets Rat pancreas |
Increase of insulin release | (Andersson, 1980; Jain and Logothetopoulos, 1978; Loubatieres-Mariani et al., 1979) |
| Adenosine | Stimulation | Rat hepatocytes | Increases gluconeogenesis | (Bartrons et al., 1984; González- Benítez et al., 2002; Zentella de Piña et al., 1989) |
| Adenosine | Stimulation | Adipocytes rat | In presence of insulin increases glucose transport activity of GLUT4 | (Vannucci et al., 1992) |
| Adenosine | Stimulation | Rat pancreas | Stimulates glucagon secretion | (Chapal et al., 1985; Chapal et al., 1984) |
|
Adenosine 2-Chloroadenosine |
Stimulation | Rat hepatocytes | Increases glycogenolysis | (González-Benítez et al., 2002; Hoffer and Lowenstein, 1986; Oetjen et al., 1990) |
| Adenosine deaminase | Inhibition by removal | Adipocytes rat | Removal of inhibition of lipolysis in lean>>>obese adipocytes | (Vannucci et al., 1989) |
| Adenosine deaminase | Inhibition by removal | Muscle soleus | improves insulin sensitivity by decreasing the concentration of insulin necessary to activate the half maximum stimulation of glycolysis | (Espinal et al., 1983) |
| Aminophylline | Inhibition | Overall body human | Stimulates insulin secretion by inhibition of glucose production | (Arias et al., 2001) |
| Aminophylline | Inhibition | Overall body Dog | Lowered glucagon release | (Schütz et al., 1978; Schütz et al., 1978 ) |
| Pentoxyfylline | Inhibition | Overall body human | Inhibits basal glucose production | (Arias et al., 2001) |
The importance of adenosine signaling with respect to glucose homeostasis and insulin sensitivity has been predominantly attributed to its ability to inhibit lipolysis in fat, and glycolysis in muscle. All four adenosine receptors are known to be expressed in muscle and fat, with the expression of A1AR being the highest (Johansson et al., 2007a; LaNoue and Martin, 1994). Furthermore adenosine can activate A1AR with EC50 values in the range between 10 ηM to 1 µM (Fredholm et al., 2001; Hasko et al., 2008). This is why adenosine signaling in both of these tissues for the longest time has been predominantly attributed to the A1AR.
Pharmacological Approach
In fat, adenosine signaling results in enhanced insulin sensitivity measured by the ability of insulin to inhibit lipolysis (Table I and II)(Green et al., 1997; Schoelch et al., 2004; Vannucci et al., 1992; Vannucci et al., 1989). The mechanism by which adenosine inhibits lipolysis and possibly enhances insulin sensitivity is mediated by the activation of cAMP-dependent protein kinase (PKA) (Londos et al., 1985; Vannucci et al., 1989). Activation of hormone sensitive lipase by glucagon or norepinephrine is inhibited by insulin. In human adipocytes, insulin inhibits the lypolytic effect of norepinephrine more efficiently when adenosine is present (Heseltine et al., 1995). Similarly to adenosine, activation of rat adipocyte-A1AR by pharmacological reagents showed the same effect on improving insulin sensitivity and consequent inhibition of lipolysis (Table II). Using pharmacological reagents selective for A1AR, Green et al, reported a 30% reduction in the responsiveness to insulin in adipocytes, as prolonged treatment impaired insulin dependent lipolysis. Furthermore, a specific, anti-lipolytic A1AR agonist, ARA ([1S,2R,3R,5R]-3-methoxymethyl-5-[6-(1-[5-trifluoromethyl-pyridin-2-yl]pyrrolidin-3-[S]-ylamino)-purin-9-yl]cyclopentane-1,2-diol), in Wistar and Zucker rats resulted in reduced lipolysis in visceral and subcutaneous fat, suggestive of increased insulin sensitivity (Schoelch et al., 2004).
Table II.
A1AR and its physiological role in glucose and lipid metabolism
| Adenosine Receptor |
Pharmacological Agent |
Activation | Model System |
Physiological effect |
References |
|---|---|---|---|---|---|
| A1AR |
CPA (n6-cyclopentyladenosine) |
Agonist | Adipocytes Rat | Decreased glucose uptake | (Cheng et al., 2000) |
| A1AR |
PIA N6-(L-2-phenylisopropyl)- Adenosine |
Agonist | Adipocytes Rat | Inhibition of lipolysis in presence of β-adrenergic hormone stimulation. Decrease insulin sensitivity measured by reduction in glucose uptake. | (Cheng et al., 2000; Vannucci et al., 1989) |
| Perfused rat pancreas | Decreases insulin secretion | (Bertrand et al.,1989b) | |||
| Perfused rat liver | Increase in hepatic glucose release | (Buxton et al., 1987) | |||
| A1AR |
8-PT (8-phenyltheophylline) |
Antagonist | Adipocytes Rat | Lipolysis increased in obese>>>lean (virtually none). | (Vannucci et al., 1989) |
| Perfused rat pancreas | Increased insulin secretion | (Bertrand et al., 1989b) | |||
| A1AR | CPA | Agonist | Hepatocytes cell line- AML-12 | Increased intracellular Triglyceride levels. Increases proteins involved in fatty acid synthesis- SREBP1 and PPARγ and consequently expression of ACL and FAS* | (Peng et al., 2009) |
| A1AR |
DPCPX (8-Cyclopentyl-1,3-dipropylxanthine) |
Antagonist | Hepatocytes cell line- AML-12 | Reversed the effect of CPA on increased triglyceride levels, SREBP-1, PPARγ, ACL and FAS | (Peng et al., 2009) |
| A1AR | BWA1433 (1,3-dipropyl-8-(4- acrylate)phenylxanthine; 4-(1,2,3,6- tetrahydro-2,6-dioxo-1,3-dipropyl-9H- purin-8-yl)cinnamic acid) | Antagonist | Muscle Gastrocnemius, soleus | Increases glucose uptake in obese animals | (Crist et al., 1998) |
| Adipocytes | Decreases glucose uptake in obese and lean animals | (Crist et al., 1998) | |||
| A1AR | ARA -[1S,2R,3R,5R]-3- methoxymethyl-5-[6-(1-[5- trifluoromethyl-pyridin-2-yl]pyrrolidin- 3-[S]-ylamino)-purin-9- yl]cyclopentane-1,2-diol | Agonist | Muscle gastrocnemius | Improvement of insulin sensitivity; an increase in glucose infusion rate and reduction of FFA levels in obese rats | (Schoelch et al., 2004) |
| Visceral and subcutaneous fat |
Reduction in lipolysis | (Schoelch et al., 2004) | |||
| A1AR | ARA | Agonist | Overall body | Reduction in FFA, glycerol, TG’s; improvement of insulin sensitivity | (Schoelch et al., 2004) |
| A1AR | BWA1433 | Antagonist | Overall body | At lower levels of insulin increase in glucose tolerance by glucose clearance; | (Crist et al., 1998) |
| A1AR | CPA | Agonist | Overall body and rat adipocytes | Increased leptin secretion | (Rice et al., 2000) |
| A1AR |
CHA (cyclohexyladenosine) |
Agonist | Perfused rat liver | Increased hepatic glucose release | (Buxton et al., 1987) |
PPARγ- Peroxisome proliferator-activated receptor gamma; SREBP-1-Sterol Regulatory Element Binding Protein-1; ACL-ATP citrate lyase; FAS-Fatty acid Synthase
The effect of adenosine signaling through the A1AR also depends on the stage of obesity (Table II). Using A1AR selective antagonist (8-phenyltheophylline), in primary adipocytes isolated from lean Zucker rats, there was a very low increase in cAMP and virtually no lipolysis observed (Vannucci et al., 1989); however, in adipocytes derived from obese rats, inhibition of A1AR resulted in high cAMP increase and concomitant lipolysis at almost maximal levels (Vannucci et al., 1989).
On the other hand, in muscle tissue activation of adenosine signaling leads to reduction of insulin sensitivity, measured by the ability of insulin to inhibit glycolysis. Insulin activation in muscle tissue is not only associated with inhibition of glycolysis but with transport of glucose and activation of glycogen synthesis. Reports show that adenosine had an effect on only glycolysis (Budohoski et al., 1984; Challis et al., 1984; Espinal et al., 1983) and glucose transport by stimulation of the insulin sensitive glucose transporters GLUT4 (Vannucci et al., 1992); glycogen synthesis was not affected by adenosine (Challis et al., 1984). In isolated soleus muscles from rats, it was found that depletion of adenosine (by adenosine deaminase) in the surrounding media improved insulin sensitivity. The observed effect on insulin sensitivity was due to decrease in the concentration of insulin necessary to activate glycolysis (Espinal et al., 1983). Using the same model and adenosine analogs, reduction in insulin sensitivity was observed when insulin levels were reduced to half of the maximum dose required to stimulate glycolysis in the muscle (Budohoski et al., 1984). Non-specific adenosine receptor antagonists (methyl xanthines) reversed the inhibitory effect of adenosine on insulin signaling (Budohoski et al., 1984). Interestingly, A1AR selective agonist (ARA) in the gastrocnemius muscle resulted in the amelioration of insulin sensitivity measured by improvement of glucose infusion rate and reduction of FFA levels in obese rats (Schoelch et al., 2004). The pattern observed does not contradict the earlier mentioned observations (Espinal et al., 1983), as here the authors used an A1AR specific agonist.
The role of adenosine in glucose clearance, however, has been reported to have contradicting results. On one hand, studies showed that non-selective adenosine receptor antagonists, or removal of adenosine by adenosine deaminase decreases insulin-activated glucose transport (Steinfelder and Pethö-Schramm, 1990). This finding is consistent with the pharmacological studies mentioned above, which indicate improvement of insulin sensitivity (Green et al., 1997; Schoelch et al., 2004; Vannucci et al., 1992; Vannucci et al., 1989). More recent studies, using euglycemic hyperinsulinemic clamp and A1AR selective antagonist (BWA1433, selective for A1 at low doses) reported that adenosine signaling through A1AR improves overall body glucose clearance in obese rats (Crist et al., 1998). In addition, with the help of radioactively labeled glucose, this group determined that there is a tissue specificity of glucose clearance under hyperinsulinemic conditions. In the gastrocnemius (fast and slow twitching fibers) and the soleus muscles (slow twitching) of rats there was an improvement of glucose uptake in obese animals after one week of treatment with A1AR antagonist. In lean animals, however, glucose uptake under one week of treatment was slightly but significantly lowered (Crist et al., 1998). On the other hand, inhibition of A1AR by selective antagonist has also been reported to improve overall body glucose tolerance (Xu et al., 1998). This observation contradicts the studies that have shown an improved insulin sensitivity and overall glucose clearance as a result of activation of A1AR by specific agonists (Crist et al., 1998; Green et al., 1997; Schoelch et al., 2004; Vannucci et al., 1992; Vannucci et al., 1989). It is possible then, that in muscle, these agonists activate the A2 adenosine receptors, an effect that can oppose the A1AR signaling. Overall, signaling by adenosine using pharmacological reagents improves glucose clearance (Crist et al., 1998; Xu et al., 1998), but may result in a tissue specific insulin resistance (Crist et al., 1998).
Mouse model approach
In vivo models have further elicited the role of adenosine signaling through the A1AR on glucose homeostasis and lipolysis. Consistent with some of the pharmacological data, lack of A1AR in primary adipocytes, contrary to the wild type, resulted in no observable lipolysis when endogenous adenosine was removed by adenosine deaminase (Johansson et al., 2008; Johansson et al., 2007b). Insulin and adenosine acted additively through cAMP to reduce lipolysis (Johansson et al., 2008). Furthermore, pharmacological activation of the A1AR was only achievable in wild type adipocytes. The last was tested by inhibition of cAMP induced lipolysis with the hormone norepinephrine. Further, induction of lipogenesis was observed in the presence of a non-selective adenosine analog, but mRNA levels of genes related to fatty acid synthesis were not affected (Johansson et al., 2008). Interestingly, plasma levels of free fatty acids, triglycerides, and glycerol were also increased in the A1AR knockout mice after administration of the adenosine analog, 2-chloroadenosine (Johansson et al., 2008). This indicates that A1AR is important in the regulation of lipolysis.
Signaling through the A1AR is also central to glucose tolerance and insulin clearance. Recent work showed that elimination of A1AR from mice at young age (8 weeks) on standard diet leads to delayed plasma glucose and insulin clearance. The effect was sustained at older ages (20–29 weeks) on both regular and high fat diet (Faulhaber-Walter et al., 2011). Using a different A1AR KO model, Johansson et al., showed that there is no difference in non-starved plasma levels of glucose, insulin, and glucagon, or glucose tolerance upon A1AR elimination when mice were not starved (Johansson et al., 2007a). This same model, however, when staved and challenged with glucose showed an improved glucose and insulin clearance on regular diet (Yang et al., 2012). A short administration of high fat diet also showed an improved course of glucose clearance but it had no effect on insulin tolerance (Yang et al., 2012). After glucose injection, A1AR knockout mice showed a sustained increase in glucagon and insulin plasma concentrations. Insulin and glucose uptake by skeletal muscle were not significantly different between the wild type and the knockout mice. The null mice showed also an enhanced second phase of pancreatic insulin secretion (Johansson et al., 2007a). A recent study by Maeda et al, suggested that in adult animals endogenous adenosine reduces plasma concentrations of insulin, glucose, and lactate via the selectively inhibiting A1AR (Maeda and Koos, 2008). These studies demonstrate that in vivo elimination or inhibition of A1AR has an effect on glucose homeostasis and the effect in contingent upon the length of the diet or the level of obesity.
In a similar fashion, genetically engineered-overexpression of adipose A1AR led to protection against obesity induced insulin resistance during high fat diet (Dong et al., 2001a). In this case, adenosine signaling through A1AR improved insulin sensitivity measured by glucose tolerance tests and insulin levels on Western diet (Dong et al., 2001b). This model also exhibited lower plasma FFA, indicative of inhibition of lipolysis (Dong et al., 2001b). These observations are in concurrence with the pharmacological studies of adipose tissue where affecting adipose-adenosine signaling improved insulin sensitivity (Green et al., 1997; Schoelch et al., 2004; Vannucci et al., 1992; Vannucci et al., 1989).
In summary, the overall effect of adenosine signaling through A1AR results in improved insulin sensitivity (Table II) (Dong et al., 2001b; Green et al., 1997; Schoelch et al., 2004; Vannucci et al., 1992; Vannucci et al., 1989), and pronounced reductions of plasma free fatty acids, glycerol, and triglycerides (Johansson et al., 2007a). Overall, A1AR is important for insulin sensitivity, glucose homeostasis and lipolysis (Dong et al., 2001b; Faulhaber-Walter et al., 2011).
Leptin, Adenosine, and A1AR
Adenosine and adenosine signaling is associated with regulation of the fat adipokine leptin. Leptin is a chemokine synthesized by adipose tissue and is responsible for control of fat metabolism. It has been associated with control of satiety and balancing caloric intake vs. energy expenditure. Plasma levels of tumor necrosis factor-alpha (TNF-alpha) and insulin have been reported to increase the circulating levels of leptin (Bradley and Cheatham, 1999; Finck et al., 1998; Kirchgessner et al., 1997). In adipocytes, increase in cAMP levels have been associated with a decrease in leptin gene expression and secretion (Deng et al., 1997; Gettys et al., 1996; Slieker et al., 1996) and, as mentioned above, A1AR inhibits cAMP in the adipose tissue. Rice et al., have reported that direct activation of A1AR by selective agonist treatment in rats increases secretion, but not gene expression, of serum leptin from isolated adipocytes (Table II) (Rice et al., 2000). Indeed, specific inhibition of adenosine signaling through the A1AR completely inhibited insulin-stimulated leptin release in isolated white adipocytes from rats. Using pharmacological reagents, it was shown that adenosine released by adipocytes acts to stimulate leptin secretion by insulin in a phospholipase C, protein kinase C (PLC-PKC) dependent manner (Cheng et al., 2000). It has been suggested that leptin induces a novel form of lipolysis by which FFA do not increase in the plasma, contrary to an increase in glycerol (Wang et al., 1999). In turn, endogenous adenosine released by white fat acts additively with insulin to inhibit lipolysis through the A1AR (Johansson et al., 2008). Leptin-induced lipolysis, in turn, was able to counteract the tonic inhibition of lipolysis by endogenous adenosine only in lean animals (Fruhbeck et al., 2001). In obese animals leptin was not able to oppose the effect of adenosine (Fruhbeck et al., 2001). Consistent with this observation, in high fat treated mice elimination of either the A1AR (Faulhaber-Walter et al., 2011) or the A2bAR (Johnston-Cox et al., 2012a) caused an increase in circulating leptin. The fact that receptors with opposing effect on cAMP have the same outcome on leptin levels suggests that the effect on leptin under HFD is predominantly due to increased fat mass accumulation.
In conclusion, there is a clearly established relationship between cAMP and leptin levels that can be controlled by adenosine, the strength of which depends on the leanness of the organism. In that sense, there is a possibility that leptin and adenosine may influence the balance between lipolysis and lipogenesis, resulting in effective homeostatic control.
A3 Adenosine Receptors
The A3 adenosine receptor (A3AR) is the other adenylyl cyclase-inhibiting receptor that has an affinity for adenosine with EC50 values in the range of 10 ηM- 1 µM (Fredholm, 2007; Fredholm et al., 2000; Hasko et al., 2008). Even though this adenosine receptor inhibits adenylyl cyclase in a similar fashion as A1AR it has not been identified as a major player in overall body glucose homeostasis or fat metabolism. Signaling through A3AR and glucose metabolism has been attributed to the ability of this receptor to get activated not only by adenosine but rather by inosine (Gomez and Sitkovsky, 2003; Jin et al., 1997; Tilley et al., 2000). Inosine is an endogenous nucleoside formed by the deamination of adenosine (Barankiewicz and Cohen, 1985) and it can accumulate under ischemic conditions (Wang et al., 1994). Since the liver is the major organ responsible for regulation of glucose homeostasis (Saltiel and Kahn, 2001), it is necessary to mention that in this organ all four adenosine receptors are present (Dixon et al., 1996), with highest levels of A3AR expression (Salvatore et al., 1993).
Pharmacological and Mouse model approaches
Studies have shown that under ischemic conditions, as a result of tissue damage, inosine is generated from the deamination of adenosine (Linden, 2001; Rubio and Berne, 1980; Silva et al., 1995). Guinzberg et al., reported that in primary hepatocytes inosine can stimulate gluconeogenesis and glycogenolysis through the A3AR (Table III) (Guinzberg et al., 2006) . Additionally, inosine is also able to stimulate glucose release from primary rat hepatocytes (Guinzberg et al., 2006). A study from the same group has demonstrated that under baseline conditions, in primary hepatocytes, inosine signaling through A3AR increases cytosolic Ca2+ and reduces cAMP, antagonizing transient signaling by adenosine (Table III). Glycogen metabolism through this receptor was also shown to be affected by adenosine but in small order (González-Benítez et al., 2002). There are no records of A3AR affecting glucose clearance, or lipid profile in transgenic or A3AR knockout mice.
Table III.
A3AR and its physiological role in glucose and lipid metabolism
| Adenosine Receptor |
Pharmacological Agent |
Activation | Model System |
Physiological effect |
References |
|---|---|---|---|---|---|
| A3AR | Inosine | Activation | Rat hepatocytes | Stimulates glycogenolysis and gluconeogenesis as | (Guinzberg et al., 2006) |
| A3AR | MRS1220- N-[9-Chloro-2-(2- furanyl)[1,2,4]-triazolo[1,5-c]quinazo lin-5- yl]benzene acetamide | Antagonist | Specific inhibition of the effect of inosine on glycogenolysis and glyconeogeneis | (Guinzberg et al., 2006) | |
| A3AR | IB-MECA - 1-Deoxy-1-[6-[[(3- iodophenyl)methyl]amino]-9H–purin-9-y l]-N- m ethyl-b-D-ribofuranuronamide | Agonist | Rat hepatocytes | Stimulates glycogenolysis and gluconeogenesis but in minor form compared to A1, A2aAR | (González-Benítez et al., 2002) |
In summary, the impact of the A3AR on glucose homeostasis, by either glucose synthesis or glucose release, may have an increased importance during anaerobic or stress elevating conditions.
A2a Adenosine Receptors
The A2a adenosine receptor (A2aAR) is a receptor with high affinity for adenosine (EC50 values in the range of 10 ηM- 1 µM), the activation of which, however, results in the elevation of cAMP (Fredholm, 2007; Fredholm et al., 2000; Hasko et al., 2008). The role of A2aAR in glucose homeostasis and lipid metabolism has not been as clearly identified as that of the A1AR. In rat hepatocytes it has been reported that adenosine can increase gluconeogenesis by elevating cyclic adenosine monophosphate (cAMP) levels through the A2AR (Bartrons et al., 1984; González-Benítez et al., 2002; Zentella de Piña et al., 1989).
Pharmacological and Mouse model approach
Studies have shown that exogenous adenosine signaling through A2aAR increases gluconeogenesis and glucose release (González-Benítez et al., 2002; Maeda and Koos, 2008; Yasuda et al., 2003). In fetal sheep, A2aAR activation increases glucose and lactate levels as a result of the increased endogenous adenosine levels under hypoxic conditions (Table IV) (Maeda and Koos, 2008). In rat hepatocytes, gluconeogenesis stimulation was also observed by the activation of A2aAR with a selective agonist (CGS-21680) in cAMP-dependent fashion (González-Benítez et al., 2002).
Table IV.
A2aAR and its physiological role in glucose and lipid metabolism
| Adenosine Receptor |
Pharmacological Agent |
Activation | Model System |
Physiological effect |
References |
|---|---|---|---|---|---|
| A2aAR |
CGS-21680 4-[2-[[6-Amino-9-(N-ethyl-b-D- ribofuranuronamidosyl)-9H -purin-2- yl]amino]ethyl]benzenepropanoic acid hydrochloride |
Agonist | Rat hepatocytes |
Stimulates gluconeogeneis and glycogenolysis |
(González-Benítez et al., 2002) |
| A2aAR |
ZM-241385 4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]t riazin-5-ylamino]ethyl)phenol |
Antagonist | Fetal sheep overall body |
Abolished induced hyperglycemia and hyperlactatemia by exogenous administration of adenosine |
(Maeda and Koos, 2008) |
| A2aAR | CGS-21680 | Agonist | THP-1 human macrophages/ murine macrophages |
Inhibited foam cell formation in stimulated macrophages, increases the mRNA levels for 27- hydroxylase and ABCA1, proteins involved in the reverse cholesterol transport |
(Reiss et al., 2008; Reiss et al., 2004) |
| A2aAR |
MRE-0094 2- [2-(4-chlorophynyl) ethosy]adenosine |
Agonist | THP-1 macrophages |
Increases the mRNA levels for 27- hydroxylase and ABCA1* |
(Reiss et al., 2004) |
| A2aAR | ZM-241385 | Antagonist | THP-1 human macrophages/ murine macrophages |
Reversed the inhibition of foam cell formation and downregulates 27- hydroxylase and ABCA1 expression |
(Reiss et al., 2004) |
ABCA1-ATP-binding cassette, sub-family A
The role of adenosine in lipid metabolism has been predominantly attributed to the activation of the A2aAR. In vitro studies showed that the A2aAR activated the reverse cholesterol transport (Reiss et al., 2008; Reiss et al., 2004), a process by which cholesterol from the periphery is transferred back to the liver by macrophages. The last is crucial for preventing foam cell formation and atherosclerosis initiation (Reiss et al., 2004). Additionally, pharmacological studies (Table IV) illustrated that activation of A2aAR in human macrophages, as well as cultured primary murine macrophages prevent foam cell formation (Reiss et al., 2008). In support, macrophages lacking A2aAR were not protected from foam cell formation by A2a agonism (Reiss et al., 2004) . The mechanism by which activation of A2aAR is able to initiate this process of cholesterol clearance involves the upregulation of 27-hydroxylase and ATP-binding cassette sub-family A member 1 (ABCA1) transporter, which are proteins involved in the reverse cholesterol transport (Reiss et al., 2008; Reiss et al., 2004).
Murine models with eliminated A2aAR have connected this adenosine receptor with elevation of plasma cholesterol levels under Western and regular chow diet (Wang et al., 2009). This finding was observed in a mouse model that lacks A2aAR on an ApoE null background. The elevation of plasma cholesterol was concentrated in the low density lipoprotein (LDL) particles. Interestingly, when A2aAR is knocked out on just C57BL background there was no difference in the cholesterol or triglycerides levels (Wang et al., 2009). Additionally, no difference in blood glucose levels was observed when A2aAR was eliminated.
In summary, in vitro murine studies have associated the A2aAR with upregulation of reverse cholesterol transport, as in vivo reports have connected A2aAR with regulation of overall body cholesterol levels under a Western diet. Pharmacological studies have shown that this receptor is also connected to gluconeogenesis and glucose release, although complete elimination of this receptor does not alter basal glucose levels. It is possible that this receptor is more important under stress related conditions. Moreover, its importance can be in tissue specific manner. More exploration is needed to explain the discrepancies between pharmacological and in vivo studies.
A2b Adenosine Receptors
The A2b adenosine receptor (A2bAR) is cAMP elevating and requires high concentration of adenosine in order to be activated. A2bAR has been of little interest due to its low affinity for adenosine (EC50 values are above 10 µM) (Fredholm, 2007; Fredholm et al., 2000; Hasko et al., 2008). The generation of the A2bAR knockout/β-galactosidase knock in (Yang et al., 2006b), and the discovery of the inducibility of this receptor under inflammation, stress or injury (St. Hilaire et al., 2008; Yang et al., 2008), led to increased interest in its biological importance. Looking at the expression of A2bAR in organs related to glucose homeostasis and lipid metabolism, it appears that pancreatic basal expression of A2bAR, as well as, all other adenosine receptors is similar levels (Nemeth et al., 2007). More specifically, A2bAR expression has been found to predominate in the islets of the pancreas (Yang et al., 2006b). Additionally, mRNA of this receptor has been not only detected in the liver (Koupenova et al., 2012; Salvatore et al., 1993) but also in fat and muscle (Dixon et al., 1996; Johansson et al., 2007a; Johnston-Cox et al., 2012b; LaNoue and Martin, 1994).
Pharmacological and Mouse model approach
With respect to glucose homeostasis, the A2bAR has been described as a receptor that has an anti-diabetic potential (Nemeth et al., 2007; Rüsing et al., 2006). Using a nonselective adenosine receptor agonist (5’-N-ethylcarboxamide (NECA)) and different A2bAR specific antagonists, the A2bAR has been associated with an increase in insulin secretion (Rüsing et al., 2006) and amelioration of diabetes (Table V) (Nemeth et al., 2007). It has been suggested that the A2bAR also stimulates glycogenolysis and gluconeogenesis in primary rat hepatocytes in a cAMP-dependent manner (Harada et al., 2001; Yasuda et al., 2003). In a Type I diabetic model, hyperglycemia was reduced predominantly through the activation of A2bAR, which sustained reduction of blood glucose in the circulation for 7 days (Nemeth et al., 2007). Furthermore, in a A2aAR knockout model NECA maintained the suppressive effect on hyperglycemia as the A1AR and A3AR were less effective on the containment of the course of diabetes development (Nemeth et al., 2007).
Table V.
A2bAR and its physiological role in glucose and lipid metabolism
| Adenosine Receptor |
Pharmacological Agent |
Activation | Place of investigation |
Physiological effect |
References |
|---|---|---|---|---|---|
| A2bAR |
PBS-53, PBS-1115 1-butyl-8-p-carboxyphenylxanthine |
Antagonist | INS-1 cells | Increased glucose- stimulated insulin release |
(Rüsing et al., 2006) |
| Overall body in GotoKakzaki rats |
Plasma insulin was increased but blood glucose was unchanged |
(Rüsing et al., 2006) | |||
| A2bAR |
MRS1754 N-(4-Cyanophenyl)-2-[4-(2,3,6,7- -tetrahydro-2,6-dioxo-1, 3-dipropyl-1H- purin-8-yl)phenoxy]-acetamide |
Antagonist | CD-1 mice with induced diabeties |
Reversed the preventative effect toward diabetes development of the adenosine analog NECA; reversed the suppressive effect on hyperglycemia. |
(Nemeth et al., 2007) |
| A2bAR |
NECA ( 5’-N-ethylcarboxamidoadenosine) In concentrations activating A2bAR (10 µM) |
Agonist Non-selective |
AML-12 | Increased intracellular Triglyceride levels. Reduces proteins involved in fatty acid oxidation- PPARα nuclear levels were decreased and consequently there was reduced expression of ACCα and CPT1. Reduced phosphorylation of AMPK* |
(Peng et al., 2009) |
|
MRS1754 (1 µM) |
Antagonist |
AML-12 |
Reversed all the effect of NECA on PPARα, ACCα, CPT1, and AMPK phosphorylation |
(Peng et al., 2009) | |
| A2bAR |
NECA, CPA, CGS21680 |
Non-selective agonist, A1AR agonist, A2aAR agonist |
Hepatocytes rat |
Increase of hepatic glucose production |
(Harada et al., 2001) |
|
FK453, KF17837, and L249313 |
Selective antagonist for A1, A2aAR, A3AR |
||||
| A2bAR | BAY 60-6853 | Selective A2bAR agonist |
Overall body mice |
It lowers liver lipid synthesis and plasma levels of cholesterol and triglycerides; it improves liver steatosis |
(Koupenova et al., 2012) |
| It improves glucose and insulin tolerance and increases leaness |
(Johnston-Cox et al., 2012) |
PPARα- Peroxisome proliferator-activated receptor alpha; ACCα- acetyl CoenzymeA carboxylase alpha; CPT1- carnitine palmitoyltransferase 1A; AMPK- AMP-activated protein kinase
Elimination of A2bAR in mice has an effect on increased fasting glucose and insulin levels as well as impaired glucose tolerance and insulin clearance post high fat diet (Johnston-Cox et al., 2012a). Interestingly, activation of A2bAR by specific agonist (BAY60–6553) in high fat diet induced obesity wild type mice lowered fasting glucose and improved their glucose and insulin tolerance (Johnston-Cox et al., 2012a). The effect of A2bAR was mediated by regulating insulin receptor substrate (IRS) levels, and a similar correlation between obesity, and levels of A2bAR and IRS were found in human adipose tissues (Johnston-Cox et al., 2012a). Interestingly, at young age (8 weeks) mice lacking the A2bAR have a superior glucose clearance (Figler et al., 2011) . This is consistent with the fact that at this age there are almost no detectable levels of A2bAR in fat or liver (Yang et al., 2006a), and one of the other adenosine receptors (such as A1) is controlling this process. Furthermore, administration of an antagonist of the A2ARs (NECA) fed in combination with high fat diet resulted in an improved fasting glucose levels in wild type mice (Figler et al., 2011). The last observation proposes that administration of pharmacological agents in vivo has different effect when administered orally or intraperitoneally, suggesting that the digestive system itself might have an effect on the metabolism of the drugs, or the drugs themselves have an impact on the digestive system before reaching their intra-body targets (such as nutrient absorption). Regardless of the course of administration, A2bAR clearly has an effect on maintaining glucose and insulin homeostasis.
The association of A2bAR with lipid metabolism can be attributed to the role of this receptor in preventing fatty liver formation post alcohol consumption (Peng et al., 2009). It is necessary to note that A2bAR is not the only adenosine receptor involved in the regulation of the fatty liver formation (Table V). Antagonism of A1AR reduced the expression of genes involved in fatty acid synthesis, and antagonism of A2bAR increased genes involved in fatty acid metabolism, as ethanol ingestion elevated A1AR, A2aAR, and A2bAR expression in the liver (Peng et al., 2009). A1AR and A2bAR agonism promoted lipid accumulation in cultured murine hepatocyte cell line (AML-12) and increased TG levels (Peng et al., 2009). The mechanism by which A2bAR and A1AR induced fatty liver after ethanol stimulation involves increasing lipogenesis (through A1AR) and decreasing fatty acid oxidation (through A2bAR). A1 and A2bAR knockout mice models (but not A2aAR) were protected from fatty liver development, consistent with the pharmacological data produced by antagonism of the receptors (Peng et al., 2009). Recent work from our group has demonstrated that A2bAR has a major protective role during high fat diet induced obesity. Indeed, A2bAR regulated liver lipid synthesis, liver steatosis and plasma levels of cholesterol and triglycerides predominantly found in the very low density lipoprotein particles (Koupenova et al., 2012). Liver specific restoration of this receptor by adenovirus as well as specific ligand activation significantly improved plasma lipid levels, and lowered liver lipid synthesis (Koupenova et al., 2012).
In conclusion, the facts that levels of A2bAR dramatically increases in liver (Koupenova et al., 2012) and fat (Johnston-Cox et al., 2012a) with high fat diet, as well as, its ability to improve glucose and insulin clearance and lipid synthesis, make this receptor a favorable drug target.
Summary: significance of adenosine and the adenosine receptors in glucose and lipid homeostasis
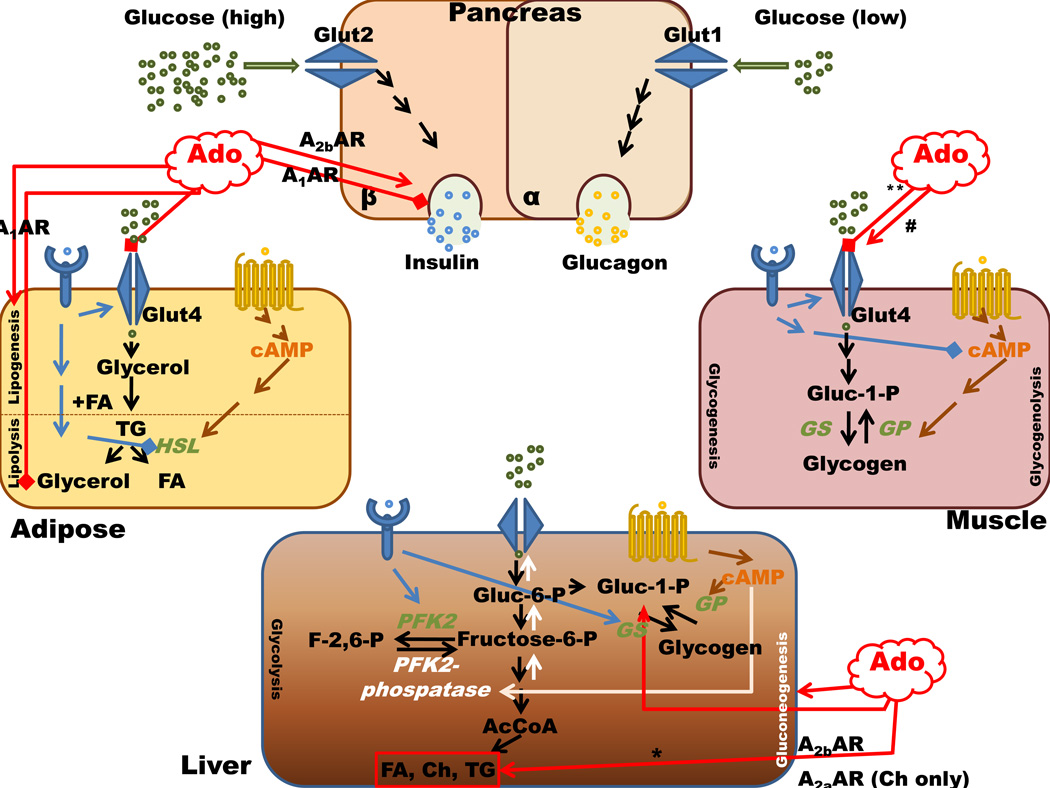
Regardless of the controversy through which receptor adenosine conveys its message, it is clear that endogenous adenosine, as a local signaling metabolite, has an important role in major processes such as inflammation, hypoxia, and glucose and lipid metabolism. It is possible that signaling through the adenosine receptors has evolved as a finely tuned buffering mechanism by which all of the four receptors contribute to the same process with opposing effect in order to regulate and prevent the consequences of differences in food intake and inflammatory stimulation (Figure 1). Targeting adenosine receptors becomes more and more promising in prophylaxis against diabetes and cardiovascular diseases. Mouse models are of particular interest in order to address the question of which receptor needs to be activated and/or inhibited in the time course of these disease states. Moreover, these findings need further conformation in human cell models. Future studies could also focus on potential synergizing or opposing signaling by which adenosine receptors exert effects on lipid and glucose homeostasis.
Figure 1. Adenosine and adenosine receptor signaling in major organs involved in glucose and lipid homeostasis.
All adenosine receptors in the liver have the ability to activate glucogenolysis and gluconeogenesis with different affinities, possibly through different second messengers. * This elevation of triglycerides is post ethanol consumption. ** This is observed in the soleus muscle, and # is observed in Gastrocnemius muscle. Stimulates ( ) Inhibits (
) Inhibits ( ). Ado- adenosine, AR- adenosine receptors; GS- glycogen synthase; GP-glycogen phosphorylase; PFK2-phospho frukto kinase 2, HSL-hormone sensitive lipase; FA-fatty acids, Ch-cholesterol, TG-triglycerides.
). Ado- adenosine, AR- adenosine receptors; GS- glycogen synthase; GP-glycogen phosphorylase; PFK2-phospho frukto kinase 2, HSL-hormone sensitive lipase; FA-fatty acids, Ch-cholesterol, TG-triglycerides.
Acknowledgement
We apologize to those whose work was not cited due to limited space.
Contract grant sponsor: National Heart, Lung and Blood Institute; Contract grant number: HL93149 (KR) and the Boston Nutrition Obesity Research Center (NIDDK grant # DK046200)
Contract grant sponsor: National Institutes of Health-T32; Contract grant number HL007969 and HL007224 (MK)
Footnotes
The authors state that there have no conflicts of interest.
Literature cited
- Allen DO, Ahmed B, Naseer K. Relationships between cyclic AMP levels and lipolysis in fat cells after isoproterenol and forskolin stimulation. J Pharmacol Exp Ther. 1986;238(2):659–664. [PubMed] [Google Scholar]
- Apasov SG, Chen J-F, Smith PT, Schwarzschild MA, Fink JS, Sitkovsky M. Study of A2A adenosine receptor gene deficient mice reveals that adenosine analogue CGS21680 possesses no A2A receptor-unrelated lymphotoxicity. British Journal of Pharmacology. 2000;131(1):43–50. doi: 10.1038/sj.bjp.0703532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias AMP, Bisschop PH, Ackermans MT, Nijpels G, Endert E, Romijn JA, Sauerwein HP. Aminophylline stimulates insulin secretion in patients with type 2 diabetes mellitus. Metabolism. 2001;50(9):1030–1035. doi: 10.1053/meta.2001.25800. [DOI] [PubMed] [Google Scholar]
- Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab. 2008;295(4):E751–E761. doi: 10.1152/ajpendo.90295.2008. [DOI] [PubMed] [Google Scholar]
- Barankiewicz J, Cohen A. Purine nucleotide metabolism in resident and activated rat macrophages in vitro. Eur J Immunol. 1985;15(6):627–631. doi: 10.1002/eji.1830150618. [DOI] [PubMed] [Google Scholar]
- Bartrons R, Van Schaftingen E, Hers H. The ability of adenosine to decrease the concentration of fructose 2,6-bisphosphate in isolated hepatocytes. A cyclic AMP-mediated effect. Biochem J. 1984;218(1):157–163. doi: 10.1042/bj2180157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G, Petit P, Bozem M, Henquin JC. Membrane and intracellular effects of adenosine in mouse pancreatic beta-cells. Am J Physiol Endocrinol Metab. 1989;257(4):E473–478. doi: 10.1152/ajpendo.1989.257.4.E473. [DOI] [PubMed] [Google Scholar]
- Boullier A, Bird DA, Chang M-K, Dennis EA, Friedman P, Gillotte-Taylor K, Horkko S, Palinski W, Quehenberger O, Shaw P, Steinberg D, Terpstra V, Witztum JL. Scavenger Receptors, Oxidized LDL, and Atherosclerosis. Ann NY Acad Sci. 2001;947(1):214–223. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Bradley RL, Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes. 1999;48(2):272–278. doi: 10.2337/diabetes.48.2.272. [DOI] [PubMed] [Google Scholar]
- Budohoski L, Challiss R, McManus B, Newsholme E. Effects of analogues of adenosine and methyl xanthines on insulin sensitivity in soleus muscle of the rat. FEBS Lett. 1984;167(1):1–4. doi: 10.1016/0014-5793(84)80820-0. [DOI] [PubMed] [Google Scholar]
- Challis R, Budohosk iL, McManus B, Newsholme E. Effects of an adenosine-receptor antagonist on insulin-resistance in soleus muscle from obese Zucker rats. Biochem J. 1984;221(3):915–917. doi: 10.1042/bj2210915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapal J, Loubatières-Mariani M, Petit P, Roye M. Evidence for an A2-subtype adenosine receptor on pancreatic glucagon secreting cells. Br J Pharmacol. 1985;86(3):565–569. doi: 10.1111/j.1476-5381.1985.tb08932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapal J, Loubatières-Mariani M, Roye M, Zerbib A. Effects of adenosine, adenosine triphosphate and structural analogues on glucagon secretion from the perfused pancreas of rat in vitro. Br J Pharmacol. 1984;83(4):927–933. doi: 10.1111/j.1476-5381.1984.tb16533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JT, Liu IM, Chi TC, Shinozuka K, Lu FH, Wu TJ, Chang CJ. Role of adenosine in insulin-stimulated release of leptin from isolated white adipocytes of Wistar rats. Diabetes. 2000;49(1):20–24. doi: 10.2337/diabetes.49.1.20. [DOI] [PubMed] [Google Scholar]
- Corssmit EP, Romijn JA, Endert E, Sauerwein HP. Pentoxifylline inhibits basal glucose production in humans. J Appl Physiol. 1994;77(6):2767–2772. doi: 10.1152/jappl.1994.77.6.2767. [DOI] [PubMed] [Google Scholar]
- Crist GH, Xu B, Lanoue KF, Lang CH. Tissue-specific effects of in vivo adenosine receptor blockade on glucose uptake in Zucker rats. FASEB J. 1998;12(13):1301–1308. doi: 10.1096/fasebj.12.13.1301. [DOI] [PubMed] [Google Scholar]
- Day Y-J, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286(2):G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- Deng C, Moinat M, Curtis L, Nadakal A, Preitner F, Boss O, Assimacopoulos-Jeannet F, Seydoux J, Giacobino J-P. Effects of {beta}-Adrenoceptor Subtype Stimulation on obese Gene Messenger Ribonucleic Acid and on Leptin Secretion in Mouse Brown Adipocytes Differentiated in Culture. Endocrinology. 1997;138(2):548–552. doi: 10.1210/endo.138.2.4922. [DOI] [PubMed] [Google Scholar]
- Dixon A, Gubitz A, Sirinathsinghji D, Richardson P, Freeman T. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118(6):1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP. Insulin-like Actions of Ribonucleic Acid, Adenylic Acid, and Adenosine. J Biol Chem. 1962;237(9):2758–2762. [PubMed] [Google Scholar]
- Dong Q, Ginsberg HN, Erlanger BF. Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes Obes Metab. 2001a;3(5):360–366. doi: 10.1046/j.1463-1326.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- Dong Q, Ginsberg HN, Erlanger BF. Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes, Obesity and Metabolism. 2001b;3(5):360–366. doi: 10.1046/j.1463-1326.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202(11):1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal J, Challiss R, EA N. Effect of adenosine deaminase and an adenosine analogue on insulin sensitivity in soleus muscle of the rat. FEBS Lett. 1983;158(1):103–106. doi: 10.1016/0014-5793(83)80685-1. [DOI] [PubMed] [Google Scholar]
- Faulhaber-Walter R, Jou W, Mizel D, Li L, Zhang J, Kim SM, Huang Y, Chen M, Briggs JP, Gavrilova O, Schnermann JB. Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes. 2011;60(10):2578–2587. doi: 10.2337/db11-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, Kim JK, LaNoue KF, Linden J. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60(2):669–679. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Kelley KW, Dantzer R, Johnson RW. In Vivo and in Vitro Evidence for the Involvement of Tumor Necrosis Factor-{alpha} in the Induction of Leptin by Lipopolysaccharide. Endocrinology. 1998;139(5):2278–2283. doi: 10.1210/endo.139.5.6012. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull Br, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2000;362(4 – 5):364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochemical Pharmacology. 2001;61(4):443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G, Gomez-Ambrosi J, Salvador J. Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J. 2001;15(2):333–340. doi: 10.1096/fj.00-0249com. [DOI] [PubMed] [Google Scholar]
- Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137(9):4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102(13):4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- González-Benítez E, Guinzberg R, Díaz-Cruz A, Piña E. Regulation of glycogen metabolism in hepatocytes through adenosine receptors. Role of Ca2+ and cAMP. European Journal of Pharmacology. 2002;437(3):105–111. doi: 10.1016/s0014-2999(02)01299-2. [DOI] [PubMed] [Google Scholar]
- Green A, Walters DJ, Belt SE. Insulin resistance in adipocytes after downregulation of Gi subtypes. Am J Physiol Endocrinol Metab. 1997;273(2):E254–E261. doi: 10.1152/ajpendo.1997.273.2.E254. [DOI] [PubMed] [Google Scholar]
- Guinzberg R, Cortes D, Diaz-Cruz A, Riveros-Rosas H, Villalobos-Molina R, Pina E. Inosine released after hypoxia activates hepatic glucose liberation through A3 adenosine receptors. Am J Physiol Endocrinol Metab. 2006;290(5):E940–E951. doi: 10.1152/ajpendo.00173.2005. [DOI] [PubMed] [Google Scholar]
- Guo Y, Bolli R, Bao W, Wu W-J, Black JRG, Murphree SS, Salvatore CA, Jacobson MA, Auchampach JA. Targeted Deletion of the A3Adenosine Receptor Confers Resistance to Myocardial Ischemic Injury and does not Prevent Early Preconditioning. Journal of Molecular and Cellular Cardiology. 2001;33(4):825–830. doi: 10.1006/jmcc.2001.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Hansen PA, Nolte LA, Holloszy JO. Removal of adenosine decreases the responsiveness of muscle glucose transport to insulin and contractions. Diabetes. 1998;47(11):1671–1675. doi: 10.2337/diabetes.47.11.1671. [DOI] [PubMed] [Google Scholar]
- Harada H, Asano O, Hoshino Y, Yoshikawa S, Matsukura M, Kabasawa Y, Niijima J, Kotake Y, Watanabe N, Kawata T, Inoue T, Horizoe T, Yasuda N, Minami H, Nagata K, Murakami M, Nagaoka J, Kobayashi S, Tanaka I, Abe S. 2-Alkynyl-8-aryl-9-methyladenines as Novel Adenosine Receptor Antagonists: Their Synthesis and Structure;Activity Relationships toward Hepatic Glucose Production Induced via Agonism of the A2B Receptor. Journal of Medicinal Chemistry. 2001;44(2):170–179. doi: 10.1021/jm990499b. [DOI] [PubMed] [Google Scholar]
- Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heseltine L, Webster JM, Taylor R. Adenosine effects upon insulin action on lipolysis and glucose transport in human adipocytes. Molecular and Cellular Biochemistry. 1995;144(2):147–151. doi: 10.1007/BF00944394. [DOI] [PubMed] [Google Scholar]
- Hillaire-Buys D, Bertrand G, Gross R, Loubatières-Mariani M-M. Evidence for an inhibitory A1 subtype adenosine receptor on pancreatic insulin-secreting cells. European Journal of Pharmacology. 1987;136(1):109–112. doi: 10.1016/0014-2999(87)90786-2. [DOI] [PubMed] [Google Scholar]
- Hoffer L, Lowenstein J. Effects of adenosine and adenosine analogues on glycogen metabolism in isolated rat hepatocytes. Biochem Pharmacol. 1986;35(24):4529–4536. doi: 10.1016/0006-2952(86)90775-6. [DOI] [PubMed] [Google Scholar]
- Ismail N, El Denshary E, Montague W. Adenosine and the regulation of insulin secretion by isolated rat islets of Langerhans. Biochem J. 1977;164(2):409–413. doi: 10.1042/bj1640409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K, Logothetopoulos J. Metabolic signals produced by purine ribonucleosides stimulate proinsulin biosynthesis and insulin secretion. Biochem J. 1978;170(3):461–467. doi: 10.1042/bj1700461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shepherd R, Duling B, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100(11):2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson SM, Lindgren E, Yang J-N, Herling AW, Fredholm BB. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue -- Interactions with insulin. European Journal of Pharmacology. 2008;597(1–3):92–101. doi: 10.1016/j.ejphar.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Johansson SM, Salehi A, Sandström ME, Westerblad H, Lundquist I, Carlsson P-O, Fredholm BB, Katz A. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochemical Pharmacology. 2007a;74(11):1628–1635. doi: 10.1016/j.bcp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Johansson SM, Yang JN, Lindgren E, Fredholm BB. Eliminating the antilipolytic adenosine A1 receptor does not lead to compensatory changes in the antilipolytic actions of PGE2 and nicotinic acid. Acta Physiologica. 2007b;190(1):87–96. doi: 10.1111/j.1365-201X.2007.01692.x. [DOI] [PubMed] [Google Scholar]
- Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG, LeBrasseur N, Ravid K. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One. 2012a;7(7):e40584. doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston-Cox HA, Koupenova M, Ravid K. A2 adenosine receptors and vascular pathologies. Arterioscler Thromb Vasc Biol. 2012b;32(4):870–878. doi: 10.1161/ATVBAHA.112.246181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner T, Uysal K, Wiesbrock S, Marino M, Hotamisligil G. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;100(11):2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupenova M, Johnston-Cox H, Vezeridis A, Gavras H, Yang D, Zannis V, Ravid K. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation. 2012;125(2):354–363. doi: 10.1161/CIRCULATIONAHA.111.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs P, Stumvoll M. Fatty acids and insulin resistance in muscle and liver. Best Practice & Research Clinical Endocrinology & Metabolism. 2005;19(4):625–635. doi: 10.1016/j.beem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- LaNoue KF, Martin LF. Abnormal A1 adenosine receptor function in genetic obesity. FASEB J. 1994;8(1):72–80. doi: 10.1096/fasebj.8.1.8299893. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Rieger JM, Linden J. A2A Adenosine Receptor Induction Inhibits IFN-{gamma} Production in Murine CD4+ T Cells. J Immunol. 2005;174(2):1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- Law WR, McLane MP, Raymond RM. Adenosine is required for myocardial insulin responsiveness in vivo. Diabetes. 1988;37(6):842–845. doi: 10.2337/diab.37.6.842. [DOI] [PubMed] [Google Scholar]
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8(11):1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- Linden J. MOLECULAR APPROACH TO ADENOSINE RECEPTORS: Receptor-Mediated Mechanisms of Tissue Protection. Annual Review of Pharmacology and Toxicology. 2001;41(1):775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Londos C, Honnor RC, Dhillon GS. cAMP-dependent protein kinase and lipolysis in rat adipocytes. III. Multiple modes of insulin regulation of lipolysis and regulation of insulin responses by adenylate cyclase regulators. J Biol Chem. 1985;260(28):15139–15145. [PubMed] [Google Scholar]
- Loubatieres-Mariani M, Chapal J, Lignon F, Valette G. Structural specificity of nucleotides for insulin secretory action from the isolated perfused rat pancreas. Eur J Pharmacol. 1979;59(3–4):277–286. doi: 10.1016/0014-2999(79)90291-7. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Apasov S, Chen J-F, Sitkovsky M. Cutting Edge: Physiologic Attenuation of Proinflammatory Transcription by the Gs Protein-Coupled A2A Adenosine Receptor In Vivo. J Immunol. 2004;173(1):21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer and Metastasis Reviews. 2007;26(2):273–279. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- Maeda T, Koos BJ. ADENOSINE A1 AND A2A RECEPTORS MODULATE INSULINEMIA,GLYCEMIA AND LACTATEMIA IN FETAL SHEEP. J Physiol Regul Integr Comp Physiol. 2008:90363–92008. doi: 10.1152/ajpregu.90363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD. Banting Lecture 2001: Dysregulation of Fatty Acid Metabolism in the Etiology of Type 2 Diabetes. Diabetes. 2002;51(1):7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Nemeth ZH, Bleich D, Csoka B, Pacher P, Mabley JG, Himer L, Vizi ES, Deitch EA, Szabo C, Cronstein BN, Hasko G. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21(10):2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetjen E, Schweickhardt C, Unthan-Fechner K, Probst I. Stimulation of glucose production from glycogen by glucagon, noradrenaline and non-degradable adenosine analogues is counteracted by adenosine and ATP in cultured rat hepatocytes. Biochem J. 1990;271(2):337–344. doi: 10.1042/bj2710337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414(6866):916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rosin DL, Smith DF, Sullivan G. Enhanced protection from renal ischemia: Reperfusion injury with A2A–adenosine receptor activation and PDE 4 inhibition. Kidney Int. 2001;59(6):2114–2125. doi: 10.1046/j.1523-1755.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- Peng Z, Borea P, Wilder T, Yee H, Chiriboga L, Blackburn M, Azzena G, Resta G, Cronstein B. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009:37409. doi: 10.1172/JCI37409. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss A, Carsons S, Anwar K, Rao S, Edelman S, Zhang H, Fernandez P, Cronstein B, Chan E. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis & Rheumatism. 2008;58(12):3675–3683. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AB, Rahman MM, Chan ESL, Montesinos MC, Awadallah NW, Cronstein BN. Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leukoc Biol. 2004;76(3):727–734. doi: 10.1189/jlb.0204107. [DOI] [PubMed] [Google Scholar]
- Rice AM, Fain JN, Rivkees SA. A1 Adenosine Receptor Activation Increases Adipocyte Leptin Secretion. Endocrinology. 2000;141(4):1442–1445. doi: 10.1210/endo.141.4.7423. [DOI] [PubMed] [Google Scholar]
- Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-[alpha] Cytokine & Growth Factor Reviews. 2003;14(5):447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Rubio R, Berne RM. Localization of purine and pyrimidine nucleoside phosphorylases in heart, kidney, and liver. Am J Physiol Heart Circ Physiol. 1980;239(6):H721–730. doi: 10.1152/ajpheart.1980.239.6.H721. [DOI] [PubMed] [Google Scholar]
- Rüsing D, Müller CE, Verspohl EJ. The impact of adenosine and A2B receptors on glucose homoeostasis. J Pharm Pharmacol. 2006;58(12):1639–1645. doi: 10.1211/jpp.58.12.0011. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B Adenosine Receptor Gene Ablation on Adenosine-Dependent Regulation of Proinflammatory Cytokines. J Pharmacol Exp Ther. 2008;324(2):694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(21):10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoelch C, Kuhlmann J, Gossel M, Mueller G, Neumann-Haefelin C, Belz U, Kalisch J, Biemer-Daub G, Kramer W, Juretschke H-P, Herling AW. Characterization of Adenosine-A1 Receptor-Mediated Antilipolysis in Rats by Tissue Microdialysis, 1H–Spectroscopy, and Glucose Clamp Studies. Diabetes. 2004;53(7):1920–1926. doi: 10.2337/diabetes.53.7.1920. [DOI] [PubMed] [Google Scholar]
- Schütz W, Raberger G, Kraupp O. Influence of aminophylline or beta-adrenoceptor blockade on glucagon release by a vasoactive adenosine analogue. Arch Int Pharmacodyn Ther. 1978;234(2):214–220. [PubMed] [Google Scholar]
- Schütz W, Raberger G, Kraupp O. Evidence for glucagon-releasing activity of vasoactive adenosine analogues in the conscious dog. Naunyn Schmiedebergs Arch Pharmacol. 1978;304(3):249–254. doi: 10.1007/BF00507965. [DOI] [PubMed] [Google Scholar]
- Silva PH, Dillon D, Van Wylen DGL. Adenosine deaminase inhibition augments interstitial adenosine but does not attenuate myocardial infarction. Cardiovasc Res. 1995;29(5):616–623. [PubMed] [Google Scholar]
- Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, Bue-Valleskey J, Stephens TW. Regulation of Expression of ob mRNA and Protein by Glucocorticoids and cAMP. J Biol Chem. 1996;271(10):5301–5304. doi: 10.1074/jbc.271.10.5301. [DOI] [PubMed] [Google Scholar]
- St. Hilaire C, Koupenova M, Carroll SH, Smith BD, Ravid K. TNF-[alpha] upregulates the A2B adenosine receptor gene: The role of NAD(P)H oxidase 4. Biochemical and Biophysical Research Communications. 2008;375(3):292–296. doi: 10.1016/j.bbrc.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfelder H, Pethö-Schramm S. Methylxanthines inhibit glucose transport in rat adipocytes by two independent mechanisms. Biochem Pharmacol. 1990;40(5):1154–1157. doi: 10.1016/0006-2952(90)90508-i. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A3 receptors on mast cells. J Clin Invest. 2000;105(3):361–367. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AL, Linden J. Cloned receptors and cardiovascular responses to adenosine. Cardiovasc Res. 1993;27(1):62–67. doi: 10.1093/cvr/27.1.62. [DOI] [PubMed] [Google Scholar]
- Ulrich Schwabe PSSRE. Facilitation by Adenosine of the Action of Insulin on the Accumulation of Adenosine 3’:5’-Monophosphate, Lipolysis, and Glucose Oxidation in Isolated Fat Cells. European Journal of Biochemistry. 1974;46(3):537–545. doi: 10.1111/j.1432-1033.1974.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Vannucci S, Nishimura H, Satoh S, Cushman S, Holman G, Simpson I. Cell surface accessibility of GLUT4 glucose transporters in insulin-stimulated rat adipose cells. Modulation by isoprenaline and adenosine. Biochem J. 1992;288(1):325–330. doi: 10.1042/bj2880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Klim CM, Martin LF, LaNoue KF. A1-adenosine receptor-mediated inhibition of adipocyte adenylate cyclase and lipolysis in Zucker rats. Am J Physiol Endocrinol Metab. 1989;257(6):E871–E878. doi: 10.1152/ajpendo.1989.257.6.E871. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang W, Zhu C, Bucher C, Blazar BR, Zhang C, Chen J-F, Linden J, Wu C, Huo Y. Inactivation of the Adenosine A2A Receptor Protects Apolipoprotein E-Deficient Mice From Atherosclerosis. Arterioscler Thromb Vasc Biol:ATVBAHA. 2009;109:188839. doi: 10.1161/ATVBAHA.109.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M-Y, Lee Y, Unger RH. Novel Form of Lipolysis Induced by Leptin. J Biol Chem. 1999;274(25):17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- Wang T, Sodhi J, Mentzer RM, Jr, Wylen DGLV. Changes in interstitial adenosine during hypoxia: relationship to oxygen supply:demand imbalance, and effects of adenosine deaminase. Cardiovasc Res. 1994;28(9):1320–1325. doi: 10.1093/cvr/28.9.1320. [DOI] [PubMed] [Google Scholar]
- Xu B, Berkich DA, Crist GH, LaNoue KF. A1 adenosine receptor antagonism improves glucose tolerance in Zucker rats. Am J Physiol Endocrinol Metab. 1998;274(2):E271–E279. doi: 10.1152/ajpendo.1998.274.2.E271. [DOI] [PubMed] [Google Scholar]
- Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proceedings of the National Academy of Sciences. 2008;105(2):792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006a;116(7):1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St. Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006b;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GK, Fredholm BB, Kieffer TJ, Kwok YN. Improved blood glucose disposal and altered insulin secretion patterns in adenosine A(1) receptor knockout mice. Am J Physiol Endocrinol Metab. 2012;303(2):E180–E190. doi: 10.1152/ajpendo.00050.2012. [DOI] [PubMed] [Google Scholar]
- Yasuda N, Inoue T, Horizoe T, Nagata K, Minami H, Kawata T, Hoshino Y, Harada H, Yoshikawa S, Asano O, Nagaoka J, Murakami M, Abe S, Kobayashi S, Tanaka I. Functional characterization of the adenosine receptor contributing to glycogenolysis and gluconeogenesis in rat hepatocytes. European Journal of Pharmacology. 2003;459(2–3):159–166. doi: 10.1016/s0014-2999(02)02832-7. [DOI] [PubMed] [Google Scholar]
- Yegutkin G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta; Epub 2008 Feb 12. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Zentella de Piña M, Díaz-Cruz A, Guinzberg R, Piña P. “Hormone-like” effect of adenosine and inosine on gluconeogenesis from lactate in isolated hepatocytes. Life Sciences. 1989;45(23):2269–2274. doi: 10.1016/0024-3205(89)90068-4. [DOI] [PubMed] [Google Scholar]