Abstract
Niemann-Pick Type C (NPC) disease is caused by a deficiency of either NPC1 or NPC2. Loss of function of either protein results in the progressive accumulation of unesterified cholesterol in every tissue leading to cell death and organ damage. Most literature on NPC disease focuses on neurological and liver manifestations. Pulmonary dysfunction is less well described. The present studies investigated how Npc1 deficiency impacts the absolute weight, lipid composition and histology of the lungs of Npc1−/− mice (Npc1nih) at different stages of the disease, and also quantitated changes in the rates of cholesterol and fatty acid synthesis in the lung over this same time span (8 to 70 days of age). Similar measurements were made in Npc2−/− mice at 70 days. All mice were of the BALB/c strain and were fed a basal rodent chow diet. Well before weaning, the lung weight, cholesterol and phospholipid (PL) content, and cholesterol synthesis rate were all elevated in the Npc1−/− mice and remained so at 70 days of age. In contrast, lung triacylglycerol content was reduced while there was no change in lung fatty acid synthesis. Despite the elevated PL content, the composition of PL in the lungs of the Npc1−/− mice was unchanged. H&E staining revealed an age-related increase in the presence of lipid-laden macrophages in the alveoli of the lungs of the Npc1−/− mice starting as early as 28 days. Similar metabolic and histologic changes were evident in the lungs of the Npc2−/− mice. Together these findings demonstrate an intrinsic lung pathology in NPC disease that is of early onset and worsens over time.
Keywords: pulmonary, alveoli, phospholipid, triacylglycerol, liver
1. Introduction
In Niemann-Pick type C (NPC) disease unesterified cholesterol (UC), gangliosides, and glycosphingolipids continually accumulate in the late endosomal/lysosomal (E/L) compartment of all cells in every organ. It is an autosomal recessive disorder primarily affecting children and is characterized by neurodegeneration, hepatic and pulmonary dysfunction, and premature death [1]. A lot of what we currently know about NPC1 and NPC2 function, and also the etiology and pathogenesis of NPC disease has come from the study of animal models with NPC1 or NPC2 deficiency, primarily in the mouse [2–14], and also from various in vitro systems as well as techniques such as X-ray crystallography [15–17]. Unequivocally, it is the sequestration of UC in the E/L compartment that is the fundamental cause of NPC disease [18]. This accumulation of UC is due to a loss of function mutation in genes encoding either NPC1 or NPC2, both of which are essential for facilitating cholesterol transport out of the late E/L compartment [17]. Disruption of this egress and the resultant buildup of UC, together with that of other types of lipids, leads to cell death and multi-organ damage. While neurodegeneration is a major cause of morbidity and mortality in NPC disease, there are cases where death occurs within about the first six months of life from either liver failure or severe pulmonary dysfunction [1].
Although there is currently no effective treatment for NPC disease, a variety of agents has been evaluated primarily in animal or in vitro models, with 2-hydroxypropyl-β-cyclodextrin (2HPβCD) showing the best efficacy thus far [19–24]. In a particularly definitive study it was demonstrated that continuous infusion of 2HPβCD into the ventricular system of Npc1−/− animals between 3 and 7 weeks of age normalized the biochemical abnormalities and completely prevented the expected neurodegeneration [25]. Earlier studies established that weekly subcutaneous administration of 2HPβCD to Npc1−/− mice, starting at 7 days of age, nearly normalized hepatic and whole animal cholesterol pools and prevented development of liver disease [26]. While there was also a slowing of cerebellar neurodegeneration and an increase in lifespan, systemic 2HPβCD treatment had little to no effect on the development of progressive pulmonary disease [26]. Subsequent studies further demonstrated lack of impact of systemically administered 2HPβCD on a number of parameters of lung function and pathology in the Npc1−/− mouse [27]. There is nothing intrinsically different about UC sequestration in the lungs in NPC1 or NPC2 deficiency because NPC2 replacement therapy in a mouse model of NPC2 disease resulted in a striking reduction in the cholesterol content of several organs including the lungs [28]. Rather, the inaction of 2HPβCD in the NPC lung may simply reflect lack of penetrance at a cellular level. Irrespective of whether this is the case, those studies revealed the paucity of published information about how NPC1 or NPC2 deficiency impacts lung cholesterol metabolism, particularly in early stage disease. In one of our initial studies in the Npcnih mouse model we found that even in 1-day old Npc1−/− pups the cholesterol concentration in most organs, including the lungs, was elevated [29]. More recently, a murine model of infantile NPC1 deficiency has been described but lungs were not included in the tissues examined [30].
The present studies represent the first systematic evaluation, in quantitative terms, of the ontogenic changes in lung mass, lipid composition, rates of cholesterol and fatty acid synthesis, and also histology in mice with NPC1 and NPC2 deficiency.
2. Materials and methods
2.1. Animals and diets
Control (Npc1+/+ and Npc2+/+) and mutant (Npc1−/− and Npc2−/−) mice were generated from respective heterozygous breeding stock on a pure BALB/c background. The NPC1 mice (Npc1nih) originated from a colony at the National Institutes of Health (Dr. Peter Pentchev), while heterozygous Npc2 breeding stock was kindly provided by Dr. Peter Lobel. Depending on the age at which they were to be studied, the mice were genotyped anywhere from 6 to 19 days of age. Unless studied beforehand, all mice were weaned at 19 to 21 days, and thereafter were fed ad libitum a cereal-based, low-cholesterol (0.02% cholesterol, 4% total fat, w/w) diet (No. 7001; Harland Teklad, Madison, WI). They were group-housed in plastic colony cages in rooms with alternating 12-h periods of dark and light and were studied in the fed state at the end of the dark phase. Unless otherwise stated there were comparable numbers of males and females in each group. All experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas Southwestern Medical Center.
2.2. Changes in lung weight as a function of age and Npc1 genotype
In the course of multiple projects with the Npc1 mouse model we recorded lung weights in a total of 188 untreated animals (94 Npc1+/+ and 94 Npc1−/−) over the age range of 8 to 80 days. The lungs were carefully excised, rinsed in physiological saline, blotted and weighed. In some of the mice studied in the age range of 57–60 days, the weight of each lobe (one on the left, and the diaphragmatic, apical, azygous, and cardiac on the right) was recorded.
2.3. Measurement of lung total cholesterol content and proportion of cholesterol present in esterified and unesterified form
Lung total cholesterol content was measured in Npc1−/− mice and their matching Npc1+/+ controls at 8, 19, 29, 35, 49, 57, 63, and 70 days of age. For the Npc2 mice such measurements were made at 70 days only. The total cholesterol concentration in the lungs (mg/g wet tissue weight) was measured by gas chromatography using stigmastanol as an internal standard [31]. The cholesterol concentration was multiplied by the respective lung weight, to give whole lung cholesterol content (mg/organ). In one study lungs were harvested specifically for measuring the proportion of the total cholesterol present in esterified and unesterified form. Lungs from Npc1 mice (57–63 days old) and Npc2 mice (70–80 days old) were weighed and extracted in chloroform:methanol (2:1 v/v) containing radiolabeled esterified and unesterified cholesterol as internal standards. The esterified and unesterified cholesterol fractions in these extracts were separated by column chromatography and quantitated as described [32].
2.4. Measurement of rates of cholesterol and fatty acid synthesis lungs in vivo
The details of the methods for these measurements are given elsewhere [31]. The ages of the Npc1 mice used in this study were 8, 23, 52, and 70 days. The mice were given an i.p. injection of [3H]water (~2 mCi /g body wt), and after 1 h, were anesthetized and exsanguinated. The lungs were removed, rinsed and weighed. They were then saponified and their content of radiolabeled digitonin-precipitable sterols (DPS) was measured [31]. The rate of cholesterol synthesis was expressed as the nmol of [3H]water incorporated into DPS per h per whole organ. After the labeled sterols were extracted, the saponified samples were acidified and extracted with hexane. Aliquots of the organic phase were taken for measurement of [3H]-fatty acid content. Rates of fatty acid synthesis were expressed in the same units as for sterol synthesis. For the Npc2 mice, the rates of lung sterol and fatty acid synthesis were measured at 70 days only.
2.5. Measurement of lung phospholipid and triacylglycerol content
These measurements were made in Npc1−/− and matching Npc1+/+ mice at 8, 25, 50, and 70 days of age. They were not the same animals as those used for the determination of either the lung cholesterol content, or the lung cholesterol and fatty acid synthesis rates. After excision, the lungs were rinsed, weighed and added to chloroform:methanol (2:1 v/v). Procedural losses were corrected for by internal standards. These were either [14C]triolein (American Radiolabeled Chemicals, Inc., St. Louis, MO), or L-α-dipalmitoyl,[choline-methyl-3H]-phosphatidylcholine (Perkin Elmer NEN Radiochemicals, Waltham, MA). An aliquot of this extract was then dried, dissolved in hexane:tert-butyl methyl ether (100:1.5 v/v) and run over a Sep-Pak RC cartridge (500mg)(Waters Corp., Milford, MA) as described elsewhere [33]. After elution of the cholesteryl esters, the solvent was changed to hexane:tert-butyl methyl ether (96:4 v/v) for elution of triacylglycerols. Finally, the eluting solvent was changed to tert-butyl methyl ether:methanol:ammonium acetate (pH 8.6) (5:8:2 v/v/v) to obtain the phospholipid fraction. This fraction, as well as the one containing the triacylglycerols, was dried and redissolved in 10 ml of chloroform:methanol (2:1 v/v). Aliquots of these solutions were used for quantitation of total triacylglycerol content using Infinity Triglycerides Liquid Stable Reagent (Thermo Fisher Scientific, Inc., Middletown, VA) [34], or of total phospholipid content by a colorimetric assay using malachite green as described elsewhere [35]. In this method all the phospholipids are digested using perchloric acid at high temperature to liberate phosphorous on a mole per mole basis. It provides a measure of total phospholipid content and not of the proportion of phospholipid species that are present. This requires the use of other techniques including LC/MS/MS as described below. The lung triacylglycerol data were expressed as mg/organ while those for phospholipid were calculated as umol/organ.
2.6. Determination of lung and liver phospholipid composition
The whole lungs and aliquots of livers from three Npc1−/− mice and three matching Npc1+/+ controls at 49 to 53 days of age were weighed and extracted in choloroform:methanol (2:1 v/v). These pooled extracts were dried under nitrogen and shipped on dry ice to Avanti Polar Lipids, Inc. (Alabaster, AL) where the phospholipid composition was determined by LC/MS/MS. The proportion of each phospholipid detected was expressed as a fraction of the total phospholipid content. For all the phospholipids except for sphingomyelin, trace quantities of their lyso form were detected. Given the uncertain origin of these lyso phospholipids they were simply added to the mass of the parent phospholipid that was measured.
2.7. Lung histology
Lungs were perfused with 10% buffered formalin and excised. The tissue was fixed in 10% buffered formalin and embedded in paraffin. These blocks were then sectioned and stained with hematoxylin and eosin (H&E). For the Npc1 mice, lung histology was obtained at 28, 49, and 70 days of age with lungs being taken from three Npc1−/− and three Npc1+/+ mice at each of these ages. For the NPC2 mice, lung histology was carried out at only 70 days in two Npc2−/− and two Npc2+/+ mice.
2.8. Analysis of data
All data are reported as the mean ± 1 SEM in the specified number of individual animals. Differences between mean values were tested for statistical significance (p<0.05) by the two-tailed Student’s t-test assuming equal variance.
3. Results
Although several mouse models of Npc1 deficiency have been described [2, 11, 14, 30] all of our studies to date have used Npc1nih mice maintained on a low-cholesterol basal rodent chow diet. These mice exhibit a marked increase in whole body cholesterol synthesis and content [29, 36]. While all organs show elevated concentrations and rates of synthesis of cholesterol, these changes are particularly pronounced in the liver [36]. Consequently the Npc1−/− mice manifest an age-related increase in plasma ALT and AST activities [34]. These changes are accompanied by progressive neurodegeneration which leads to a shortened life span of around 80 to 90 days [13, 26]. The features of Npc2−/− mice parallel those lacking Npc1 but their lifespan is generally longer, depending partly on the strain of mouse [12, 28, 37].
3.1. Npc1−/− mice show a significant increase in lung mass starting before weaning
At 8 days after birth the weight of the lungs in the Npc1−/− mice (0.080 ± 0.003 g, n=10) was about the same as that in matching Npc1+/+ controls (0.079 ± 0.003 g, n=12) (Fig. 1A). However, at all ages after 8 days the lung mass in the Npc1−/− mice was consistently greater than in their Npc1+/+ controls. From the data in Fig. 1A it can be calculated that across the age span from 19–22 to 77–80 days, the average weight of the lungs in the Npc1−/− mice (0.165 ± 0.010 g) was significantly higher than in the matching Npc1+/+ controls (0.134 ± 0.006 g, p<0.05). At least in mice in the age range of 57–60 days, the greater lung mass in the mutants was not confined to any one lobe although the bulk of the additional tissue was in the left and diaphragmatic lobes (Fig. 1B).
Fig. 1.
Absolute whole lung weights in Npc1+/+ and Npc1−/− mice over the age range of 8 to 80 days, and weights of individual lung lobes in Npc1+/+ and Npc1−/− mice at 57–60 days. The data for lung weights from all the present experiments were pooled with those from previous projects in this laboratory using Npc1 mice. Except for the 8-day time point, the data are grouped for animals over successive spans of 3 days each (A). Lung weights were obtained from a total of 188 mice (94 for each genotype). The numbers of mice at each age varied considerably. At the 8, 19–22, 27–30, 48–51, 52–55, and 68–71 day time points there was a minimum of 9 mice of each genotype. For all other points there was a minimum of 4 mice of each genotype except in the group at 61–64 days where there were just two Npc1+/+ mice. Separate groups of mice in the age range of 57–60 days were used for the measurement of the weights of individual lobes (B). These data are from 4 mice of each genotype. All values represent the mean ± 1 SEM. The asterisk denotes that the value for the Npc1−/− mice is significantly different from that for the Npc1+/+ controls at that particular age (p<0.05).
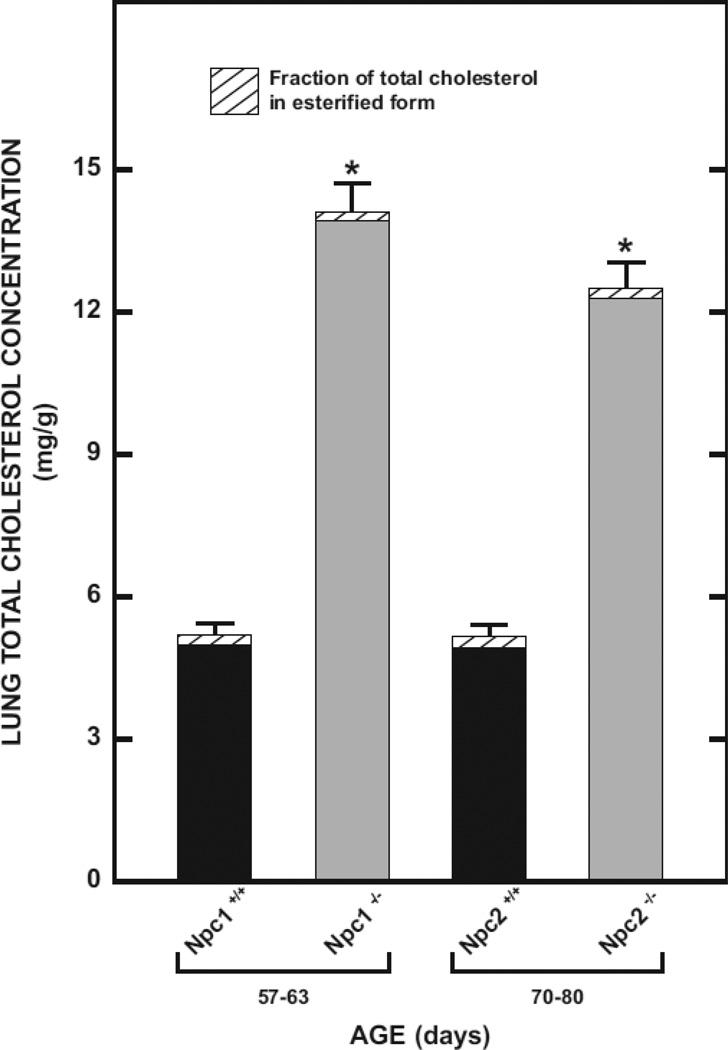
3.2. Marked increase in lung total cholesterol concentration in Npc1−/− and Npc2−/− mice is due only to the unesterified component
Before carrying out detailed measurements of genotypic and ontogenic changes in lung cholesterol content and synthesis, and other parameters of lipid metabolism, we first quantitated the magnitude of the increase in the total cholesterol concentration (mg/g) in the lungs of both Npc1−/− and Npc2−/− mice exhibiting early clinical symptoms of disease. Furthermore, we also wanted to confirm that it was expansion of the unesterified cholesterol component that was driving the elevation in cholesterol concentration in the lungs of both models. The data show unequivocally that the 2.7-fold and 2.4-fold increase in total cholesterol concentrations in the Npc1−/− and Npc2−/− mice respectively, was entirely accounted for by unesterified sterol (Fig. 2). These data also make the point that while changes in whole lung cholesterol content (mg/organ) described below reflect both greater tissue mass and cholesterol concentrations, it is the latter that accounts for much of the expansion of the lung cholesterol pool in NPC deficiency. One earlier study with Npc1−/− mice showed their hepatic esterified cholesterol concentrations were not elevated unless they were fed a high cholesterol diet [36]. However, another study using Npc1−/− primary hepatocytes did find some accumulation of cholesteryl ester in these cells [38].
Fig. 2.
Total, unesterified, and esterified cholesterol concentrations in the lungs of Npc1+/+, Npc1−/−, Npc2+/+, and Npc2−/− mice. The unesterified and esterified cholesterol concentrations were measured as described in Materials and Methods and summed to give the total cholesterol concentration. In each histogram the solid shading represents the level of unesterified cholesterol. Values represent the mean ± 1 SEM of measurements in four mice in each group. The asterisk denotes that the values for the Npc1−/− and Npc2−/− mice are significantly different from the value for the Npc1+/+ and Npc2+/+ mice, respectively (p<0.05).
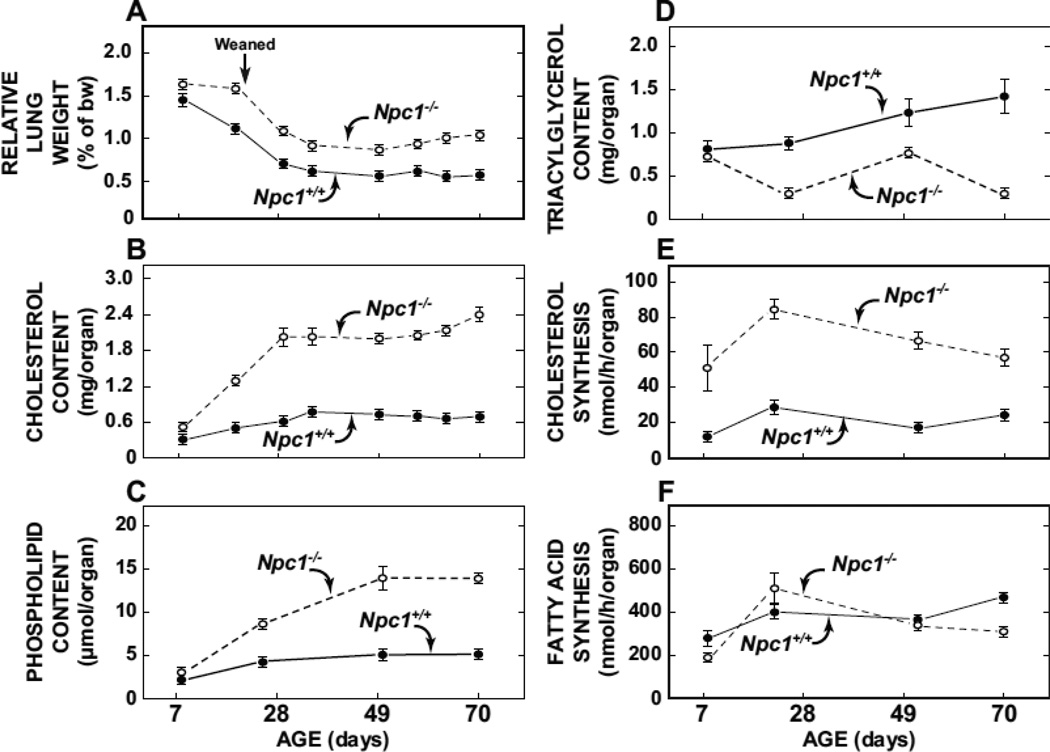
3.3. Ontogenic changes in lipid composition and synthesis rates in the lungs of Npc1−/− mice are not uniform for all the major classes of lipids
The data in Fig. 3A show lung weights relative to body weights in Npc1−/− and Npc1+/+ from 8 to 70 days which was generally the age range over which various parameters of lung cholesterol and lipid metabolism were measured. It should be noted that after about 49 days of age the genotypic difference in relative lung weight becomes exaggerated because of the steady decrease in the body weight of the Npc1−/− mice in late stage disease.
Fig. 3.
Age-related changes in the relative weight, lipid composition, and synthesis rates of cholesterol and fatty acid in the lungs of Npc1+/+ and Npc1−/− mice. The data for relative lung weight (A) and whole lung cholesterol content (B) were obtained from mice at 8, 19, 29, 35, 49, 57, 63 and 70 days of age. For the measurement of lung phospholipid (C) and triacylglycerol (D) content tissue was taken at only four ages (8, 25, 50, and 70 days). This was also the case for the measurement of the rates of lung cholesterol synthesis (E) and fatty acid synthesis (F) (8, 23, 52, 70 days). These rates were measured in vivo using [H]water as described in Materials and Methods. The groups of mice used for this procedure were separate from those used for the chemical composition assays. For all parameters the values at each time point represent the mean ± 1 SEM of measurements in a minimum of four mice.
In the 8-day old mutant mice a higher lung cholesterol content was already apparent (Fig. 3B). At 23 days lung cholesterol content in the mutants was 3.3-fold more than in the matching Npc1+/+ mice (Fig 3B). This difference remained at about 3-fold until 70 days of age when it increased to 3.7-fold. While the data are not shown, in 80-day old Npc1−/− mice whole lung cholesterol was 4.3-fold higher than in their Npc1+/+ littermates (3.2 ± 0.2 vs 0.75 ± 0.05 mg/organ). Although measured at fewer time points, the genotypic differences in lung phospholipid content nevertheless generally followed the same pattern as those found for cholesterol. At both 49 and 70 days lung phospholipid content in the Npc1−/− mice was about 2.7- to 2.8-fold higher than in matching Npc1+/+ controls (Fig. 3C). In sharp contrast, in the case of lung triacylglycerol content, the values for the Npc1−/− mice were consistently less than those for the Npc1+/+ animals from about the time of weaning onwards (Fig. 3D). In addition to the lipid composition measurements, separate groups of mice at 8, 23, 52, and 70 days of age were used for the determination in vivo of the rates of cholesterol and fatty acid synthesis in lung, expressed on a whole organ basis. At just 8 days of age lung cholesterol synthesis in the mutants exceeded that in the Npc1+/+ controls by 4.5-fold, and remained more than 2-fold higher at all the other age points (Fig. 3E). In marked contrast there was no discernible genotypic difference in lung fatty acid synthesis at any age (Fig. 3F).
3.4. Lung phospholipid composition did not show a genotypic difference but in the livers of the Npc1−/− mice the proportion of sphingomyelin was markedly elevated
Given that phospholipids are the most abundant lipid class in the lung, and also that other investigators had earlier reported an elevation in hepatic sphingomyelin levels in the livers of Npc1−/− mice [39], we carried out a phospholipid composition analysis by LC/MS/MS in pooled lipid extracts from the lungs and livers of Npc1−/− and Npc1+/+ at 49–53 days of age. There were no discernable genotypic differences in lung phospholipids, but in the liver the relative proportion of sphingomyelin (SM) in the Npc1−/− mice was 7.3-fold higher than in the Npc1+/+ controls (data not shown).
3.5. Histology of the lungs revealed an increasing presence of alveolar lipid-laden macrophages in the Npc1−/− mice as they age
The finding of marked changes in the lipid composition of the lungs in the Npc1−/− mice starting well before adulthood prompted an examination of lung histology in Npc1 mice of both genotypes at 28, 49, and 70 days by H&E staining. In the Npc1+/+ mice over this age range no macrophage infiltration was observed (Fig. 4A–C). However, in marked contrast, lungs from the 28-day old Npc1−/− mice revealed the presence of a few scattered, yet abnormal appearing lipid-laden macrophages within the alveoli (Fig. 4D). This feature was much more evident at 49 days (Fig. 4E), and at 70 days (Fig. 4F). In both cases an increasing presence of alveolar lipid-laden macrophages is readily discernible.
Fig. 4.
Representative histological sections of lung tissue from Npc1+/+ and Npc1−/− mice at three different ages. (A) The arrow points to a normal alveolar macrophage in the Npc1+/+ mouse. (D–F) the arrows point to abnormal lipid-laden macrophages within the alveoli of Npc1−/− mice. The scale bar equals 100µm (A), and all six images are at the same magnification (×400). For each age, lung histology was done on three mice of each genotype.
3.6. Lung mass, cholesterol metabolism and histology in Npc2−/− mice show changes comparable to those manifest in Npc1−/− mice
The striking age-related changes in lipid metabolism and histology in the lungs of the Npc1−/− mice prompted an abbreviated investigation of these same parameters in the lungs of Npc2−/− mice at the single age point of 70 days. In our earlier studies comparing Npc1 and Npc2 mice, both on the BALB/c background, the median lifespan of the Npc2−/− mice (146 days) was notably longer than that of the Npc1−/− mice (89 days) [12]. Thus, at 70 days, Npc2−/− mice have less advanced disease than those with NPC1 deficiency at that age. Nevertheless the relative lung weight in the Npc2−/− mice at 70 days (0.86 ± 0.04%) was almost 50% more than that in the Npc2+/+ (0.58 ± 0.02%) controls (Fig. 5A), but below the relative lung weight seen in 70-day-old Npc1−/− mice (1.1 ± 0.1%) (Fig. 3A). In the Npc2−/− mice lung cholesterol content was elevated 2.7-fold (Fig. 5B) and the rate of cholesterol synthesis was 61% greater than in their Npc2+/+ controls (Fig. 5C). There was no corresponding genotypic difference in the rate of fatty acid synthesis in the lung (Fig. 5D). H&E staining showed a clear difference histologically in the lungs of the Npc2+/+ (Fig. 5E) and Npc2−/− (Fig. 5F) mice. In the latter case lipid-laden macrophages were evident as seen in the lungs of the Npc1−/− mice.
Fig. 5.
Lung relative weight, cholesterol content, rates of cholesterol and fatty acid synthesis and histology in Npc2+/+ and Npc2−/− mice at 70 days of age. (A–D) the organ weight and metabolic data were obtained from a single study using six Npc2+/+ and six Npc2−/−, all at 70 days of age. For all of these parameters the values are mean ± 1 SEM. The asterisk denotes a statistically significant difference (p<0.05). Separate mice were used to obtain lungs for histology. The two lower images show representative histological sections of lung tissue in Npc2+/+ (E) and Npc2−/− (F) mice. The arrow points to a lipid-laden macrophage within the alveoli of the Npc2−/− mouse. The scale bar equals 100µm (E) and both images are at the same magnification (×400).
4. Discussion
Four main conclusions can be drawn from the current work. The first of these relates to changes in the mass of the lungs from early to late stage disease. An early publication describing lipid metabolism in the Npc1−/− mouse (then known as the NCTR-BALB/c mouse) noted a 2.3-fold higher relative lung weight in the mutants at about 70 days of age [3]. A comparable genotypic difference was seen in the relative lung weights in the 70 day old Npc1−/− mice in the present studies (Fig. 3A). It should be emphasized that such relative organ weight data in late stage NPC disease can be difficult to interpret because, after about 7 weeks of age, the Npc1−/− mice show an accelerated loss of body weight. Hence, relative organ weights, whether they be for lung, liver or spleen rise, but mostly this is not due to a continuing expansion of organ mass. The more important point to be made here, however, is that, although hepatosplenomegaly is a hallmark of NPC disease, there is also a decisive increase in lung mass. In the present studies this was clearly the case for the Npc1−/− mice (Fig. 1), and for the Npc2−/− mice as well (Fig. 5A). What the present studies also reveal is that the expansion in lung mass in the Npc1−/− mice is clearly evident from about the time of weaning. It is noteworthy that in a young NPC2-deficient human subject a marked increase in lung weight was reported [40].
The second conclusion concerns the striking ontogenic differences in lung cholesterol content and synthesis which were evident starting in early neonatal life. As documented by many laboratories, and as again confirmed here (Fig. 2), the increase in tissue cholesterol content in all organs, including the lungs, in both NPC1 and NPC2 disease reflects an expansion of the unesterified cholesterol fraction [3, 13, 14, 26]. By 50 days of age the lung cholesterol content in the Npc1−/− mice was 2.8-fold more than in matching Npc1+/+ controls. This cholesterol sequestration in the lungs is detectable as early as one day after birth in the Npc1−/− mice [29]. It was therefore not surprising to find that, at just 8 days of age, cholesterol synthesis in the lungs of the mutant mice was already four-fold above normal. The rapidly developing lung in the neonate has a critical need for cholesterol both for tissue growth and surfactant production. Ordinarily, most of this need would be met through the uptake of lipoproteins by receptor-mediated and bulk-phase endocytosis. When cholesterol entering tissues through these pathways becomes sequestered in the late E/L compartment because of either NPC1 or NPC2 deficiency this is compensated for in all tissues, including the lungs, by an increase in de novo sterol synthesis [8, 36]. This effect was selective for cholesterol synthesis because the rate of fatty acid synthesis in the lungs of the Npc1−/− mice was about the same as that in the Npc1+/+ controls at all ages (Fig. 3F).
The next conclusion relates to the divergence in the genotypic- and age-related changes in lung phospholipid (Fig. 3C) and triacylglycerol (Fig. 3D) content. For these data, as well as those for cholesterol (Fig. 3B), it should be emphasized that since the lungs were not lavaged before harvesting, the tissue lipid composition partially reflects that of the residual surfactant. However, data for other types of murine models of pulmonary dysfunction have shown that the phospholipid composition of alveolar lavage fluid mirrors that of the lung tissue [41–43]. Moreover, the total amount of phospholipid found in alveolar lavage fluid was reported to be about 10% of that contained in the lung tissue and fluid combined [41]. Similarly, the cholesterol content of alveolar lavage is very small compared to that of lung tissue [44]. It remains to be determined why the total phospholipid content of the lungs in the mutants rises by about the same magnitude, and follows a similar pattern of age-related changes as seen for cholesterol. Based on studies in primary hepatocytes from Npc1−/− mice a probable explanation is that there is an increase in phospholipid synthesis [38]. If so, this would potentially act as a compensatory mechanism to balance the increase in membrane cholesterol content thereby helping to preserve membrane structure and function. Alternatively, there may be a reduction in surfactant phospholipid degradation [45].
The lung triacylglycerol (TAG) content data (Fig. 3D) are very different from those found for phospholipid (Fig. 3C). In the pioneering work of Pentchev et al. [2], a dramatic depletion of tissue TAG content in several organs, including lung, was found in 72-day old Npc1−/− mice. In our earlier studies with 56-day old Npc1−/− mice we described a marked reduction in hepatic TAG content [34]. Several subsequent studies with hepatocytes have confirmed this finding [38, 46, 47]. One of these concluded that the diminished TAG levels resulted from the accelerated rate of utilization of acetate for cholesterol synthesis [47]. However, the measurement of TAG synthesis in primary hepatocytes from Npc1−/− mice showed that it is not significantly altered [38]. In the case of the lung we show here that lung TAG content in the Npc1−/− mice is normal at birth but declines markedly thereafter. Clearly this is not due to a reduction in fatty acid synthesis (Fig. 3F).
The fourth conclusion relates to the type of cells in the lungs that are affected in NPC disease. Using H&E stain, abnormal alveolar macrophages are seen as early as 28 days and accumulate over time. Surprisingly, infiltration with intra-alveolar lipidladen macrophages seen in 70 day old Npc1−/− mice is equally as severe as in Npc2−/− mice of the same age. This poses a dilemma because case reports of NPC2 patients are dominated by lung involvement characterized by alveolar foamy macrophage accumulation [40, 48, 49]. On the other hand, our observations are similar to those reported in a newly published study in both mouse and feline models of NPC1 and NPC2 disease [45]. Further evaluation with electron microscopy in our laboratory has identified macrophages with lipid accumulation and vacuolar inclusions infiltrating the lung parenchyma and enlarged, pleomorphic lamellar bodies within the Type II pneumocytes (unpublished data). These findings are consistent with those of the newly reported work of Roszell et al [45], as well as of an earlier study [50].
The final point to be made concerns the directions that future research on lung disease in NPC1 and NPC2 deficiency might reasonably follow. One of these is the delineation of changes in gene expression levels for a constellation of parameters including surfactant production, cholesterol metabolism, phospholipid synthesis and transport, apoptosis, macrophage infiltration, and inflammation. Ideally, such measurements should be carried out in early and late stage disease in one of the murine models for NPC deficiency as defined by the ontogenic data presented here. Another area of investigation clearly needed has to do with the cause(s) of the failure of systematically administered 2HPβCD to mobilize unesterified cholesterol sequestered in the E/L compartment in the various types of alveolar and epithelial cells in the lung parenchyma. If limited penetrance is the underlying cause then new technical approaches for delivering finely aerosolized 2HPβCD through inhalation will need to be designed and tested. The existing literature on pulmonary dysfunction in humans with NPC disease [40, 48, 49, 51–53] clearly underscores the need for more research at both a basic and clinical level.
Highlights.
NPC1-deficient mice have increased lung mass starting during neonatal development
Lung content of cholesterol and phospholipid in npc1−/− mice increase with age
Lungs of npc1−/− mice show increase in synthesis of cholesterol but not fatty acids
Lungs of npc1−/− mice show an increase in lipid-laden macrophages in the alveoli
Effects of NPC2 deficiency on lung lipid metabolism resemble those in npc1−/− mice
Acknowledgements
We thank Mario Saucedo, Carolyn Crumpton, Taylor Wagner, Stephen Ostermann and Monti Schneiderman for excellent technical assistance. This research was supported principally by US Public Health Service Grant RO1HL09610 (SDT). Other support was provided by the Ara Parseghian Medical Research Foundation (CMR).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- bw
body weight
- E/L
endosomal/lysosomal
- 2HPβCD
2-hydroxypropyl-β-cyclodextrin
- Npc1
Niemann-Pick C1
- Npc2
Niemann-Pick C2
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
- SM
sphingomyelin
- TAG
triacylglycerol
- UC
unesterified cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Charina M. Ramirez, Email: charina.ramirez@utsouthwestern.edu.
Adam M. Lopez, Email: adam.lopez@utsouthwestern.edu.
Lam Q. Le, Email: lam.le@ttuhsc.edu.
Kenneth S. Posey, Email: Kenneth.posey@utsouthwestern.edu.
Arthur G. Weinberg, Email: arthur.weinberg@childrens.com.
References
- 1.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pentchev PG, Gal AE, Booth AD, Omodeo-Sale F, Fouks J, Neumeyer BA, Quirk JM, Dawson G, Brady RO. A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase. Biochim. Biophys. Acta. 1980;619:669–679. doi: 10.1016/0005-2760(80)90116-2. [DOI] [PubMed] [Google Scholar]
- 3.Morris MD, Bhuvaneswaran C, Shio H, Fowler S. Lysosome lipid storage disorder in NCTR-BALB/c mice. I. Description of the disease and genetics. Am. J. Pathol. 1982;108:140–149. [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwamura M, Awakura T, Shimada A, Umemura T, Kagota K, Kawamura N, Naiki M. Type C Niemann-Pick disease in a boxer dog. Acta Neuropathol. 1993;85:345–348. doi: 10.1007/BF00227733. [DOI] [PubMed] [Google Scholar]
- 5.Cruz JC, Chang TY. Fate of endogenously synthesized cholesterol in Niemann-Pick type C1 cells. J. Biol. Chem. 2000;275:41309–41316. doi: 10.1074/jbc.M008272200. [DOI] [PubMed] [Google Scholar]
- 6.Sleat DE, Wiseman JA, El-Banna M, Price SM, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garver WS, Jelinek D, Oyarzo JN, Flynn J, Zuckerman M, Krishnan K, Chung BH, Heidenreich RA. Characterization of liver disease and lipid metabolism in the Niemann-Pick C1 mouse. J. Cell. Biochem. 2007;101:498–516. doi: 10.1002/jcb.21200. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Xie C, Richardson JA, Turley SD, Dietschy JM. Receptor-mediated and bulk-phase endocytosis cause macrophage and cholesterol accumulation in Niemann-Pick C disease. J. Lipid Res. 2007;48:1710–1723. doi: 10.1194/jlr.M700125-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Rimkunas VM, Graham MJ, Crooke RM, Liscum L. In vivo antisense oligonucleotide reduction of NPC1 expression as a novel mouse model for Niemann Pick type C-associated liver disease. Hepatology. 2008;47:1504–1512. doi: 10.1002/hep.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vite CH, Ding W, Bryan C, O'Donnell P, Cullen K, Aleman D, Haskins ME, van Winkle T. Clinical, electrophysiological, and serum biochemical measures of progressive neurological and hepatic dysfunction in feline Niemann-Pick type C disease. Pediatr. Res. 2008;64:544–549. doi: 10.1203/PDR.0b013e318184d2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaptzan T, West SA, Holicky EL, Wheatley CL, Marks DL, Wang T, Peake KB, Vance J, Walkley SU, Pagano RE. Development of a Rab9 transgenic mouse and its ability to increase the lifespan of a murine model of Niemann-Pick type C disease. Am. J. Pathol. 2009;174:14–20. doi: 10.2353/ajpath.2009.080660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez CM, Liu B, Aqul A, Taylor AM, Repa JJ, Turley SD, Dietschy JM. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J. Lipid Res. 2011;52:688–698. doi: 10.1194/jlr.M013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie X, Brown MS, Shelton JM, Richardson JA, Goldstein JL, Liang G. Amino acid substitution in NPC1 that abolishes cholesterol binding reproduces phenotype of complete NPC1 deficiency in mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:15330–15335. doi: 10.1073/pnas.1112751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maue RA, Burgess RW, Wang B, Wooley CM, Seburn KL, Vanier MT, Rogers MA, Chang CC, Chang TY, Harris BT, Graber DJ, Penatti CA, Porter DM, Szwergold BS, Henderson LP, Totenhagen JW, Trouard TP, Borbon IA, Erickson RP. A novel mouse model of Niemann-Pick type C disease carrying a D1005G-Npc1 mutation comparable to commonly observed human mutations. Hum. Mol. Genet. 2012;21:730–750. doi: 10.1093/hmg/ddr505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J. Biol. Chem. 2006;281:31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peake KB, Vance JE. Defective cholesterol trafficking in Niemann-Pick C-deficient cells. FEBS Lett. 2010;584:2731–2739. doi: 10.1016/j.febslet.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Erickson RP. Current controversies in Niemann-Pick C1 disease: steroids or gangliosides; neurons or neurons and glia. J. Appl. Genet. 2013;54:215–224. doi: 10.1007/s13353-012-0130-0. [DOI] [PubMed] [Google Scholar]
- 19.Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helquist P, Wiest O. Current status of drug therapy development for Niemann-Pick type C disease. Drug Future. 2009;34:315–331. [Google Scholar]
- 21.Rosenbaum AI, Maxfield FR. Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J. Neurochem. 2011;116:789–795. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B. Therapeutic potential of cyclodextrins in the treatment of Niemann–Pick type C disease. Clin. Lipidol. 2012;7:289–301. doi: 10.2217/clp.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peake KB, Vance JE. Normalization of cholesterol homeostasis by 2-hydroxypropyl-β-cyclodextrin in neurons and glia from Niemann-Pick C1 (NPC1)-deficient mice. J. Biol. Chem. 2012;287:9290–9298. doi: 10.1074/jbc.M111.326405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AM, Liu B, Mari Y, Liu B, Repa JJ. Cyclodextrin mediates rapid changes in lipid balance in Npc1−/− mice without carrying cholesterol through the bloodstream. J. Lipid Res. 2012;53:2331–2342. doi: 10.1194/jlr.M028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aqul A, Liu B, Ramirez CM, Pieper AA, Estill SJ, Burns DK, Liu B, Repa JJ, Turley SD, Dietschy JM. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011;31:9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez CM, Liu B, Taylor AM, Repa JJ, Burns DK, Weinberg AG, Turley SD, Dietschy JM. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 2010;68:309–315. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muralidhar A, Borbon IA, Esharif DM, Ke WJ, Manacheril R, Daines M, Erickson RP. Pulmonary function and pathology in hydroxypropyl-beta-cyclodextin-treated and untreated Npc1−/− mice. Mol. Genet. Metab. 2011;103:142–147. doi: 10.1016/j.ymgme.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen GK, Dagnaes-Hansen F, Holm IE, Meaney S, Symula D, Andersen NT, Heegaard CW. Protein replacement therapy partially corrects the cholesterol-storage phenotype in a mouse model of Niemann-Pick type C2 disease. PLoS One. 2011;6:e27287. doi: 10.1371/journal.pone.0027287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie CL, Burns DK, Turley SD, Dietschy JM. Cholesterol is sequestered in the brains of mice with Niemann-Pick Type C disease but turnover is increased. J. Neuropath. Exp. Neur. 2000;59:1106–1117. doi: 10.1093/jnen/59.12.1106. [DOI] [PubMed] [Google Scholar]
- 30.Parra J, Klein AD, Castro J, Morales MG, Mosqueira M, Valencia I, Cortes V, Rigotti A, Zanlungo S. Npc1 deficiency in the C57BL/6J genetic background enhances Niemann-Pick disease type C spleen pathology. Biochem. Biophys. Res. Commun. 2011;413:400–406. doi: 10.1016/j.bbrc.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7 alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]
- 32.Beltroy EP, Liu B, Dietschy JM, Turley SD. Lysosomal unesterified cholesterol content correlates with liver cell death in murine Niemann-Pick type C disease. J. Lipid Res. 2007;48:869–881. doi: 10.1194/jlr.M600488-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton JG, Comai K. Rapid separation of neutral lipids, free fatty acids and polar lipids using prepacked silica Sep-Pak columns. Lipids. 1988;23:1146–1149. doi: 10.1007/BF02535281. [DOI] [PubMed] [Google Scholar]
- 34.Beltroy EP, Richardson JA, Horton JD, Turley SD, Dietschy JM. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology. 2005;42:886–893. doi: 10.1002/hep.20868. [DOI] [PubMed] [Google Scholar]
- 35.Chalvardjian A, Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal. Biochem. 1970;36:225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- 36.Xie C, Turley SD, Pentchev PG, Dietschy JM. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am. J. Physiol. 1999;276:E336–E344. doi: 10.1152/ajpendo.1999.276.2.E336. [DOI] [PubMed] [Google Scholar]
- 37.Sleat DE, Wiseman JA, El-Banna M, Price SM, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulinski A, Vance JE. Lipid homeostasis and lipoprotein secretion in Niemann-Pick C1-deficient hepatocytes. J. Biol. Chem. 2007;282:1627–1637. doi: 10.1074/jbc.M610001200. [DOI] [PubMed] [Google Scholar]
- 39.Schedin S, Sindelar PJ, Pentchev P, Brunk U, Dallner G. Peroxisomal impairment in Niemann-Pick type C disease. J. Biol. Chem. 1997;272:6245–6251. doi: 10.1074/jbc.272.10.6245. [DOI] [PubMed] [Google Scholar]
- 40.Elleder M, Houstkova H, Zeman J, Ledvinova J, Poupetova H. Pulmonary storage with emphysema as a sign of Niemann-Pick type C2 disease (second complementation group) Report of a case, Virchows Arch. 2001;439:206–211. doi: 10.1007/s004280100407. [DOI] [PubMed] [Google Scholar]
- 41.Gross NJ, Smith DM. Impaired surfactant phospholipid metabolism in hyperoxic mouse lungs. J. Appl. Physiol. 1981;51:1198–1203. doi: 10.1152/jappl.1981.51.5.1198. [DOI] [PubMed] [Google Scholar]
- 42.Ikegami M, Dhami R, Schuchman EH. Alveolar lipoproteinosis in an acid sphingomyelinase-deficient mouse model of Niemann-Pick disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L518–L525. doi: 10.1152/ajplung.00258.2002. [DOI] [PubMed] [Google Scholar]
- 43.Besnard V, Wert SE, Stahlman MT, Postle AD, Xu Y, Ikegami M, Whitsett JA. Deletion of Scap in alveolar type II cells influences lung lipid homeostasis and identifies a compensatory role for pulmonary lipofibroblasts. J. Biol. Chem. 2009;284:4018–4030. doi: 10.1074/jbc.M805388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L980–L989. doi: 10.1152/ajplung.00234.2005. [DOI] [PubMed] [Google Scholar]
- 45.Roszell BR, Tao JQ, Yu KJ, Gao L, Huang S, Ning Y, Feinstein SI, Vite CH, Bates SR. Pulmonary abnormalities in animal models due to Niemann-Pick type C1 (NPC1) or C2 (NPC2) disease. PLoS One. 2013;8:e67084. doi: 10.1371/journal.pone.0067084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Uronen RL, Lundmark P, Orho-Melander M, Jauhiainen M, Larsson K, Siegbahn A, Wallentin L, Zethelius B, Melander O, Syvanen AC, Ikonen E. Niemann-Pick C1 modulates hepatic triglyceride metabolism and its genetic variation contributes to serum triglyceride levels. Arterioscler. Thromb. Vasc. Biol. 2010;30:1614–1620. doi: 10.1161/ATVBAHA.110.207191. [DOI] [PubMed] [Google Scholar]
- 48.Bjurulf B, Spetalen S, Erichsen A, Vanier MT, Strom EH, Stromme P. Niemann-Pick disease type C2 presenting as fatal pulmonary alveolar lipoproteinosis: morphological findings in lung and nervous tissue. Med. Sci. Monit. 2008;14:CS71–CS75. [PubMed] [Google Scholar]
- 49.Griese M, Brasch F, Aldana VR, Cabrera MM, Goelnitz U, Ikonen E, Karam BJ, Liebisch G, Linder MD, Lohse P, Meyer W, Schmitz G, Pamir A, Ripper J, Rolfs A, Schams A, Lezana FJ. Respiratory disease in Niemann-Pick type C2 is caused by pulmonary alveolar proteinosis. Clin. Genet. 2010;77:119–130. doi: 10.1111/j.1399-0004.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 50.Manabe T, Yamane T, Higashi Y, Pentchev PG, Suzuki K. Ultrastructural changes in the lung in Niemann-Pick type C mouse. Virchows Arch. 1995;427:77–83. doi: 10.1007/BF00203741. [DOI] [PubMed] [Google Scholar]
- 51.Minai OA, Sullivan EJ, Stoller JK. Pulmonary involvement in Niemann-Pick disease: case report and literature review. Respir. Med. 2000;94:1241–1251. doi: 10.1053/rmed.2000.0942. [DOI] [PubMed] [Google Scholar]
- 52.Morisot C, Millat G, Coeslier A, Bourgois B, Fontenoy E, Dobbelaere D, Verot L, Haouari N, Vaillant C, Gottrand F, Bogaert E, Thelliez P, Klosowski S, Djebara A, Bachiri A, Manouvrier S, Vanier MT. [Fatal neonatal respiratory distress in Niemann-Pick C2 and prenatal diagnosis with mutations in gene HE1/NPC2] Arch. Pediatr. 2005;12:434–437. doi: 10.1016/j.arcped.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Guillemot N, Troadec C, de Villemeur TB, Clement A, Fauroux B. Lung disease in Niemann-Pick disease. Pediatr. Pulmonol. 2007;42:1207–1214. doi: 10.1002/ppul.20725. [DOI] [PubMed] [Google Scholar]