Abstract
Tropomyosin (Tm) is the key regulatory component of the thin-filament and plays a central role in the cardiac muscle's cooperative activation mechanism. Many mutations of cardiac Tm are related to hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), and left ventricular noncompaction (LVNC). Using the thin-filament extraction/reconstitution technique, we are able to incorporate various Tm mutants and protein isoforms into a muscle fiber environment to study their roles in Ca2+ regulation, cross-bridge kinetics, and force generation. The thin-filament reconstitution technique poses several advantages compared to other in vitro and in vivo methods: (1) Tm mutants and isoforms are placed into the real muscle fiber environment to exhibit their effect on a level much higher than simple protein complexes; (2) only the primary and immediate effects of Tm mutants are studied in the thin-filament reconstituted myocardium; (3) lethal mutants of Tm can be studied without causing a problem; and (4) inexpensive. In transgenic models, various secondary effects (myocyte disarray, ECM fibrosis, altered protein phosphorylation levels, etc.) also affect the performance of the myocardium, making it very difficult to isolate the primary effect of the mutation. Our studies on Tm have demonstrated that: (1) Tm positively enhances the hydrophobic interaction between actin and myosin in the “closed state”, which in turn enhances the isometric tension; (2) Tm's seven periodical repeats carry distinct functions, with the 3rd period being essential for the tension enhancement; (3) Tm mutants lead to HCM by impairing the relaxation on one hand, and lead to DCM by over inhibition of the AM interaction on the other hand. Ca2+ sensitivity is affected by inorganic phosphate, ionic strength, and phosphorylation of constituent proteins; hence it may not be the primary cause of the pathogenesis. Here, we review our current knowledge regarding Tm's effect on the actomyosin interaction and the early molecular pathogenesis of Tm mutation related to HCM, DCM, and LVNC.
Keywords: HCM, DCM, LVNC, Hypertrophic cardiomyopathy, Dilated cardiomyopathy, Left ventricular noncompaction, Cross-bridge kinetics, Elementary steps, Sinusoidal analysis
Introduction
Tropomyosin (Tm) is a regulatory protein of contraction in striated muscles. Together with troponin (Tn) complex, Tm interacts with actin and regulates the actomyosin (AM) interaction. The cooperative Ca2+ activation process in cardiomyocytes involves sequential conformational changes of sarcomeric proteins, which is an extremely complex process. The precise mechanism through which Tm modulates the conformational changes has been one of the vigorously researched subjects for the past 20 years, and numerous proposals have been made to account for the role of Tm in normal cardiac functions as well as in the diseased states. Here, we review our current knowledge on cardiac Tm in three aspects: (1) the mechanism relating to how Tm promotes the AM interaction and enhances force generation; (2) the role of each period of Tm in the thin-filament activation mechanism; and (3) the mechanism how Tm's mutations change the cardiac function and lead to the disease phenotype. There have been several review articles written in these areas recently (Wieczorek et al. 2008; Tardiff 2011; Jagatheesan et al. 2010; Nevzorov and Levitsky 2011; Lehman and Craig 2008; Hitchcock-DeGregori 2008; Kobayashi et al. 2008), hence we try to avoid unnecessary duplications.
Using thin-filament extraction/reconstitution technique and sinusoidal analysis to investigate Tm functions
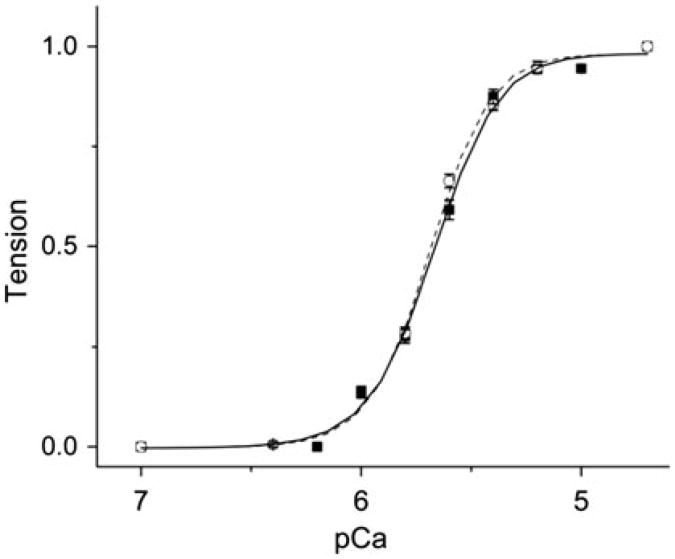
Thin-filament extraction and reconstitution technique has been employed in Kawai laboratory to investigate Tm's functions for more than 10 years since first report (Fujita et al. 2002) and reviewed (Kawai and Ishiwata 2006). This technique, for the first time, offered us an opportunity to replace Tm in cardiac muscle fibers (also called cardiac muscle strips, myocardium) to study the effect of Tm mutants on tension generation and cross-bridge kinetics. Our recent publication on disease related TnT mutants also proved this technique could be applied to replace Tn complex within the thin-filament (Bai et al. 2013). Our previous studies have shown that the thin-filament extraction/reconstitution technique to be a highly reliable method (Fujita et al. 1996, 2002, 2004). The reconstituted fibers fully recover protein compositions as examined by SDS-PAGE, and the sarcomere structure as examined by electronic microscopy (EM). Furthermore, the contractile and regulatory functions of the native muscle fibers are 100 % restored after the reconstitution in muscle fiber studies (Kawai and Ishiwata 2006). Figure 1 shows the pCa-tension plot of the native myocardium and the reconstituted myocardium using bovine cardiac actin, Tm and Tn. The pCa50 value was identical between the native fibers (5.69 ± 0.01) and fibers reconstituted with BVC actin, Tm and Tn (5.68 ± 0.02). The cooperativity of the BVC actin, Tm and Tn-reconstituted myocardium (2.71 ± 0.23) was slightly less than that of the native fibers (3.22 ± 0.18), but with no statistical significance (P>0.1). Other techniques, such as stopped-flow florescence measurements (Heeley et al. 2006), light scattering measurements (Ishii and Lehrer 1990), co-sedimentation (binding affinity) assay (Mirza et al. 2007), in vitro motility assay (Barua et al. 2012; Oguchi et al. 2011), the ATP hydrolysis rate assay (Chang and Potter 2005), and transgenic (Tg) animal models (Muthuchamy et al. 1999) have offered valuable information on Tm's role in the AM binding and cardiac diseases. Compared to other techniques mentioned above, the thin-filament extraction/reconstitution ensures that Tm is placed in the native protein environment without any secondary effects, such as fibrosis, myocyte disarray, and altered post-translation modifications which are inevitable in transgenic animal models with disease causing mutations. Those lethal mutants of Tm which cannot be studied using a transgenic model can be readily studied with the thin-filament extraction/reconstitution technique. The use of muscle fibers allows us to directly measure the tension and its transients, the most important characters and the final outcome of contraction.
Fig. 1.
pCa-tension plot of the native cardiac muscle fibers and fibers reconstituted with BVC actin + Tm + Tn. (circle) Native fibers. (square) Fibers in which thin-filament was removed and reconstituted with bovine cardiac actin, Tm, and Tn. The symbols represent the data points and the curves represent best fit values to the Hill equation. (This figure is re-plotted from Bai et al. 2013)
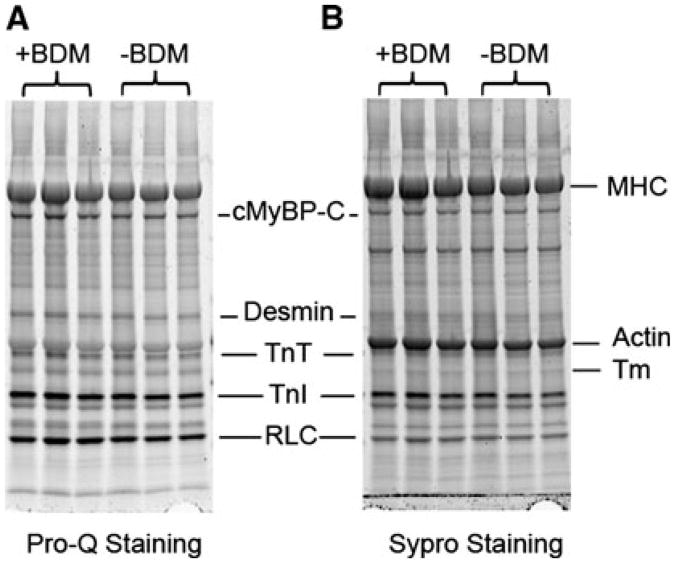
During the skinning and reconstitution processes of bovine cardiac muscle fibers, 30 mM of 2, 3-butanedione 2-monoxime (BDM) has been used to fully inhibit the actin-myosin interaction. However, BDM was reported to be a general protein phosphatase and able to alter protein phosphorylation status in cardiac muscle (Stapleton et al. 1998; Waurick et al. 1999). At the same time, phosphorylation of sarcomeric proteins has been shown to play an important role in maintaining normal cardiac function (Sadayappan et al. 2005; Layland et al. 2005). Therefore, it is necessary to justify the use of BDM in our experiments. We have performed phosphorylation analysis on myofilament proteins in skinned cardiac muscle fibers using SDS-PAGE with Pro-Q diamond and Sypro Ruby stains. A comparison between muscle fibers skinned using the solution containing 30 mM BDM and the ones without BDM is shown in Fig. 2. The results demonstrate that using BDM in the skinning solution has no effect on phosphorylation status of any sarcomeric proteins. We also have reported a similar result by using a 2D gel on the phosphorylation status of αTm when it was treated by 40 mM BDM overnight; the phospholylation level did not change with the BDM treatment (Lu et al. 2010). In conclusion, BDM does not change the phosphorylation status of sarcomeric proteins, hence it does not act as a general phosphatase.
Fig. 2.
The effect of BDM on the phosphorylation status of sarcomeric proteins. (a) Pro-Q diamond stain showing phosphorylated proteins in skinned fibers, (b) Sypro Ruby stain showing the total proteins. In both a and b, left 3 lanes represent fibers skinned using the solution containing 30 mM BDM, and right 3 lanes represent fibers skinned using solution which did not contain BDM
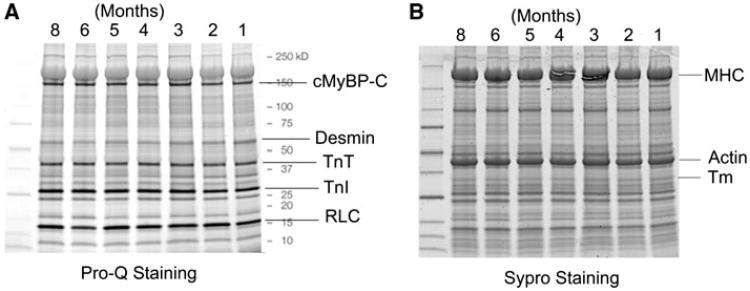
Because we use a new cow heart once every month or so, and results are sometimes compared from different hearts, we should establish that the hearts are not any different from one another. For this reason, myofilament proteins and their phosphorylation levels were compared for the seven available heart muscles in storage for up to 8 months. Figure 3a is Pro-Q diamond stain to show the levels of phosphorylation, and Fig. 3b is the Sypro Ruby stain to show the total proteins. Each lane corresponds to a different heart, which was stored for the specified months. The data in Fig. 3 indicate that the total amount of myofilament proteins, as well as their phosphorylation levels, are invariant across seven different bovine heart muscles we have examined.
Fig. 3.
Phosphorylation analysis of sarcomeric proteins of skinned fibers from 7 different cow hearts, which were stored in the solution containing 30 mM of BDM for the specified durations (1–8 months). a Pro-Q diamond stain showing phosphorylated proteins, and b Sypro Ruby stain showing the total proteins
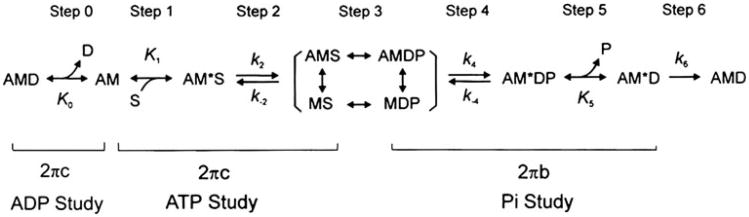
Sinusoidal analysis has been applied to the thin-filament reconstituted fibers to investigate the cross-bridge kinetics (Fujita et al. 2002; Kawai et al. 1993), which allows us to learn how Tm mutants change elementary steps of the cross-bridge cycle. Figure 4 shows the six state cross-bridge model that has been used to characterize elementary steps of WT Tm and its mutants (Bai et al. 2012, 2011).
Fig. 4.
Elementary steps of the cross-bridge cycle. Uppercase letters K indicate the association or equilibrium constants, and lowercase letters k indicate the rate constants. Collectively they are referred to as the “kinetic constants”. A actin, M myosin, D MgADP, S MgATP, and P Pi phosphate
In this analysis method, small amplitude sinusoidal length changes are applied to the reconstituted myocardium at 18 different frequencies (f). The tension transients resulting from the length changes are analyzed and the quantity called complex modulus Y(f) is obtained. Y(f) is the ratio of the stress change to the strain change represented in the frequency domain.
Process B Process C.
| (1) |
where ; 2πb and 2πc(b<c) are the apparent rate constants of processes B and C, respectively; B and C are their respective magnitudes (amplitudes), and H is a constant. Y∞ = H−B + C is the modulus extrapolated to the infinite frequency; all of these are real numbers. B, C, H, and Y∞ have the same units as Y(f) and isometric tension, and thus are usually normalized to Ta. Ta is the active tension generated by the actin-filament reconstituted fibers. In this way, errors associated with the cross-sectional area of fibers can be minimized. The Y(f) measured includes the effect of series compliance, which may (Martyn et al. 2002) or may not (Wang et al. 1999) affect the apparent rate constants. However, the series compliance is not a concern in this report because it is expected not to differ between the mutant and WT proteins.
2πb and 2πc are studied as functions of MgATP (S), Pi (P) and MgADP (D) concentrations. The data are fitted to the following equations derived from Fig. 4 (Kawai and Halvorson 1991).
| (2) |
| (3) |
where
| (4) |
In the cross-bridge scheme (Fig. 4), K1 is the ATP association constant, K0 is the ADP association constant, k2 is the forward rate constant of the cross-bridge detachment step 2, and k-2 is its reversal step. k4 is the forward rate constant of the force generation (isomerization) step 4, and k-4 is its reversal step. K5 is the Pi association constant. σ in Eq. [4] is calculated from K1 and K2 obtained from the MgATP study and S = 5 mM, the condition of the Pi study.
The number of actively cycling cross bridges and the tension supported by each cross bridge can be deduced with the kinetic constants and S, P, and D dependence of isometric tension (Kawai et al. 1993). Tension per cross bridge are used as a direct indicator of the AM binding. When combined with temperature studies, the ionic and hydrophobic surface area changes of the AM interaction can be deduced (Kawai 2003). A part of the interacting surface area change comes from Tm's allosteric effect on actin. These thermodynamic parameters can provide information on dynamic structural changes among Tm, actin, and myosin during isometric contraction.
Tm's allosteric effect on the AM interaction
For more than 40 years, Tm has been known to enhance the AM interaction at the saturating Ca2+ concentration (Bremel and Weber 1972; Lehrer and Morris 1982; Eaton 1976). This is called the positive allosteric effect of Tm on the AM interaction. It has been shown that Tm increases AM binding affinity by three-sevenfold in the presence of Tn and Ca2+ (Williams and Greene 1983). Fujita and his colleagues demonstrated, in skinned fibers, that the isometric tension increased by ∼50 % after reconstitution with Tm and Tn, compared to tension generated by unregulated actin filament and the thick filament (Fujita et al. 2002). In vitro motility assay also demonstrated a similar increase in tension and sliding velocity when Tm and Tn were reconstituted (Oguchi et al. 2011; Bing et al. 2000a; Kawai et al. 2006; Gordon et al. 1998; VanBuren et al. 1999). From a structural point of view, this allosteric effect may happen because Tm binding to the AM complex recruits more hydrophobic residues to the AM interface, leading to an enhanced AM binding (Holmes 1995). At the low Ca2+ concentration in the presence of Tn, Tm sterically blocks the myosin binding site of actin (Lehman et al. 2009; Huxley 1973). At saturating Ca2+ concentration, Tm moves away from the blocked position and allows the initial binding between actin and myosin to take place (Lehman et al. 2009). This binding changes the actin conformation, which in turn allows a further azimuthal shift of Tm's position; this shift promotes the stronger AM binding and may recruit more hydrophoic residues into the AM interface. AM consequently achieves the strong binding state, in which cross-bridges are fully active (Lehman et al. 2009; Holmes 1995). Structural studies have shown that most amino acid residues in the AM interface are hydrophobic (Behrmann et al. 2012; Holmes et al. 2004; Rayment et al. 1993) and there is thermodynamic evidence to this (Zhao and Kawai 1994; Kawai 2003). It has been thought that Tm directly interacts with myosin in the rigor state and stabilize the AM interaction (Behrmann et al. 2012).
The initial binding between actin and myosin involves ionic amino acid residues and it is presumably a “weak” interaction. This is convenient because the ionic interaction takes place over a long distance. It has been known for some time that actin's N-terminus has four negative charges (Asp-1, Glu-2, Asp-3, Glu-4) (Sutoh et al. 1991), which interact with myosin's loop 2 (residues 633–642) with 5 Lys residues (Furch et al. 1998). Another ionic interaction between next actin's Glu-99 and myosin's positively charged segment Lys-567 through His-578 is possible (Bookwalter and Trybus 2006; Holmes et al. 2004). The actin sequence is based on rabbit skeletal actin (virtually the same as human cardiac actin), and the myosin sequence on chicken skeletal myosin. The ionic interaction is followed by the hydrophobic and stereospecific interaction, which is only effective at a short distance, and corresponds to the “strong” interaction. The primary binding site on actin consists of helix-turn-helix (residues 337–351) in subdomain 1, and that on myosin is helix-turn-helix of the lower 50 K domain (residues 526–559); The secondary binding site on actin consists of segments Ala-26 through Ala-29 and Pro-333 through Tyr-337, and that on myosin is in its upper 50 K domain called “cardiomyopathy loop” (around residues 405–414) (Holmes et al. 2004). The weak interaction between N-terminus of actin and the loop 2 of myosin is known to induce the strong interaction subsequently, because a decrease in the number of N-terminal negative charges of actin results in a decrease in isometric tension and stiffness (Lu et al. 2005). The strong interaction is detected as isometric tension or stiffness measured by low frequency (<1 kHz). Unfortunately, how Tm affects these interactions, or at which point the “closed” to “open” transition occurs, is not yet known, because the AM interaction is based on observations without Tm, except for the recent study of the rigor complex between actin, myosin, and Tm (Behrmann et al. 2012).
Physiological and mechanical evidence for the enhanced hydrophobic interaction by Tm comes from temperature studies of skinned cardiac muscle fibers. Ca2+ activatable tension increases with an increase in temperature (called endothermic force generation) (Coupland et al. 2001; Zhao and Kawai 1994; Ranatunga 1996), because of an increase in the hydrophobic interaction which requires heat to be absorbed (Kawai 2003). When unregulated and regulated cardiac muscle fibers are compared, we reported that the slope of the temperature-tension plot increased by ∼3 times upon reconstitution of Tm and Tn, indicating increased hydrophobic interaction of AM (Fujita and Kawai 2002). Sinusoidal analysis also demonstrated that tension supported by each cross-bridge was enhanced to ∼2 times upon the reconstitution of Tm/Tn to the actin-filament reconstituted muscle fibers (Fujita et al. 2002). This increase may come in part from the direct Tm-myosin interaction that is known to occur during the rigor state (Behrmann et al. 2012). Further thermodynamic analysis under different temperatures provided evidence that in the presence of Tm/Tn, the activation energy of 2πc (apparent rate constant of fast tension recovery step) decreased significantly compared to that in the absence of Tm/Tn (Fujita and Kawai 2002), indicating that the elementary step of the cross-bridge cycle associated with 2πc (cross-bridge detachment step 2, Fig. 1) becomes easier to occur in the presence of Tm/Tn than in their absence.
Tm's positive allosteric effect on AM binding can also be induced without Tn. In the unregulated cardiac muscle fibers (no Tm/Tn), a reconstitution with Tm increases the isometric tension by ∼20 % (Fujita et al. 2004). Further reconstitution with Tn increases the tension by another ∼25 % (Fujita et al. 2004, 2002). In the absence of Tm/Tn, cross bridge detachment rate was decreased and the equilibrium constant of force generation step (K4, Fig. 1) was increased, leading to ∼30 % more strongly binding cross-bridges compared to that in the presence of Tm/Tn (Fujita et al. 2002). However, adding Tm alone to unregulated cardiac muscle fibers shifts the number of active cycling cross bridges to the same as that in the presence of both Tm and Tn (Fujita et al. 2004). These results indicate that Tm is the key protein in the positive allosteric effect, and an addition of the Tn complex further enhances this effect as an amplifier.
Isoforms and post-translational modification of Tm in cardiac muscle fibers
In striated muscles, there are four major isoforms of Tm: α-Tm, β-Tm, γ-Tm, and κ-Tm. However, γ-Tm is not expressed in cardiomyocytes. While β-Tm is expressed during the development of the heart, α-Tm is the predominant isoform in adult cardiac muscles (Muthuchamy et al. 1993). A recent study showed that in adult human heart, there are 90–94 % α-Tm, 3–5 % β-Tm, and 3–5 % κ-Tm. κ-Tm is a novel Tm isoform which is generated from alternative splicing of TPM1 gene (Rajan et al. 2010). Different isoforms of Tm bear functional differences despite of the fact that they are highly homologous in their amino acid sequences. κ-Tm isoform showed almost no actin binding affinity, which indicates that the presence and expression level of this isoform may be important for regulating the overall Tm function (Rajan et al. 2010). This isoform also decreased Ca2+ sensitivity and caused cardiac dysfunction in transgenic mice expressing 90 % κ-Tm (Rajan et al. 2010). Replacing α-Tm with β-Tm in skinned cardiac muscle fibers resulted in an increased Ca2+ sensitivity and a decreased cooperativity (Lu et al. 2010). Transgenic mice expressing 55 % β-Tm showed no change in cardiac morphology and function, but also caused an increase in myofilament Ca2+ sensitivity (Muthuchamy et al. 1995). Transgenic mice expressing 50 % γ-Tm and 50 % α-Tm caused both systolic and diastolic dysfunction and a decrease in Ca2+ sensitivity (Pieples et al. 2002). Structural analysis showed that different Tm isoform composition shifted the position and movement of Tm's coiled-coil on the actin filament (Lehman et al. 2009, 2000). However, except for the recent report that κ-Tm expression is increased in DCM patients' heart (Karam et al. 2011), it is still not clear how the ratio among different isoform of Tm in the human heart affects normal cardiac functions or development of diseases.
Phosphorylation of sarcomeric proteins has been shown to be an important mechanism to modulate contraction, relaxation, and Ca2+ activation processes (Solaro and Kobayashi 2011). Both α-Tm and β-Tm have a potential phosphorylation site at Ser-283, a region critical for the Tm-TnT interaction, and the head-to-tail overlap between adjacent Tm's coiled-coil on the actin-filament (Mak et al. 1978; Heeley 1994). α-Tm phosphorylation may be essential in heart development as majority of α-Tm is phosphorylated during the fetal stage, whereas only 10–30 % of α-Tm is phosphorylated in adult cardiomyocytes (Heeley et al. 1982; Yuan et al. 2008). β-Tm, although carrying 86 % sequence identity with α-Tm, is not shown to be phosphorylated in either fetal stage or adult cardiomyocytes (Heeley et al. 1982). The functional effect of α-Tm phosphorylation was studied using different approaches and the results varied accordingly. In vitro studies showed that phosphorylation of α-Tm had no effect on Tm-actin binding (Heeley et al. 1989), but promoted the head-to-tail interaction between two neighboring Tms (Rao et al. 2009). Pseudo-phosphorylation of α-Tm is shown to decrease the relaxation rate in myofibrils (Nixon et al. 2012). Skinned fiber study showed that 100 % phosphorylated α-Tm increased the Ca2+ sensitivity without changing the contractility compared to the cardiac muscle fiber containing 100 % unphosphorylated α-Tm (Lu et al. 2010). Sinusoidal analysis showed that the cross-bridge kinetics during the steady state remained largely unchanged by the phosphorylation of α-Tm (Lu et al. 2010). Tg mice bearing α-Tm S283A mutation (mimic unphosphorylated state) showed mild hypertrophy without a change in cardiac function, Ca2+ sensitivity, or cooperativity compared to non Tg mice (Schulz et al. 2012). From our thin-filament reconstitution experiments, we conclude that the Tm phosphorylation is a fast method of increasing the Ca2+ sensitivity, whereas isoform switch from α-Tm to β-Tm is a slow method of increasing the Ca2+ sensitivity (Lu et al. 2010).
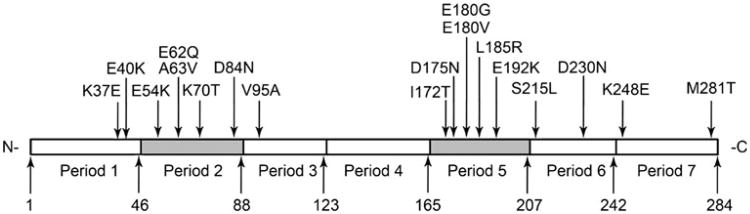
Function of different periods of Tm on cooperative activation and disease
Tm molecule has 7 loosely similar “periods” (P1-P7, Fig. 5). Each Tm dimer interacts with 7 adjacent actin molecules in the thin-filament, and each period is defined as a region along the Tm molecule which interacts with one of the 7 adjacent actin molecules (McLachlan and Stewart 1975). The length of each period varies from 35 to 46 residues. Each of these 7 periods has been shown to contribute differently to the Tm-actin binding and Tm's regulatory role in the AM binding (Hitchcock-DeGregori et al. 2002). Tm's one period deletion mutants (Δ2, Δ3, Δ4, Δ5, and Δ6) have been generated using E. coli to study the function of each period. In vitro motility assay showed that Δ2, Δ3, and Δ6 decreased sliding velocity, and Δ2 and Δ3 decreased force compared to those of WT; Δ6 caused a significantly impaired relaxation (Oguchi et al. 2011). In the thin-filament reconstituted myocardium, Δ2 showed no effect on force generation, but Δ3 decreased the maximal isometric force by ∼40 % compared to that of WT (Lu et al. 2003; Kawai et al. 2009). Temperature studies suggested that Δ3 failed to positively promote the hydrophobic interaction between actin and myosin, therefore leading to decreased force/cross-bridge and decreased isometric tension (Kawai et al. 2009). Sinusoidal analysis showed Tm Δ3 increased K4 (equilibrium constant of force generation step, Fig. 3) to ∼2× compared to that of WT. Deletion of the period 2 mildly decreased the Ca2+ sensitivity, and deletion of the period 3 significantly increased the Ca2+ sensitivity. However, deleting periods 2 and 3 simultaneously decreased Ca2+ sensitivity (Lu et al. 2003). Deletion of the period 4 decreased the AM ATPase rate (Hitchcock-DeGregori et al. 2002). Deletion of the period 5 totally destroyed Tm's binding affinity to actin, indicating the role of the period 5 in the actin-Tm interaction (Hitchcock-DeGregori et al. 2002). In summary, the period 3 is essential to enhance the hydrophobic interaction between actin and myosin; the period 5 is essential for actin binding; and the period 6 is essential for Tm's inhibitory role under the relaxed state. Period 2 seems to have no significant effects compared to periods 3, 5, and 6, therefore, this period may have only a limited function.
Fig. 5.
Seven periodic repeats of α-tropomyosin and the location of mutations that are known to lead to cardiomyopathies in humans. The residue number of each period's C-terminus is also indicated
The significance of individual periods of Tm may also be reflected by the distribution of disease causing Tm mutations in each period (Fig. 5; Table 1). There are 5 known HCM causing Tm mutations which are located in period 5a (each period's N-terminal half is termed as “a”, and C-terminal half as “b”), which has been shown to be critical for the Tm-actin interaction (Hitchcock-DeGregori et al. 2002). Several charged residues in Tm period 5a, which are in close proximity to these 5 disease causing mutations, have been recently reported to be directly involved in the Tm-myosin interface during the rigor state (Behrmann et al. 2012). Although in vitro studies indicated the deletion of the period 2, compared with the deletion of other periods, had the smallest impact on Tm's functions, 5 mutations in period 2 have been related to DCM and HCM. There is only 1 HCM related mutation identified in period 3, which is shown to be the key of Tm's positive allosteric effect on the AM interaction. So far, there is no known disease causing mutations in period 4 of Tm. It is plausible that more mutations were retained in the period that is not important for the function of the protein, whereas fewer mutations survived if they occurred at the critical functional domain of the protein in the course of evolution. In summary, Tm mutations in the same period can result in the DCM, HCM, or LVNC phenotype (Table 1), indicating that the disease pathogenesis doesn't depend on the internal Tm period. The only exception to this rule is the period 5a, in which all known mutations result in HCM.
Table 1. Inherited cardiomyopathy causing mutations in α-Tm.
| Mutation | Phenotype | Period | Heptad | Function | Reference |
|---|---|---|---|---|---|
| K37E | LVNC | 1b | b | Actin binding | (Chang et al. 2011) |
| E40K | DCM | 1b | e | Actin binding | (Olson et al. 2001) |
| E54K | DCM | 2a | e | Actin binding | (Olson et al. 2001) |
| E62Q | HCM | 2a | f | (Jongbloed et al. 2003) | |
| A63V | HCM | 2a | g | (Nakajima-Taniguchi et al. 1995; Yamauchi-Takihara et al. 1996) | |
| K70T | HCM | 2b | g | (Yamauchi-Takihara et al. 1996) | |
| D84N | DCM | 2b | g | Actin binding | (van de Meerakker et al. 2012) |
| V95A | HCM | 3a | d | (Karibe et al. 2001) | |
| I172T | HCM | 5a | d | (Van Driest et al. 2003) | |
| D175N | HCM | 5a | g | TnT Interactiion; actin-Tm-myosin interface | (Yamauchi-Takihara et al. 1996; Andersen et al. 2009; Garcia-Castro et al. 2009; Thierfelder et al. 1994) |
| E180G | HCM | 5a | e | TnT Interaction; actin-Tm-myosin interface | (Thierfelder et al. 1994) |
| E180V* | HCM* | 5a | e | TnT Interaction; actin-Tm-myosin interface | (Regitz-Zagrosek et al. 2000) |
| L185R | HCM | 5a | c | TnT Interaction | (Van Driest et al. 2003; Earing et al. 2003) |
| E192K | LVNC | 5b | c | (Probst et al. 2011) | |
| S215L | HCM | 6a | e | (Morita et al. 2008) | |
| D230N | DCM | 6b | f | (Lakdawala et al. 2010) | |
| K248E | LVNC | 7a | c | (Probst et al. 2011) | |
| M281T | HCM | 7b | a | Tm head-to-tail binding | (Van Driest et al. 2003) |
18 mutations in Tm have been shown to cause primary cardiomyopathy in humans. 11 of these mutations lead to HCM, 4 lead to DCM, and 3 lead to LVNC
DCM dilated cardiomyopathy, HCM hypertrophic cardiomyopathy, LVNC left ventricular noncompaction of the myocardium
Patient bearing this Tm mutation was diagnosed to have HCM until 30 years old. The symptom progressively turned to DCM-like at the age of 40, showing severe systolic dysfunction and thinning of the interventricular septum (Regitz-Zagrosek et al. 2000)
Molecular pathogenesis of Tm mutations leading to inherited cardiomyopathies
So far, 18 Tm mutations are known to cause HCM, DCM, or LVNC in humans. These mutations scatter in different periods of Tm and interact with different Tm binding proteins (Table 1; Fig. 5). However, despite extensive investigations, the mechanism of how the mutations within Tm lead to various disease phenotypes remains unknown.
HCM Tm mutations
Hypertrophic cardiomyopathy (HCM) is a disease of the heart muscle characterized by the abnormally thickened left ventricular wall and interventricular septum. Familial forms of HCM are the most common inherited heart disease caused by sarcomeric protein mutations (Elliott and McKenna 2004; Ho and Seidman 2006). HCM prevalence is ∼0.2 % in the total population and is the leading cause of sudden death in young adults (Ommen and Gersh 2009; Maron et al. 2003). The most notable clinical manifestation of HCM is left ventricular hypertrophy. However, individuals carrying HCM causing genes can remain asymptomatic, or can experience severe heart failure and sudden cardiac death (Marian and Roberts 2001; Barron 1999). So far, more than 400 mutations within the sarcomeric proteins have been identified to be associated with HCM, 11 of which occur in Tm. In different countries, Tm mutation accounts for 5–11 % of all familial HCM cases (Tardiff 2005; Nakajima-Taniguchi et al. 1995; Yamauchi-Takihara et al. 1996; Jaaskelainen et al. 1998). This difference in prevalenceis likely due to the “founder” effect in the specific population (Wieczorek et al. 2008). The phenotype of the Tm mutation related HCM is generally benign, but also it can be severe depending on the specific mutation (Tardiff 2005; Nakajima-Taniguchi et al. 1995; Yamauchi-Takihara et al. 1996; Jaaskelainen et al. 1998).
Of the 11 HCM Tm mutations, six of them (E62Q, K70T, D175N, E180G, E180V, and L185R) cause charge changes. Because Tm-actin interaction is largely electrostatic, the charge changes are considered critical to cause abnormal Tm-actin interaction (Brown and Cohen 2005; Lorenz et al. 1995). Four of the mutations (D175N, E180G, E180V, and L185R) are located in or close proximity to the region involved in the actin-Tm-myosin interface and possibly interfere with the electrostatic interaction (Behrmann et al. 2012). This region of Tm has also been shown to interact with TnT and plays a key role in the electrostatic interaction between Tm and actin (Barua et al. 2011). Other five HCM mutations which do not cause a charge change in Tm (A63V, V95A, I172T, S215L and M281T) all cause changes in the hydrophobicity. Hydrophobic interaction is critical for stabilizing the Tm's coiled-coil structure. It has been shown that Ala clusters in Tm molecule is critical for maintaining its flexibility and replacing Ala residues in the a and d position of the heptad repeat with more hydrophobic residues significantly improve the stability of Tm's coiled-coil (Singh and Hitchcock-DeGregori 2003). Hydrophobic interaction is also involved in Tm (period 5 and 6) and actin binding (Brown et al. 2005; Miki et al. 2011).
The underlying molecular mechanism how these Tm mutants lead to HCM phenotype has been extensively investigated. The most studied mutants include V95A, D175N and E180G. Structural analysis has shown that A63V, K70T, D175N and E180G decreased molecular stability (Ly and Lehrer 2012; Heller et al. 2003). Fluorescence polarization measurement showed that D175N and E180G shifted the Tm's position in the thin-filament towards the open position (Borovikav et al. 2011). Solution studies indicated that V95A decreased ATPase activity (Mathur et al. 2011). D175N and E180G reduced the Tm-actin and Tm-TnT affinities, but they exhibited no effect on the ATP hydrolysis rate (Mathur et al. 2011). In vitro motility assay also showed that D175N and E180G increased the Ca2+ sensitivity in the fraction of motile filaments (Bing et al. 2000b).
Tg mouse models have been created for D175N and E180G (Prabhakar et al. 2001, 2003,Muthuchamy et al. 1999). D175N mutation caused only mild hypertrophic phenotype: increased end diastolic pressure and increased Ca2+ sensitivity in skinned fiber preparations (Muthuchamy et al. 1999). E180G caused more severe and progressive hypertrophy and fibrosis, leading to cardiac death in 4.5–6 months (Prabhakar et al. 2001). E180G mice also showed increased end diastolic pressure and Ca2+ sensitivity (Prabhakar et al. 2001). Both mutants caused a significant decrease in contraction and relaxation rate (Muthuchamy et al. 1999; Prabhakar et al. 2001). A human patient study also indicated D175N to be one of the benign HCM-causing mutations based on the prognosis and symptom (Van Driest et al. 2002).
HCM Tm mutant proteins V95A, D175N, and E180G were incorporated into skinned cardiac muscle fibers by using the thin-filament removal and reconstitution protocol to investigate the underlying molecular pathogenesis at the earliest stage (Bai et al. 2011). Our results demonstrated that all these three Tm mutants exhibit abnormal increase in the number of active cycling cross-bridges at pCa 8, leading to the increased tension at the relaxed state, which causes diastolic dysfunction (Bai et al. 2011). Diastolic pressure overload in the affected myocardium may stimulate cardiac growth, causing abnormal release of angiotensin II and aldosterone (Sadoshima et al. 1993), and eventually lead to the HCM phenotype: left ventricular hypertrophy (Koide et al. 1999) and abnormal extracellular matrix formation (Sadoshima et al. 1993). V95A and D175N also cause decreased Ca2+ activatable tension, which contributes to a systolic problem. V95A and E180G significantly increase the Ca2+ sensitivity compared to that of WT and D175N. Our results also demonstrated that these Tm mutations altered the steady-state cross-bridge kinetics, with V95A showed the most severe effect (Bai et al. 2011). Interestingly, V95A also exhibited severe symptom and poor prognosis in human patients (Karibe et al. 2001). By comparing the observed parameters (Table 1 in (Bai et al. 2011)) and results from human patient studies (Karibe et al. 2001; Coviello et al. 1997), we conclude that both impaired relaxation and elevated Ca2+ sensitivity cause diastolic problem leading to the HCM phenotype. Other changes in contractility and cross-bridge kinetics may also contribute to the severity of HCM symptoms and disease prognosis.
DCM Tm mutations
Dilated cardiomyopathy (DCM) is characterized by dilated left ventricle and systolic dysfunction (Fuster et al. 1981). DCM affects ∼36.5 people in 100,000 and causes ∼10,000 death annually (Gillum 1986; Codd et al. 1989). It is estimated that 25–30 % of DCM cases are caused by genetic defects (Grunig et al. 1998; Zimmerman et al. 2010; Michels et al. 1992; Marston 2011). Mutations in more than 20 proteins, including β-myosin heavy chain, cardiac myosin binding protein-C (cMyBP-C), α-actin, troponin, titin, desmin, vinculin, and Tm, have been shown to cause DCM in humans (Hershberger et al. 2009). However, the number of DCM causing mutations is small compared to that causing HCM, hence there are fewer studies on DCM related mutations than its HCM counterpart. So far, there are only four Tm mutations which have been identified to cause DCM: E40K, E54K, D84N and D230N (Table 1; Fig. 5). All four DCM related Tm mutations are located on the surface of Tm coiled-coil and carry a charge change. Both E40 and E54 are located at the e position of the heptad repeat and respectively interact with R35 (g) and K49 (g) on the other Tm chain to stabilize the Tm's coiled-coil structure (Brown et al. 2001). D84 is located at the g position of the heptad repeat and interacts with E75 (e) of the other Tm chain, which increases the flexibility of Tm's coiled-coil (Whitby and Phillips 2000). D230 is located in the outer surface of Tm (f position) and possibly involved in the cTnT interaction (Tardiff 2011). E40K, E54K and D84N are also located in the actin-binding motif of the Tm (McLachlan and Stewart 1976). Furthermore, E40K is located in Tm period 1b, which interacts with actin in the presence of Ca2+, whereas E54K is located in Tm period 2a, which interacts with actin in the absence of Ca2+ (Holthauzen et al. 2004). Previous studies showed that E40K and E54K decreased the Ca2+ sensitivity of myocardium (Chang et al. 2005), and the decreased Ca2+ sensitivity was thought to be critical to DCM pathogenesis (Willott et al. 2010; Mirza et al. 2007). In a different study, E40K caused a decreased maximum ATPase activity, but E54K showed only diminished Tm-actin affinity in the absence of Tn and myosin (other parameters remained unchanged) (Mirza et al. 2007). Co-sedimentation assay showed that D84N decreased the Tm's affinity to F-actin by 25 % (van de Meerakker et al. 2012). ATPase assay showed that D230N led to a significant decrease in both maximal ATPase activity and Ca2+ sensitivity (Lakdawala et al. 2010).
To investigate the in vivo effects of these DCM causing Tm mutants, Tg mouse models have been generated for E54K and D230N mutations (Rajan et al. 2007; Tal et al. 2012). E54K mice showed typical DCM phenotype with both systolic and diastolic dysfunction. Isolated cardiac myofibers from E54K mice showed significantly decreased tension generation and Ca2+ sensitivity (Rajan et al. 2007). D230N mice showed early dilation and systolic failure without any histological changes (Tal et al. 2012). Secondary effect of these mutations on the transgenic models was also studied. Real-time RT-PCR proved that the expression level of β-myosin heavy chain and skeletal actin was increased in E54K mice; however, the expression level of two Ca2+ handling proteins, sarcoplasmic reticulum Ca2+ ATPase and ryanodine receptor, was decreased in E54K mice (Rajan et al. 2007). Isolated myocytes from D230N mice showed no changes in Ca2+ transients, indicating this mutation has no effect on the Ca2+ handling proteins (Tal et al. 2012).
We have also studied Tm mutation E40K and E54K using thin-filament reconstituted myocardium (Bai et al. 2012). Our results demonstrated that E40K and E54K didn't change the Ca2+ sensitivity compared to that of the WT, which was mainly dues to 8 mM phosphate (Pi) included in the activating solution (see below). However, E40K and E54K caused an over-inhibition effect on force generation and decreased the maximal tension (pCa 4.66) by ∼20 % compared to that of the WT. This over-inhibition effect also decreased tension at pCa 7.0 by ∼50 % compared to that of the WT, from which we conclude that the over-inhibition occurs regardless of [Ca2+]. Sinusoidal analysis demonstrated that under pCa 4.66, E40K decreased tension by decreasing the number of actively cycling cross-bridges without changing force/cross-bridge, whereas E54K decreased tension both by decreasing the number of actively cycling cross-bridges and force/cross-bridge. At pCa 7.0, both E40K and E54K decreased tension by decreasing the number of actively cycling cross-bridges. These results demonstrated that E40K and E54K can directly decrease the isometric tension of the myocardium, leading to the systolic dysfunction. The decreased tension under pCa 7.0 may contribute to the altered filling pattern of the left ventricle in DCM patients. Kinetic analysis showed that both E40K and E54K changed the steady state cross-bridge kinetics, with E40K had more severe effect than E54K.
Our results demonstrated that E40K and E54K caused no change in Ca2+ sensitivity. This result is at variance with the generally accepted concept that DCM causing sarcomeric protein mutations lead to a decreased Ca2+ sensitivity in the affected myocardium. This inconsistency can be resolved in part by different activating solutions used in different laboratories. 8 mM phosphate (Pi) was used in our activating solutions, while other investigators did not use Pi in their solutions (Chang et al. 2005; Mirza et al. 2005, 2007; Rajan et al. 2007). The Pi concentration under physiological conditions in cardiomyoctes has been reported to be ∼6 mM (Opie et al. 1971; Roth et al. 1989). Therefore, adding Pi in the activation solution better mimics the physiological condition in the heart, as described (Bai et al. 2012). The inconsistency can also be caused by different approaches used. Actin-Tm-myosin S1 complex used for the ATPase assay in solution may be too simple to account for the complex interactions which take place in cardiomyocytes. Isolated muscle fibers from Tg models may be a better system to study the effect of Tm mutations on cardiac performance. At the same time, the Tg models generally involves many secondary effects caused by the mutation, including altered protein expression levels (Rajan et al. 2007), and altered post-translational modifications (Sfichi-Duke et al. 2010). These secondary effects have been shown to significantly alter muscle regulation and performances (Jacques et al. 2008; Layland et al. 2005; Sadayappan et al. 2005), making it difficult to identify the primary effect of the mutation. In fact, Tardiff (2011) states in her recent review said that “the predicted result of focusing on the earliest stages of cardiac remodeling (before the activation of potentially complex signaling cascades) would be that the observed molecular and biophysical effects of thin filament mutations would provide a more robust mechanistic link between genotype and phenotype.” Using thin-filament reconstituted myocardium, we can assure that only Tm is replaced with mutants, and all the other components in the myocardium remain unchanged (Kawai and Ishiwata 2006). Therefore, direct and primary effects of the mutation on regulation and contraction can be studied without complications. Altogether, we conclude that the over inhibition of the AM interaction is the immediate and primary cause of DCM in E40K and E54K Tm, that causes systolic (and perhaps diastolic) problems. A decreased Ca2+ sensitivity in transgenic models may not be the major cause of DCM in these mutations, because this phenomenon was observed in the absence of Pi, which is unphysiological.
Tm mutation in left ventricular noncompaction (LVNC)
LVNC occurs with both DCM and HCM and is classified as the primary cardiomyopathy by The American Heart Association and The European Society of Cardiology. The major symptom of LVNC involves numerous and prominent ventricular trabeculations and deep intertrabecular recesses (Sarma et al. 2010; Chin et al. 1990; Oechslin et al. 2000). LVNC can be caused by genetic mutations of proteins including taffazin, β-dystrobrevin, lamin A/C, myosin, and cMyBP-C (Zaragoza et al. 2007). Recently, 3 mutations of Tm (E37K, E192K, and K248E) have been identified to cause LVNC in humans (Chang et al. 2011; Probst et al. 2011). All three mutants are located at the outer surface of Tm's coiled-coil (E37K is at the b position of the heptad repeat, whereas E192K and K248E are located at the c position) and bear an opposite charge change. The functional consequence of these Tm mutants at the molecular level has not been worked out yet. It would be very interesting to investigate the molecular mechanism of how these Tm mutations lead to a specific phenotype, which is neither DCM nor HCM.
Tm mutations lead to DCM or HCM through different molecular mechanisms
The genotype-phenotype connection between a Tm mutation and familial cardiomyopathy (DCM or HCM) has been established by patient screening and Tg models. However, how these distinctively different phenotypes originate from mutations within the same protein or even the same region of the protein remains unknown. Mechanical stress caused by these mutations has been generally considered the key to the cardiac remodeling in HCM and DCM pathogenesis (Bernardo et al. 2010). Tm plays the key role in the mechanical performance of the heart, therefore, it is natural to assume different mutations in Tm leads to different mechanical changes in myocardium and finally lead to different disease phenotypes.
It has been suggested that mutations that increase the Ca2+ sensitivity lead to HCM, and mutations that decrease the Ca2+ sensitivity lead to DCM (Willott et al. 2010). In the case of Tm, both Tg mice models and in vitro studies seem to support this hypothesis as D175N and E180G increased Ca2+ sensitivity and E40K, E54K and D230N decreased Ca2+ sensitivity (Muthuchamy et al. 1999; Prabhakar et al. 2003; Rajan et al. 2007; Bing et al. 2000b; Mirza et al. 2007; Chang et al. 2005). However, studies using human DCM myocardium showed an increased Ca2+ sensitivity (Wolff et al. 1996; Lee et al. 2010). A recent Tg study provided solid counter evidence for this hypothesis: Tg mice bearing one TnT mutation, which caused significant increased Ca2+ sensitivity in skinned fibers, showed no change in phenotype or survival rate compared to the non-Tg controls (Gollapudi et al. 2012). Our study also showed that Tm mutants E40K (DCM), E54K (DCM), D175N (HCM) and cardiac TnT mutant ΔK210 (DCM) had no effect on Ca2+ sensitivity under the physiological condition (Bai et al. 2011, 2012, 2013). Furthermore, it has been demonstrated that Ca2+ sensitivity can be regulated by many other factors, such as the mutant protein expression level (McConnell et al. 1999), altered phosphorylation status of sarcomeric proteins (Sadayappan et al. 2005), cardiac remodeling (Wang et al. 1994), and even exercise (Wisloff et al. 2002). Although most disease-related sacomeric-protein mutations have been found to associate with Ca2+ sensitivity changes, it is still too early to assume that increasing or decreasing Ca2+ sensitivity will lead to a specific disease phenotype. A most recent study also proved that E54K didn't change the Ca2+ sensitivity and D230N even increased the Ca2+ sensitivity (Memo et al. 2013). Therefore, we conclude that in disease related Tm mutations, Ca2+ sensitivity change may not be the key factor in the pathogenesis.
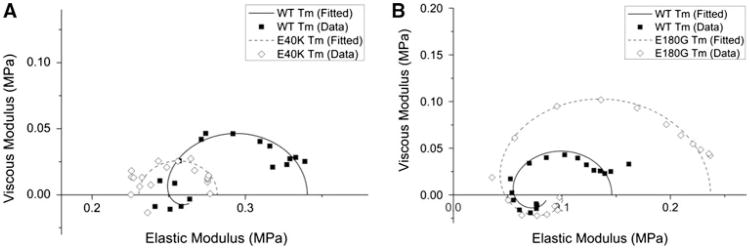
Using thin-filament reconstitution protocol, we were able to compare the immediate effect of HCM and DCM related Tm mutations on cardiac contraction and regulation (Table 1 in Ref (Bai et al. 2012)). This comparison showed that the effects of each individual mutation were diverse and complex, which suggest that the underlying molecular pathogenesis is different for each mutation. However, we have pointed out a distinct difference between HCM and DCM related Tm mutants: HCM related mutants lead to an insufficient inhibition under the relaxed condition, and the DCM related mutants lead to an over-inhibition under both relaxed state and activated state. In Fig. 6, the Nyquist plots of Tm mutants E40K and E180G collected at pCa 8.0 (relaxing condition) are shown and compared to that of the WT Tm. It is clear that DCM and HCM related Tm mutants showed distinctively different effects in the Nyquist plots under the relaxing condition. Figure 6a shows that, in a DCM Tm mutant (E40K) the diameter of the Nyquist plot decreased to ∼50 %, indicating the number of active cycling cross-bridges decreased to ∼50 % compared to the WT; we found similar effects on another DCM mutant E54K (Bai et al. 2012). Figrue 6b shows that, in an HCM Tm mutant (E180G) the diameter of the Nyquist plot significantly increased to ∼2–2.3×, indicating the number of active cycling cross-bridges increased to ∼2–2.3× compared to the WT; we found similar effects on other HCM mutants V95A and D175N (Bai et al. 2011). Insufficient inhibition by the HCM mutants under the relaxed condition implies Tm's “blocked” position on actin is changed and the myosin binding site of actin is partially exposed, leading to an incomplete relaxation. This impaired relaxation of myocardium can cause a permanent pressure overload in the heart and lead to a pathological change to result in the hypertrophy (Bernardo et al. 2010). Over-inhibition by the DCM mutants indicates Tm's “blocked” position is more difficult to become “closed” or “open” state, leading to an impaired force generation, consequently a DCM phenotype. Similar conclusions have also been arrived when examining HCM and DCM causing myosin mutations: HCM causing mutations enhanced myosin function, but DCM causing mutations depressed myosin function (Debold et al. 2007). We believe this difference (over-inhibition in DCM and insufficient inhibition in HCM) contributes significantly to the disease pathogenesis.
Fig. 6.
Nyquist plots of Tm mutants (dashed line) and WT (solid line) at 25 °C and pCa 8.0. Frequency range used is 0.13–100 Hz. The frequency increases in the clockwise direction, and the rightmost point corresponds to 100 Hz. Note the difference in scales for both axes between panel a and b. (This figure is re-plotted from Bai et al. 2011, 2012)
Both DCM and HCM Tm mutants changed the kinetic constants of elementary steps of the cross-bridge cycle. However, this effect largely depended on individual mutants, and not on a specific disease phenotype (Table 1 in Ref (Bai et al. 2012)). Among HCM mutants, V95A disturbed the cross-bridge kinetics by promoting ADP dissociation, weakening ATP binding, diminishing cross-bridge detachment, and accelerating Pi release (decreased K0, K1, K2, and K5); In D175N, K0 and K2 did not change; In E180G, K0 increased, but K2 did not change (Bai et al. 2011). Among DCM mutants, E40K changed all equilibrium constants (decrease in K0, K4, and K5; increase in K1 and K2); but E54K only promoted ATP binding to myosin (increased K1) (Bai et al. 2012). In the case of V95A, the severely disturbed cross-bridge kinetics coincided with severe disease symptoms and poor prognosis (Karibe et al. 2001). Our results demonstrate that the cross-bridge kinetics are affected differently among the same disease phenotype.
Conclusions
The thin-filament removal and reconstitution technique has been shown to be a powerful method to investigate the immediate and early effect of mutation on cardiac muscle contraction and regulation before the complex signaling cascades start. We found that, in Tm mutants, inadequate inhibition of the AM interaction by regulatory proteins during diastole causes HCM, and over-inhibition of the AM interaction during systole causes DCM. Ca2+ sensitivity may not be the primary cause of the pathogenesis, because this is affected by the amount of inorganic phosphate in the myocyte, ionic strength, and phosphorylation status of myofilament proteins. The sinusoidal analysis technique is a good method to characterize the cross-bridge kinetics and the elementary steps of the cross-bridge cycle. Both methods are valuable tools to investigate the molecular mechanisms of contraction.
Acknowledgments
This work was supported by grant from the NIH (HL70041) to MK. The content of this study is solely the responsibility of the authors, and does not necessarily represent the official view of the awarding organization.
Contributor Information
Fan Bai, Email: masataka-kawai@uiowa.edu.
Li Wang, Email: fan-bai@uiowa.edu.
Masataka Kawai, Email: li-wang-1@uiowa.edu.
References
- Andersen PS, Havndrup O, Hougs L, Sorensen KM, Jensen M, Larsen LA, Hedley P, Thomsen AR, Moolman-Smook J, Christiansen M, Bundgaard H. Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives. Hum Mutat. 2009;30(3):363–370. doi: 10.1002/humu.20862. [DOI] [PubMed] [Google Scholar]
- Bai F, Weis A, Takeda AK, Chase PB, Kawai M. Enhanced active cross-bridges during diastole: molecular pathogenesis of tropomyosin's HCM mutations. Biophys J. 2011;100(4):1014–1023. doi: 10.1016/j.bpj.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Groth HL, Kawai M. DCM-related tropomyosin mutants E40 K/E54 K over-inhibit the actomyosin interaction and lead to a decrease in the number of cycling cross-bridges. PLoS ONE. 2012;7(10):e47471. doi: 10.1371/journal.pone.0047471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Caster HM, Pinto JR, Kawai M. Analysis of the molecular pathogenesis of cardiomyopathy-causing cTnT mutants I79N, ΔE96, and ΔK210. Biophys J. 2013;104(9):1979–1988. doi: 10.1016/j.bpj.2013.04.001. 10.1016/j.bpj. 2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron JT. Hypertrophic cardiomyopathy. Curr Treat Options Cardiovasc Med. 1999;1:277–282. doi: 10.1007/s11936-999-0044-2. [DOI] [PubMed] [Google Scholar]
- Barua B, Pamula MC, Hitchcock-DeGregori SE. Evolutionarily conserved surface residues constitute actin binding sites of tropomyosin. Proc Natl Acad Sci USA. 2011;108(25):10150–10155. doi: 10.1073/pnas.1101221108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua B, Winkelmann DA, White HD, Hitchcock-DeGregori SE. Regulation of actin-myosin interaction by conserved periodic sites of tropomyosin. Proc Natl Acad Sci USA. 2012;109(45):18425–18430. doi: 10.1073/pnas.1212754109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann E, Muller M, Penczek PA, Mannherz HG, Manstein DJ, Raunser S. Structure of the rigor actin-tropomyosin-myosin complex. Cell. 2012;150(2):327–338. doi: 10.1016/j.cell.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Therapeut. 2010;128(1):191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Bing W, Knott A, Marston SB. A simple method for measuring the relative force exerted by myosin on actin filaments in the in vitro motility assay: evidence that tropomyosin and troponin increase force in single thin filaments. Biochemical Journal. 2000a;350:693–699. doi: 10.1042/0264-6021:3500693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing W, Knott A, Redwood C, Esposito G, Purcell I, Watkins H, Marston S. Effect of hypertrophic cardiomyopathy mutations in human cardiac muscle alpha -tropomyosin (Asp175Asn and Glu180Gly) on the regulatory properties of human cardiac troponin determined by in vitro motility assay. J Mol Cell Cardiol. 2000b;32(8):1489–1498. doi: 10.1006/jmcc.2000.1182. [DOI] [PubMed] [Google Scholar]
- Bookwalter CS, Trybus KM. Functional consequences of a mutation in an expressed human alpha-cardiac actin at a site implicated in familial hypertrophic cardiomyopathy. J Biol Chem. 2006;281(24):16777–16784. doi: 10.1074/jbc.M512935200. [DOI] [PubMed] [Google Scholar]
- Borovikav YS, Rysev NA, Karpicheva OE, Redwood CS. Hypertrophic cardiomyopathy-causing Asp175asn and Glu180gly Tpm1 mutations shift tropomyosin strands further towards the open position during the ATPase cycle. Biochem Bioph Res Co. 2011;407(1):197–201. doi: 10.1016/j.bbrc.2011.02.139. [DOI] [PubMed] [Google Scholar]
- Bremel RD, Weber A. Cooperation within actin filament in vertebrate skeletal-muscle. Nature-New Biol. 1972;238(82):97. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brown JH, Cohen C. Regulation of muscle contraction by tropomyosin and troponin: how structure illuminates function. Adv Protein Chem. 2005;71:121–159. doi: 10.1016/S0065-3233(04)71004-9. [DOI] [PubMed] [Google Scholar]
- Brown JH, Kim KH, Jun G, Greenfield NJ, Dominguez R, Volkmann N, Hitchcock-DeGregori SE, Cohen C. Deciphering the design of the tropomyosin molecule. Proc Natl Acad Sci USA. 2001;98(15):8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Zhou ZC, Reshetnikova L, Robinson H, Yammani RD, Tobacman LS, Cohen C. Structure of the mid-region of tropomyosin: bending and binding sites for actin. Proc Natl Acad Sci USA. 2005;102(52):18878–18883. doi: 10.1073/pnas.0509269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10(3):225–235. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- Chang AN, Harada K, Ackerman MJ, Potter JD. Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in α-tropomyosin. J Biol Chem. 2005;280:34343–34349. doi: 10.1074/jbc.M505014200. [DOI] [PubMed] [Google Scholar]
- Chang B, Nishizawa T, Furutani M, Fujiki A, Tani M, Kawaguchi M, Ibuki K, Hirono K, Taneichi H, Uese K, Onuma Y, Bowles NE, Ichida F, Inoue H, Matsuoka R, Miyawaki T. Noncom-paction study c Identification of a novel TPM1 mutation in a family with left ventricular noncompaction and sudden death. Mol Genet Metab. 2011;102(2):200–206. doi: 10.1016/j.ymgme.2010.09.009. 10.1016/j.ymgme.2010. 09.009. [DOI] [PubMed] [Google Scholar]
- Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- Codd MB, Sugrue DD, Gersh BJ, Melton LJ. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy: a population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80:564–572. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- Coupland ME, Puchert E, Ranatunga KW. Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J Physiol. 2001;536(Pt 3):879–891. doi: 10.1111/j.1469-7793.2001.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello DA, Maron BJ, Spirito P, Watkins H, Vosberg HP, Thierfelder L, Schoen FJ, Seidman Seidman CE. Clinical features of hypertrophic cardiomyopathy caused by mutation of a “hot spot” in the α-tropomyosin gene. J Am Coll Cardiol. 1997;29(3):635–640. doi: 10.1016/s0735-1097(96)00538-4. [DOI] [PubMed] [Google Scholar]
- Debold EP, Schmitt JP, Patlak JB, Beck SE, Moore JR, Seidman JG, Seidman C, Warshaw DM. Hypertrophic and dilated cardiomyopathy mutations differentially affect the molecular force generation of mouse alpha-cardiac myosin in the laser trap assay. Am J Physiol Heart Circulatory Physiol. 2007;293(1):H284–H291. doi: 10.1152/ajpheart.00128.2007. [DOI] [PubMed] [Google Scholar]
- Earing MG, Ackerman MJ, O'Leary PW. Diastolic ventricular dysfunction as a marker for hypertrophic cardiomyopathy in a family with a novel alpha-tropomyosin mutation. J Am Soc Echocardiog. 2003;16(6):698–702. doi: 10.1016/S0894-7317(03)00285-2. [DOI] [PubMed] [Google Scholar]
- Eaton BL. Tropomyosin Binding to F-Actin Induced by Myosin Heads. Science. 1976;192(4246):1337–1339. doi: 10.1126/science.131972. [DOI] [PubMed] [Google Scholar]
- Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363(9424):1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- Fujita H, Kawai M. Temperature effect on isometric tension is mediated by regulatory proteins tropomyosin and troponin in bovine myocardium. J Physiol. 2002;539(Pt 1):267–276. doi: 10.1113/jphysiol.2001.013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Yasuda K, Niitsu S, Funatsu T, Ishiwata S. Structural and functional reconstitution of thin filaments in the contractile apparatus of cardiac muscle. Biophys J. 1996;71:2307–2318. doi: 10.1016/S0006-3495(96)79465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Sasaki D, Ishiwata S, Kawai M. Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys J. 2002;82(2):915–928. doi: 10.1016/S0006-3495(02)75453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Lu X, Suzuki M, Ishiwata S, Kawai M. The effect of tropomyosin on force and elementary steps of the cross-bridge cycle in reconstituted bovine myocardium. J Physiol. 2004;556(Pt 2):637–649. doi: 10.1113/jphysiol.2003.059956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furch M, Geeves MA, Manstein DJ. Modulation of actin affinity and actomyosin adenosine triphosphatase by charge changes in the myosin motor domain. Biochemistry. 1998;37(18):6317–6326. doi: 10.1021/Bi972851y. [DOI] [PubMed] [Google Scholar]
- Fuster V, Gersh BJ, Giuliani ER, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. Am J Cardiol. 1981;47(3):525–531. doi: 10.1016/0002-9149(81)90534-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro M, Coto E, Reguero JR, Berrazueta JR, Alvarez V, Alonso B, Sainz R, Martin M, Moris C. Mutations in sarcomeric genes MYH7, MYBPC3, TNNT2, TNNI3, and TPM1 in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol. 2009;62(1):48–56. [PubMed] [Google Scholar]
- Gillum RF. Idiopathic cardiomyopathy in the United States, 1970–1982. Am Heart J. 1986;111(4):752–755. doi: 10.1016/0002-8703(86)90111-0. [DOI] [PubMed] [Google Scholar]
- Gollapudi SK, Mamidi R, Mallampalli SL, Chandra M. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys J. 2012;103(5):940–948. doi: 10.1016/j.bpj.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Chen Y, Liang B, LaMadrid M, Luo Z, Chase PB. Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv Exp Med Biol. 1998;453:187–196. doi: 10.1007/978-1-4684-6039-1_22. [DOI] [PubMed] [Google Scholar]
- Grunig E, Tasman JA, Kucherer H, Franz W, Kubler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol. 1998;31(1):186–194. doi: 10.1016/s0735-1097(97)00434-8. [DOI] [PubMed] [Google Scholar]
- Heeley DH. Investigation of the effects of phosphorylation of rabbit striated-muscle alpha–alpha-tropomyosin and rabbit skeletal-muscle troponin-T. Eur J Biochem. 1994;221(1):129–137. doi: 10.1111/j.1432-1033.1994.tb18721.x. [DOI] [PubMed] [Google Scholar]
- Heeley DA, Moir AJ, Perry SV. Phosphorylation of tropomyosin during development in mammalian striated muscle. FEBS Lett. 1982;146(1):115–118. doi: 10.1016/0014-5793(82)80716-3. [DOI] [PubMed] [Google Scholar]
- Heeley DH, Watson MH, Mak AS, Dubord P, Smillie LB. Effect of phosphorylation on the interaction and functional properties of rabbit striated muscle alpha alpha-tropomyosin. J Biol Chem. 1989;264(5):2424–2430. [PubMed] [Google Scholar]
- Heeley DH, Belknap B, White HD. Maximal activation of skeletal muscle thin filaments requires both rigor myosin S1 and calcium. J Biol Chem. 2006;281(1):668–676. doi: 10.1074/jbc.M505549200. [DOI] [PubMed] [Google Scholar]
- Heller MJ, Nili M, Homsher E, Tobacman LS. Cardiomyopathic tropomyosin mutations that increase thin filament Ca2+ sensitivity and tropomyosin N-domain flexibility. J Biol Chem. 2003;278(43):41742–41748. doi: 10.1074/jbc.M303408200. [DOI] [PubMed] [Google Scholar]
- Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA. Genetic evaluation of cardiomyopathy–a heart failure society of america practice guideline. J Card Fail. 2009;15(2):83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Hitchcock-DeGregori SE. Tropomyosin: function follows structure. Adv Exp Med Biol. 2008;644:60–72. doi: 10.1007/978-0-387-85766-4_5. [DOI] [PubMed] [Google Scholar]
- Hitchcock-DeGregori SE, Song Y, Greenfield NJ. Functions of tropomyosin's periodic repeats. Biochemistry. 2002;41(50):15036–15044. doi: 10.1021/bi026519k. [DOI] [PubMed] [Google Scholar]
- Ho CY, Seidman CE. A contemporary approach to hypertrophic cardiomyopathy. Circulation. 2006;113(2):e858–e862. doi: 10.1161/CIRCULATIONAHA.105.591982. [DOI] [PubMed] [Google Scholar]
- Holmes KC. The actomyosin interaction and its control by tropomyosin. Biophys J. 1995;68(4):S2–S7. [PubMed] [Google Scholar]
- Holmes KC, Schroder RR, Sweeney HL, Houdusse A. The structure of the rigor complex and its implications for the power stroke. Philos T Roy Soc B. 2004;359(1452):1819–1828. doi: 10.1098/rstb.2004.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthauzen LM, Correa F, Farah CS. Ca2+-induced rolling of tropomyosin in muscle thin filaments: the alpha- and beta-band hypothesis revisited. J Biol Chem. 2004;279(15):15204–15213. doi: 10.1074/jbc.M308904200M308904200. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Structural changes in actin-containing and myosin-containing filaments during contraction. Cold Spring Harb Sym. 1973;37:361–376. [Google Scholar]
- Ishii Y, Lehrer SS. Excimer fluorescence of pyrenyliodoacetamide-labeled tropomyosin: a probe of the state of tropomyosin in reconstituted muscle thin-filaments. Biochemistry. 1990;29(5):1160–1166. doi: 10.1021/bi00457a010. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen P, Soranta M, Miettinen R, Saarinen L, Pihlajamaki J, Silvennoinen K, Tikanoja T, Laakso M, Kuusisto J. The cardiac beta-myosin heavy chain gene is not the predominant gene for hypertrophic cardiomyopathy in the Finnish population. J Am Coll Cardiol. 1998;32(6):1709–1716. doi: 10.1016/s0735-1097(98)00448-3. [DOI] [PubMed] [Google Scholar]
- Jacques AM, Copeland O, Messer AE, Gallon CE, King K, McKenna WJ, Tsang VT, Marston SB. Myosin binding protein C phosphorylation in normal, hypertrophic and failing human heart muscle. J Mol Cell Cardiol. 2008;45(2):209–216. doi: 10.1016/j.yjmcc.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Wieczorek DF. Investigations into tropomyosin function using mouse models. J Mol Cell Cardiol. 2010;48(5):893–898. doi: 10.1016/j.yjmcc.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed RJ, Marcelis CL, Doevendans PA, Schmeitz-Mulkens JM, Van Dockum WG, Geraedts JP, Smeets HJ. Variable clinical manifestation of a novel missense mutation in the alpha-tropomyosin (TPM1) gene in familial hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41(6):981–986. doi: 10.1016/s0735-1097(02)03005-x. [DOI] [PubMed] [Google Scholar]
- Karam CN, Warren CM, Rajan S, de Tombe PP, Wieczorek DF, Solaro RJ. Expression of tropomyosin-kappa induces dilated cardiomyopathy and depresses cardiac myofilament tension by mechanisms involving cross-bridge dependent activation and altered tropomyosin phosphorylation. J Muscle Res Cell Motil. 2011;31(5–6):315–322. doi: 10.1007/s10974-010-9237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karibe A, Tobacman LS, Strand J, Butters C, Back N, Bachinski LL, Arai AE, Ortiz A, Roberts R, Homsher E, Fananapazir L. Hypertrophic cardiomyopathy caused by a novel α-tropomyosin mutation (V95A) is associated with mild cardiac phenotype abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis. Circulation. 2001;103(1):65–71. doi: 10.1161/01.cir.103.1.65. [DOI] [PubMed] [Google Scholar]
- Kawai M. What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres? J Muscle Res Cell Motil. 2003;24(2–3):127–138. doi: 10.1023/a:1026093212111. [DOI] [PubMed] [Google Scholar]
- Kawai M, Halvorson HR. Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas. Biophys J. 1991;59:329–342. doi: 10.1016/S0006-3495(91)82227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Ishiwata S. Use of thin filament reconstituted muscle fibres to probe the mechanism of force generation. J Muscle Res Cell Motil. 2006;27:455–468. doi: 10.1007/s10974-006-9075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Saeki Y, Zhao Y. Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res. 1993;73(1):35–50. doi: 10.1161/01.res.73.1.35. [DOI] [PubMed] [Google Scholar]
- Kawai M, Kido T, Vogel M, Fink RH, Ishiwata S. Temperature change does not affect force between regulated actin filaments and heavy meromyosin in single-molecule experiments. J Physiol. 2006;574(Pt 3):877–887. doi: 10.1113/jphysiol.2006.111708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Lu X, Hitchcock-DeGregori SE, Stanton KJ, Wandling Michael W. Tropomyosin period 3 is essential for enhancement of isometric tension in thin filament-reconstituted bovinemyocardium. J Biophys. 2009;2009:1–17. doi: 10.1155/2009/380967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Jin L, de Tombe PP. Cardiac thin filament regulation. Pflugers Arch. 2008;457(1):37–46. doi: 10.1007/s00424-008-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Carabello BA, Conrad CC, Buckley JM, DeFreyte G, Barnes M, Tomanek RJ, Wei CC, Dell'Italia LJ, GC IV, Zile MR. Hypertrophic response to hemodynamic overload: role of load vs. renin-angiotensin system activation. Am J Physiol Heart Circ Physiol. 1999;276:H350–H358. doi: 10.1152/ajpheart.1999.276.2.h350. [DOI] [PubMed] [Google Scholar]
- Lakdawala NK, Dellefave L, Redwood CS, Sparks E, Cirino AL, Depalma S, Colan SD, Funke B, Zimmerman RS, Robinson P, Watkins H, Seidman CE, Seidman JG, McNally EM, Ho CY. Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: the distinctive natural history of sarcomeric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55(4):320–329. doi: 10.1016/j.jacc.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66(1):12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Lee S, Lu R, Muller-Ehmsen J, Schwinger RH, Brixius K. Increased Ca2+ sensitivity of myofibrillar tension in ischaemic vs dilated cardiomyopathy. Clin Exp Pharmacol Physiol. 2010;37(12):1134–1138. doi: 10.1111/j.1440-1681.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Lehman W, Craig R. Tropomyosin and the steric mechanism of muscle regulation. Adv Exp Med Biol. 2008;644:95–109. doi: 10.1007/978-0-387-85766-4_8. [DOI] [PubMed] [Google Scholar]
- Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302(3):593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- Lehman W, Galinska-Rakoczy A, Hatch V, Tobacman LS, Craig R. Structural basis for the activation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2009;388(4):673–681. doi: 10.1016/j.jmb.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer SS, Morris EP. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment-1 atpase. J Biol Chem. 1982;257(14):8073–8080. [PubMed] [Google Scholar]
- Lorenz M, Poole KJV, Popp D, Rosenbaum G, Holmes KC. An atomic model of the unregulated thin filament obtained by X-ray fiber diffraction on oriented actin-tropomyosin gels (Vol 246, Pg 108, 1995) J Mol Biol. 1995;249(2):509–519. doi: 10.1006/jmbi.1994.0070. [DOI] [PubMed] [Google Scholar]
- Lu X, Tobacman LS, Kawai M. Effects of tropomyosin internal deletion Delta23Tm on isometric tension and the cross-bridge kinetics in bovine myocardium. J Physiol. 2003;553(Pt 2):457–471. doi: 10.1113/jphysiol.2003.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Bryant MK, Bryan KE, Rubenstein PA, Kawai M. Role of the N-terminal negative charges of actin in force generation and cross-bridge kinetics in reconstituted bovine cardiac muscle fibres. J Physiol. 2005;564(Pt 1):65–82. doi: 10.1113/jphysiol.2004.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Heeley DH, Smillie LB, Kawai M. The role of tropomyosin isoforms and phosphorylation in force generation in thin-filament reconstituted bovine cardiac muscle fibres. J Muscle Res Cell Motil. 2010;31(2):93–109. doi: 10.1007/s10974-010-9213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly S, Lehrer SS. Long-range effects of familial hypertrophic cardiomyopathy mutations E180G and D175N on the properties of tropomyosin. Biochemistry-US. 2012;51(32):6413–6420. doi: 10.1021/Bi3006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak A, Smillie LB, Barany M. Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci USA. 1978;75(8):3588–3592. doi: 10.1073/pnas.75.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2001;33:655–670. doi: 10.1006/jmcc.2001.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, Spirito P, Cate FJT, Wigle ED. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–1713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- Marston SB. How do mutations in contractile proteins cause the primary familial cardiomyopathies? J Cardiovasc Transl. 2011;4(3):245–255. doi: 10.1007/s12265-011-9266-2. [DOI] [PubMed] [Google Scholar]
- Martyn DA, Chase PB, Regnier M, Gordon AM. A simple model with myofilament compliance predicts activation dependent crossbridge kinetics in skinned skeletal fibers. Biophys J. 2002;83:3425–3434. doi: 10.1016/S0006-3495(02)75342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur MC, Chase PB, Chalovich JM. Several cardiomyopathy causing mutations on tropomyosin either destabilize the active state of actomyosin or alter the binding properties of tropomyosin. Biochem Bioph Res Co. 2011;406(1):74–78. doi: 10.1016/j.bbrc.2011.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, Turnbull DH, Georgakopoulos D, Kass D, Bond M, Niimura H, Schoen FJ, Conner D, Fischman DA, Seidman CE, Seidman JG. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest. 1999;104(12):1771. doi: 10.1172/JCI7377C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan AD, Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975;98:293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- McLachlan AD, Stewart M. The 14-fold periodicity in alpha-tropomyosin and the interaction with actin. J Mol Biol. 1976;103(2):271–298. doi: 10.1016/0022-2836(76)90313-2. [DOI] [PubMed] [Google Scholar]
- Memo M, Leung MC, Ward DG, Dos Remedios C, Morimoto S, Zhang L, Ravenscroft G, McNamara E, Nowak KJ, Marston SB, Messer AE. Familial dilated cardiomyopathy mutations uncouple troponin i phosphorylation from changes in myofibrillar Ca2+—sensitivity. Cardiovasc Res. 2013 doi: 10.1093/cvr/cvt071. [DOI] [PubMed] [Google Scholar]
- Michels VV, Moll PP, Miller FA, Tajik AJ, Chu JS, Driscoll DJ, Burnett JC, Rodeheffer RJ, Chesebro JH, Tazelaar HD. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992;326(2):77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- Miki M, Makimura S, Saitoh T, Bunya M, Sugahara Y, Ueno Y, Kimura-Sakiyama C, Tobita H. A three-dimensional FRET analysis to construct an atomic model of the actin-tropomyosin complex on a reconstituted thin filament. J Mol Biol. 2011;414(5):765–782. doi: 10.1016/j.jmb.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, Robinson P, Redwood C, Watkins H. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J Biol Chem. 2005;280(31):28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- Mirza M, Robinson P, Kremneva E, Copeland ON, Nikolaeva O, Watkins H, Levitsky D, Redwood C, EL-Mezgueldi M, Marston S. The Effect of Mutations in α-Tropomyosin (E40 K and E54 K) That Cause Familial Dilated Cardiomyopathy on the Regulatory Mechanism of Cardiac Muscle Thin Filaments. J Biol Chem. 2007;282:13487–13497. doi: 10.1074/jbc.M701071200. [DOI] [PubMed] [Google Scholar]
- Morita H, Rehm HL, Menesses A, McDonough B, Roberts AE, Kucherlapati R, Towbin JA, Seidman JG, Seidman CE. Shared genetic causes of cardiac hypertrophy in children and adults. New Engl J Med. 2008;358(18):1899–1908. doi: 10.1056/Nejmoa075463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Pajak L, Howles P, Doetschman T, Wieczorek DF. Developmental analysis of tropomyosin gene expression in embryonic stem cells and mouse embryos. Mol Cell Biol. 1993;13(6):3311–3323. doi: 10.1128/mcb.13.6.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Grupp IL, Grupp G, OToole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF. Molecular and physiological effects of overexpressing striated muscle beta-tropomyosin in the adult murine heart. J Biol Chem. 1995;270(51):30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP, Wolska B, Evans C, Solaro RJ, Wieczorek DF. Mouse model of a familial hypertrophic cardiomyopathy mutation in α-tropomyosin manifests cardiac dysfunction. Circ Res. 1999;85:47–56. doi: 10.1161/01.res.85.1.47. [DOI] [PubMed] [Google Scholar]
- Nakajima-Taniguchi C, Matsui H, Nagata S, Kishimoto T, Yamauchitakihara K. Novel missense mutation in alpha-tropomyosin gene found in japanese patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol. 1995;27(9):2053–2058. doi: 10.1016/0022-2828(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Nevzorov IA, Levitsky DI. Tropomyosin: double helix from the protein world. Biochemistry Biokhimiia. 2011;76(13):1507–1527. doi: 10.1134/S0006297911130098. [DOI] [PubMed] [Google Scholar]
- Nixon BR, Liu B, Scellini B, Tesi C, Piroddi N, Ogut O, John Solaro R, Ziolo MT, Janssen PM, Davis JP, Poggesi C, Biesiadecki BJ. Tropomyosin Ser-283 pseudo-phosphorylation slows myofibril relaxation. Arch Biochem Biophys. 2012 doi: 10.1016/j.abb.2012.11.010. 0.1016/j.abb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- Oguchi Y, Ishizuka J, Hitchcock-DeGregori SE, Ishiwata S, Kawai M. The role of tropomyosin domains in cooperative activation of the actin-myosin interaction. J Mol Biol. 2011;414(5):667–680. doi: 10.1016/j.jmb.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TM, Kishimoto NY, Whitby FG, Michels VV. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J Mol Cell Cardiol. 2001;33(4):723–732. doi: 10.1006/jmcc.2000.1339. [DOI] [PubMed] [Google Scholar]
- Ommen SR, Gersh BJ. Sudden cardiac death risk in hypertrophic cardiomyopathy. Eur Heart J. 2009;30(21):2558–2559. doi: 10.1093/eurheartj/ehp307. [DOI] [PubMed] [Google Scholar]
- Opie LH, Mansford KR, Owen P. Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J. 1971;124(3):475–490. doi: 10.1042/bj1240475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieples K, Arteaga G, Solaro RJ, Grupp I, Lorenz JN, Boivin GP, Jagatheesan G, Labitzke E, Detombe PP, Konhilas JP, Irving TC, Wieczorek DF. Tropomyosin 3 expression leads to hypercontractility and attenuates myofilament length-dependent Ca2(+) activation. Am J Physiol-Heart C. 2002;283(4):H1344–H1353. doi: 10.1152/ajpheart.00351.2002. [DOI] [PubMed] [Google Scholar]
- Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro JR, Wieczorek DF. A familial hypertrophic cardiomyopathy α-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001;33:1815–1828. doi: 10.1006/jmcc.2001.1445. [DOI] [PubMed] [Google Scholar]
- Prabhakar R, Petrashevskaya N, Schwartz A, Aronow B, Boivin GP, Molkentin JD, Wieczorek DF. A mouse model of familial hypertrophic cardiomyopathy caused by a alpha-tropomyosin mutation. Mol Cell Biochem. 2003;251(1–2):33–42. [PubMed] [Google Scholar]
- Probst S, Oechslin E, Schuler P, Greutmann M, Boye P, Knirsch W, Berger F, Thierfelder L, Jenni R, Klaassen S. Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ Cardiovas Genet. 2011;4(4):367–374. doi: 10.1161/CIRCGENETICS.110.959270. 10.1161/CIRCGENETICS.110. 959270. [DOI] [PubMed] [Google Scholar]
- Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res. 2007;101(2):205–214. doi: 10.1161/CIRCRESAHA.107.148379. [DOI] [PubMed] [Google Scholar]
- Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D'Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation. 2010;121(3):410–416. doi: 10.1161/CIRCULATIONAHA.109.889725. 10.1161/Circulationaha. 109.889725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW. Endothermic force generation in fast and slow mammalian (rabbit) muscle fibers. Biophys J. 1996;71(4):1905–1913. doi: 10.1016/S0006-3495(96)79389-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VS, Marongelli EN, Guilford WH. Phosphorylation of tropomyosin extends cooperative binding of myosin beyond a single regulatory unit. Cell Motil Cytoskelet. 2009;66(1):10–23. doi: 10.1002/cm.20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Erdmann J, Wellnhofer E, Raible J, Fleck E. Novel mutation in the alpha-tropomyosin gene and transition from hypertrophic to hypocontractile dilated cardiomyopathy. Circulation. 2000;102(17):E112–E116. doi: 10.1161/01.cir.102.17.e112. [DOI] [PubMed] [Google Scholar]
- Roth K, Hubesch B, Meyerhoff DJ, Naruse S, Gober JR, Lawry TJ, Boska MD, Matson GB, Weiner MW. Noninvasive quantitation of phosphorus metabolites in human-tissue by Nmr-spectroscopy. J Magn Reson. 1989;81(2):299–311. [Google Scholar]
- Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97(11):1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. 10.1161/01.RES.0000 190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- Sarma RJ, Chana A, Elkayam U. Left ventricular noncompaction. Prog Cardiovasc Dis. 2010;52(4):264–273. doi: 10.1016/j.pcad.2009.11.001. 10.1016/j.pcad.2009. 11.001. [DOI] [PubMed] [Google Scholar]
- Schulz EM, Correll RN, Sheikh HN, Lofrano-Alves MS, Engel PL, Newman G, Schultz Jel J, Molkentin JD, Wolska BM, Solaro RJ, Wieczorek DF. Tropomyosin dephosphorylation results in compensated cardiac hypertrophy. J Biol Chem. 2012;287(53):44478–44489. doi: 10.1074/jbc.M112.402040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfichi-Duke L, Garcia-Cazarin ML, Sumandea CA, Sievert GA, Balke CW, Zhan DY, Morimoto S, Sumandea MP. Cardiomyopathy-causing deletion K210 in cardiac troponin T alters phosphorylation propensity of sarcomeric proteins. J Mol Cell Cardiol. 2010;48(5):934–942. doi: 10.1016/j.yjmcc.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Hitchcock-DeGregori SE. Local destabilization of the tropomyosin coiled coil gives the molecular flexibility required for actin binding. Biochemistry-Us. 2003;42(48):14114–14121. doi: 10.1021/bi0348462. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Kobayashi T. Protein phosphorylation and signal transduction in cardiac thin filaments. J Biol Chem. 2011;286(12):9935–9940. doi: 10.1074/jbc.R110.197731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton MT, Fuchsbauer CM, Allshire AP. BDM drives protein dephosphorylation and inhibits adenine nucleotide exchange in cardiomyocytes. Am J Physiol. 1998;275(4 Pt 2):H1260–H1266. doi: 10.1152/ajpheart.1998.275.4.H1260. [DOI] [PubMed] [Google Scholar]
- Sutoh K, Ando M, Sutoh K, Toyoshima YY. Site-directed mutations of dictyostelium actin—disruption of a negative charge cluster at the N-terminus. Proc Natl Acad Sci USA. 1991;88(17):7711–7714. doi: 10.1073/pnas.88.17.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal L, Jimenez J, Tardiff JC. DCM-Linked D230N Tropomyosin mutation results in early dilatation and systolic dysfunction in mice. Biophys J. 2012;10235(3(S1)):6a. [Google Scholar]
- Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10(3):237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- Tardiff JC. Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res. 2011;108(6):765–782. doi: 10.1161/CIRCRESAHA.110.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seldman JG, Seidman CE. α-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77(5):701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- van de Meerakker JB, Christiaans I, Barnett P, Lekanne Deprez RH, Ilgun A, Mook OR, Mannens MM, Lam J, Wilde AA, Moorman AF, Postma AV. A novel alpha-tropomyosin mutation associates with dilated and non-compaction cardiomyopathy and diminishes actin binding. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbamcr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Van Driest SL, Ackerman MJ, Ommen SR, Shakur R, Will ML, Nishimura RA, Tajik AJ, Gersh BJ. Prevalence and severity of “benign” mutations in the beta-myosin heavy chain, cardiac troponin T, and alpha-tropomyosin genes in hypertrophic cardiomyopathy. Circulation. 2002;106(24):3085–3090. doi: 10.1161/01.cir.0000042675.59901.14. [DOI] [PubMed] [Google Scholar]
- Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation. 2003;108(4):445–451. doi: 10.1161/01.CIR.0000080896.52003.DF. [DOI] [PubMed] [Google Scholar]
- VanBuren P, Palmiter KA, Warshaw DM. Tropomyosin directly modulates actomyosin mechanical performance at the level of a single actin filament. Proc Natl Acad Sci USA. 1999;96(22):12488–12493. doi: 10.1073/pnas.96.22.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Flemal K, Qiu Z, Ablin L, Grossman W, Morgan JP. Ca2+ handling and myofibrillar Ca2+ sensitivity in ferret cardiac myocytes with pressure-overload hypertrophy. Am J Physiol. 1994;267(3 Pt 2):H918–H924. doi: 10.1152/ajpheart.1994.267.3.H918. [DOI] [PubMed] [Google Scholar]
- Wang GW, Ding W, Kawai M. Does thin filament compliance diminish the cross-bridge kinetics? A study in rabbit psoas fibers. Biophys J. 1999;76:978–984. doi: 10.1016/S0006-3495(99)77261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waurick R, Knapp J, Van Aken H, Boknik P, Neumann J, Schmitz W. Effect of 2,3-butanedione monoxime on force of contraction and protein phosphorylation in bovine smooth muscle. Naunyn-Schmiedeberg's archives of pharmacology. 1999;359(6):484–492. doi: 10.1007/pl00005380. [DOI] [PubMed] [Google Scholar]
- Whitby FG, Phillips GN. Crystal structure of tropomyosin at 7 Angstroms resolution. Proteins. 2000;38(1):49–59. doi: 10.1002/(Sici)1097-0134(20000101). [DOI] [PubMed] [Google Scholar]
- Wieczorek DF, Jagatheesan G, Rajan S. The role of tropomyosin in heart disease. Adv Exp Med Biol. 2008;644:132–142. doi: 10.1007/978-0-387-85766-4_11. [DOI] [PubMed] [Google Scholar]
- Williams DL, Jr, Greene LE. Comparison of the effects of tropomyosin and troponin-tropomyosin on the binding of myosin subfragment 1 to actin. Biochemistry. 1983;22(11):2770–2774. doi: 10.1021/bi00280a027. [DOI] [PubMed] [Google Scholar]
- Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J Mol Cell Cardiol. 2010;48(5):882–892. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54(1):162–174. doi: 10.1016/s0008-6363(01)00565-x. [DOI] [PubMed] [Google Scholar]
- Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996;98(1):167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi-Takihara K, Nakajima-Taniguchi C, Matsui H, Fujio Y, Kunisada K, Nagata S, Kishimoto T. Clinical implications of hypertrophic cardiomyopathy associated with mutations in the alpha-tropomyosin gene. Heart. 1996;76(1):63–65. doi: 10.1136/hrt.76.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Sheng Q, Tang H, Li Y, Zeng R, Solaro RJ. Quantitative comparison of sarcomeric phosphoproteomes of neonatal and adult rat hearts. Am J Physiol Heart Circ Physiol. 2008;295(2):H647–H656. doi: 10.1152/ajpheart.00357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza MV, Arbustini E, Narula J. Noncompaction of the left ventricle: primary cardiomyopathy with an elusive genetic etiology. Curr Opin Pediatr. 2007;19(6):619–627. doi: 10.1097/MOP.0b013e3282f1ecbc. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kawai M. Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J. 1994;67(4):1655–1668. doi: 10.1016/S0006-3495(94)80638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RS, Cox S, Lakdawala NK, Cirino A, Mancini-DiNardo D, Clark E, Leon A, Duffy E, White E, Baxter S, Alaamery M, Farwell L, Weiss S, Seidman CE, Seidman JG, Ho CY, Rehm HL, Funke BH. A novel custom resequencing array for dilated cardiomyopathy. Genet Med. 2010;12(5):268–278. doi: 10.1097/GIM.0b013e3181d6f7c0. [DOI] [PMC free article] [PubMed] [Google Scholar]