Abstract
Polymyxin B (PB) is increasingly used as the last treatment for multidrug-resistant (MDR) Gram-negative bacterial infections. In this study, serum and epithelial lining fluid (ELF) pharmacokinetics and the efficacy of a PB liposomal formulation were investigated. Two groups of 24 Swiss Webster mice were intravenously administered PB liposomes or PB aqueous solution at ca. 3 mg/kg. Serum and ELF samples were collected for up to 6 h to quantify major PB components. Three groups of neutropenic mice (n = 6/group) were infected with a clinical MDR Pseudomonas aeruginosa strain followed by intravenous administration of PB liposomes or PB aqueous solution at 3 mg/kg every 6 h or sham (drug-free) liposomes every 6 h. Bacterial burden in animal lung tissues was quantified after 24 h of therapy and was compared using one-way ANOVA. Survival of infected animals over time (n = 10/group) was evaluated by Kaplan–Meier analysis and log-rank test. In the pharmacokinetic study, the AUC ratio in ELF between liposome and aqueous solution groups ranged from 4.6 to 11.1 for various major PB components. In the efficacy study, for strain PA 9019 a significantly lower bacterial burden was seen in the liposomal group (3.8 ± 0.7 vs. 7.9 ± 0.8 log10 CFU/g in the aqueous solution group), which subsequently prolonged survival of infected animals. In this study, treatment with a PB liposomal formulation yielded higher drug penetration into pulmonary ELF, which resulted in superior efficacy. However, further investigations on the clinical utility of the PB liposomal formulation are warranted.
Keywords: Multidrug resistance, Pulmonary, Pseudomonas aeruginosa, Epithelial lining fluid
1. Introduction
Infections caused by multidrug-resistant (MDR) Gram-negative bacteria such as Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae present a critical clinical challenge worldwide [1,2]. Among the different infections caused by MDR Gram-negative bacteria, pulmonary infections are especially problematic and are associated with a high mortality rate [3–5]. Since no first-line antibiotics are effective, polymyxin B (PB) is often used as the last-resort treatment for infections caused by MDR Gram-negative bacteria [6,7].
PB [US Pharmacopeia (USP)] is commercially available as a mixture of several closely related polypeptides, obtained from cultures of various Bacillus polymyxa strains and related species [8]. The major components of PB (USP) are polymyxin B1, B2, B3 and isoleucine-B1 (PB1, PB2, PB3 and ile-PB1, respectively) [9], the proportions of which have been reported to be 73.5%, 13.7%, 4.2% and 8.6%, respectively [10]. Since most clinical isolates of Gram-negative bacilli (including those that are MDR) remain susceptible to PB [11–13], intravenous (i.v.) PB is commonly used for the treatment of critically ill patients with pulmonary infections [14]. Despite good in vitro susceptibility, previous studies have demonstrated that PB is associated with reduced efficacy in the treatment of pulmonary infections [14–16]. A possible explanation for the poor therapeutic outcomes is the limited penetration of PB into the site of infection, i.e. epithelial lining fluid (ELF).
Liposome encapsulation may potentially alter the pharmacokinetics and biodistribution of antimicrobials compared with standard formulations [17,18]. Increased uptake by activated tissue macrophages would allow higher antimicrobial concentrations to be achieved at the site of infection [19,20] and presumably improve treatment efficacy. In this study, PB was encapsulated in liposomes by a modified method of reversed-phase evaporation. Serum and ELF pharmacokinetic (PK) profiles were compared between the liposomal formulation and standard aqueous solution in mice. In addition, treatment efficacy was evaluated in a neutropenic murine pneumonia model of P. aeruginosa. Improving drug delivery to the site of infection is expected to enhance the effectiveness of PB for pulmonary infections due to MDR Gram-negative bacteria.
2. Materials and methods
2.1. Chemicals and reagents
DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL). Polymyxin B sulfate (USP) powder, 1.25% 2,2,2-tribromoethanol (TBE) and trichloroacetic acid (TCA) were purchased from Sigma-Aldrich (St Louis, MO). Carbutamide was purchased from Aldrich (Milwaukee, WI). Liquid chromatography/mass spectrometry (LC/MS)-grade acetonitrile and water were obtained from Mallinckrodt Baker (Phillipsburg, NJ). LC/MS-grade formic acid was purchased from Fluka Analytical (Buchs, Germany).
2.2. Bacteria
Two P. aeruginosa strains were used. PA 9019 was a bloodstream isolate from Houston, Texas, which was previously found to be resistant to all first-line agents [21]. Pseudomonas aeruginosa ATCC 27853 (PA 27853) was obtained from the American Type Culture Collection (Rockville, MD). The PB minimum inhibitory concentrations (MICs) for PA 9019 and PA 27853 were previously determined to be 4 mg/L and 2 mg/L, respectively [22]. According to the Clinical and Laboratory Standards Institute (CLSI) [23], an isolate with an MIC ≤ 2 mg/L would be considered as susceptible and an isolate with MIC of 4 mg/L would be considered as intermediate.
2.3. Preparation of liposomal polymyxin B formulation
PB was encapsulated in liposomes by a modified method of reversed-phase evaporation. The specific method of liposome preparation is under protection by a provisional patent application 61/684,276 (unpublished, filing date 17 August 2012). Briefly, PB was added to a solution of DPPC and cholesterol in chloroform. A water-in-oil emulsion was formed and chloroform was evaporated under pressure to form a uniform liposomal suspension. Finally, this liposomal dispersion was extruded through a high-pressure extruder (Northern Lipids, Inc., Burnaby, BC, Canada) and free PB was removed by centrifugation at 48 400 × g for 1 h (Beckman Coulter, Indianapolis, IN). The concentration of PB in each liposome batch was determined by a validated ultraperformance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS) method.
2.4. Pharmacokinetic studies
The PK investigation in a small number of uninfected immunocompetent animals was used as a screening tool to justify subsequent efficacy investigations in a murine neutropenic model. The animal protocol was approved by the University of Houston (Houston, TX) Institutional Animal Care and Use Committee. All of the animals received food and water ad libitum and were cared for in accordance with National Research Council recommendations. Two groups of 24 female ND4 Swiss Webster mice (20–23 g) (Harlan Laboratory, Indianapolis, IN) were intravenously administered PB liposomes or aqueous solution (USP) at approximately 3 mg/kg through the tail vein. At each time point (0.1, 0.5, 1, 2, 4 and 6 h post dose), four mice were sacrificed for blood and ELF sample collection. Blood samples were clotted on crushed ice and the serum was obtained by centrifugation. ELF samples were obtained by bronchoalveolar lavage. All of the serum and ELF samples were stored at –80 °C until analysis.
2.5. Serum and epithelial lining fluid sample analysis
Four major PB components in serum and ELF samples were assayed by a validated UPLC-MS/MS method. An ACQUITY UPLC HSS C18 column (Waters, Milford, MA) was used with 0.1% formic acid/acetonitrile as mobile phases. Analysis was performed in positive ionisation mode with multiple reactions monitoring (MRM) scan type. Briefly, the serum and ELF samples (200 μL) were spiked with 0.2 mL of carbutamide (internal standard). Then, 200 μL of 5% TCA was added to precipitate the proteins, followed by 1 min of vortexing. After centrifugation at 18 000 × g for 15 min, the supernatant was transferred to a new tube and was evaporated to dryness under a stream of nitrogen.
The residue was reconstituted in 0.1 mL of mobile phase (acetonitrile:0.1% formic acid, 50:50) and then centrifuged at 18 000 × g for 15 min. Then, 10 μL of supernatant was injected into the UPLC-MS/MS for quantitative analysis. The linear concentration range was 0.006–3.2 mg/L both for serum and ELF samples. Samples with concentrations higher than the linear range were diluted before the assay. The intraday and interday variance was <11% for all of the components both in serum and ELF. The concentration of drug in ELF was corrected using the concentrations of urea in bronchoalveolar lavage fluid (BALF) and serum, respectively [24]. Concentrations of urea in serum and BALF samples were quantified with a commercially available assay kit (QuantiChrom™ Urea Assay Kit; BioAssay Systems, Hayward, CA) and were measured on a Synergy2 microplate reader (BioTek Instruments, Winooski, VT). Drug exposures observed both in serum and ELF were normalised by the specific dose of each batch of liposomes to account for the variances among different PK experiments. Naïve data averaging was used; the best-fit PK parameters as well as drug exposure in serum and ELF were calculated by WinNonlin 3.3 (Pharsight Corp., Mountain View, CA) using a one-compartment model and non-compartmental analysis, respectively.
2.6. Experimental pneumonia model
Animals were housed in isolation boxes to decrease the risk of infection from extraneous pathogens. To reduce the influence of innate immune function on the observed outcomes, transient neutropenia was induced using two doses of intraperitoneal cyclophosphamide: 150 mg/kg administered 4 days prior to infection and 100 mg/kg administered 1 day prior to infection. This procedure was reported to result in transient neutropenia for 1 week after the last injection [25]. Animals were anaesthetised by a single intraperitoneal injection of 1.25% TBE at a dosage of 250 mg/kg. Overnight bacterial cultures were inoculated in cation-adjusted Mueller–Hinton broth (BBL, Sparks, MD), grown to log-phase growth and diluted to ca. 104.5 CFU/mL (PA 9019) or 106 CFU/mL (PA 27853) with sterile normal saline on the basis of absorbance at 630 nm. The bacterial inocula selected were determined by previous lethal inoculum studies [21] and were intended to mimic a window of opportunity in which pharmacological intervention might have an impact on outcome. Bacteria were washed once in sterile saline and were inoculated (10 μL) into the trachea of anaesthetised animals under laryngoscopic guidance [21]. Two hours after bacterial infection, three mice were sacrificed at baseline to ascertain the infective inoculum.
2.7. Bacterial burden studies
Two hours after bacterial infection, three to six mice in each treatment group were intravenously administered one of the following every 6 h: (i) PB liposomes (3 mg/kg); (ii) PB aqueous solution (3 mg/kg); or (iii) sham (drug-free) liposomes. The selected dosing regimen was guided by previous investigations based on the highest tolerated i.v. dose and logistic feasibility (i.e. the number of injections given via the tail vein). All infected mice were euthanised after 24 h by CO2 asphyxiation, and the lungs from each mouse were aseptically collected for quantitative culture. Prior to being cultured, the lungs were homogenised in 10 mL of sterile saline. Lung homogenate suspensions were centrifuged (4000 × g at 10 °C for 15 min), decanted and reconstituted with sterile saline at 10 times the original volume. Samples were subsequently serially diluted (10×) and plated on Mueller–Hinton agar plates (Hardy Diagnostics, Santa Maria, CA). Colony counts were enumerated after incubation at 35 °C in a humidified incubator for 24 h. The reliable lower limit of detection was 1000 CFU/g. Statistical analysis was performed using the Kruskal–Wallis test and Dunn's multiple comparison test. A P-value of ≤0.05 was considered to be statistically significant.
2.8. Survival studies
Two hours after infection with PA 9019, ten mice in each treatment group were intravenously administered (0.2 mL) with either of the following every 6 h: (i) PB liposomes (3 mg/kg); (ii) PB aqueous solution (3 mg/kg); or (iii) sham (drug-free) liposomes for 24 h. The mice were examined every 8 h for up to 96 h. Moribund mice were humanely sacrificed at each inspection time, and death was recorded as occurred at the next inspection time. Lungs from each mouse were aseptically collected for quantitative culture as described previously, either upon death or at the end of the experiment. Survival over time was evaluated by Kaplan–Meier analysis and log-rank test. A P-value of ≤0.05 was considered to be statistically significant.
3. Results
3.1. Serum pharmacokinetics
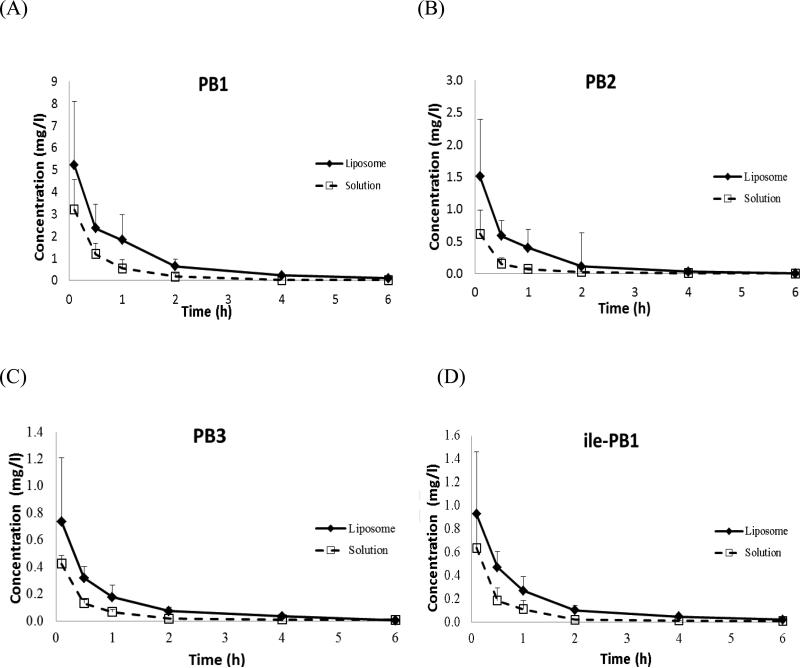
The serum concentration–time profiles (normalised by the total dose) following administration of PB liposomes and aqueous solution are shown in Fig. 1. All four major PB components in serum could be quantified for up to 6 h post dose; the PK profiles were satisfactorily characterised by a one-compartment model. The best-fit PK parameters for each component are presented in Table 1. Compared with the solution group, a relatively slower clearance of all of the components was found in the liposome group.
Fig. 1.
Serum concentrations of the major polymyxin components (A) PB1, (B) PB2, (C) PB3 and (D) isoleucine-B1 (ile-PB1) following intravenous administration of polymyxin B liposomes (◆) and aqueous solution (US Pharmacopeia) (❑). N = 4, data shown as mean ± standard deviation.
Table 1.
Best-fit pharmacokinetic (PK) parameters of the major polymyxin components PB1, PB2, PB3 and isoleucine-B1 (ile-PB1) following intravenous administration of polymyxin B (n = 4)
| PK parameter | Component | Liposome | Solution (USP) |
|---|---|---|---|
| AUC0–6h (mg·h/L) | PB1 | 4.77 | 2.76 |
| PB2 | 1.07 | 0.30 | |
| PB3 | 0.39 | 0.33 | |
| ile-PB1 | 0.64 | 0.64 | |
| T1/2 (h) | PB1 | 0.60 | 0.32 |
| PB2 | 0.44 | 0.21 | |
| PB3 | 0.30 | 0.28 | |
| ile-PB1 | 0.42 | 0.29 | |
| CL (mL/h/kg) | PB1 | 444.10 | 790.06 |
| PB2 | 308.28 | 899.90 | |
| PB3 | 489.78 | 664.39 | |
| ile-PB1 | 562.65 | 575.61 | |
| Vss (ml/kg) | PB1 | 382.90 | 373.06 |
| PB2 | 197.77 | 274.74 | |
| PB3 | 209.87 | 267.30 | |
| ilePB1 | 338.41 | 241.10 |
USP, US Pharmacopeia; AUC0–6h, area under the concentration–time curve from 0–6 h; T1/2, elimination half-life; CL, clearance; Vss, volume of distribution at steady state.
3.2. Comparative polymyxin B exposures in epithelial lining fluid
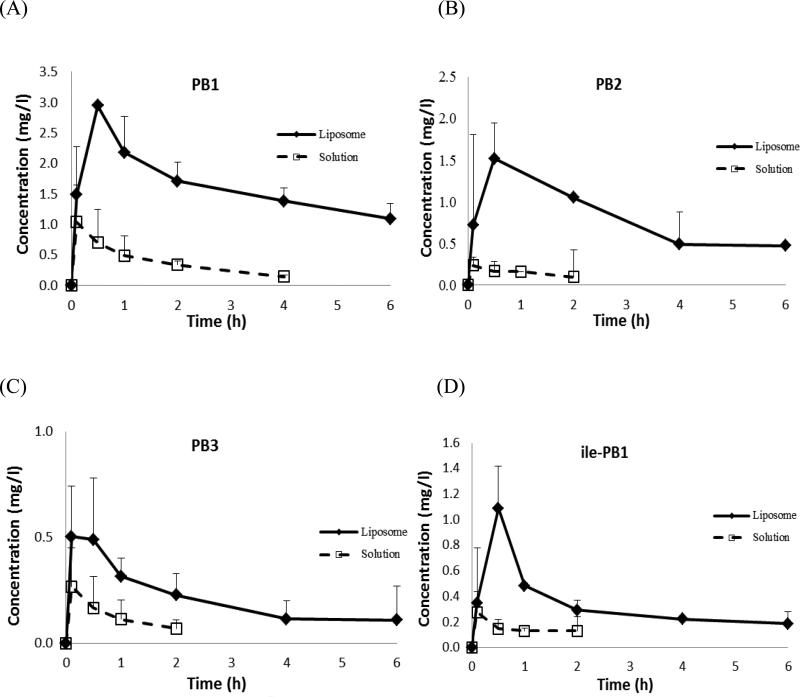
The ELF concentration–time profiles of PB1, PB2, PB3 and ile-PB1 following i.v. administration of PB liposomes and aqueous solution are displayed in Fig. 2. PB1 could be quantified for up to 4 h, whilst PB2, PB3 and ile-PB1 could only be quantified for up to 2 h post dose in the solution group. In contrast, all of the components could be quantified up to the last sampling time point in the liposome group. Drug exposures over 6 h for the liposome group was ca. 7-fold (range 4.6–11.1-fold) higher than that calculated for the solution group (Table 2).
Fig. 2.
Epithelial lining fluid concentrations of the major polymyxin components (A) PB1, (B) PB2, (C) PB3 and (D) isoleucine-B1 (ile-PB1) following intravenous administration of polymyxin B liposomes (◆) and aqueous solution (US Pharmacopeia) (❑). N = 4, data shown as mean ± standard deviation.
Table 2.
Comparative epithelial lining fluid exposures of the major polymyxin components PB1, PB2, PB3 and isoleucine-B1 (ile-PB1) in mice following intravenous administration of polymyxin B (n = 4)
| Component | AUC0–6h (mg·h/L)a |
AUC ratio (liposome:solution) | |
|---|---|---|---|
| Liposome | Solution | ||
| PB1 | 9.75 | 1.57 | 6.21 |
| PB2 | 3.44 | 0.31 | 11.10 |
| PB3 | 1.28 | 0.26 | 4.92 |
| ile-PB1 | 2.00 | 0.44 | 4.55 |
The area under the concentration–time curve from 0–6 h (AUC0–6h) was calculated from the average concentration–time profile. Each time concentration was averaged by naïve pooling. Undetectable concentrations were deemed to be zero.
3.3. Comparative efficacy in neutropenic pneumonia model
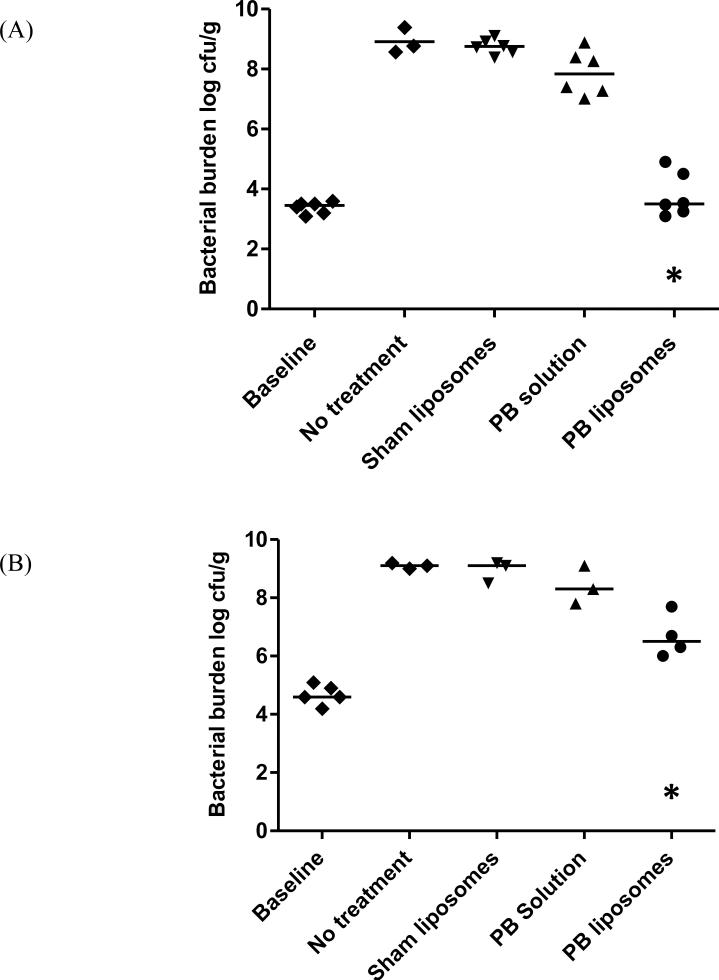
The bacterial burdens after 24 h in various treatment groups are displayed in Fig. 3. At the start of therapy, the animals had 3.1–3.6 log10 CFU/g (PA 9019) and 4.2–5.1 log10 CFU/g (PA 27853) in lung tissues. After 24 h, the bacterial burden in lung tissues of the sham liposome control group increased to 8.4–9.1 log10 CFU/g (PA 9019) and 8.5–9.2 log10 CFU/g (PA 27853); these increases were similar to no-treatment controls (P > 0.05). For PA 9019, a significant difference in bacterial burden was found between the liposome group (3.8 ± 0.7 log10 CFU/g) and the sham liposome control group (8.7 ± 0.2 log10 CFU/g) (P < 0.001). However, only a minimal antimicrobial effect was observed in the solution group compared with the sham liposome control group. A similar trend was observed in PA 27853. These results were generally consistent with our postulation that only a limited drug exposure could be achieved at the site of infection with the highest tolerated dose.
Fig. 3.
Comparison of bacterial burdens in lung tissue after 24 h of treatment for Pseudomonas aeruginosa strains (A) PA 9019 and (B) PA 27853. * Significantly different compared with the polymyxin B (PB) solution and sham liposome groups (P < 0.05). Each data point represents one animal; the horizontal line in each group depicts the median bacterial burden.
3.4. Survival studies
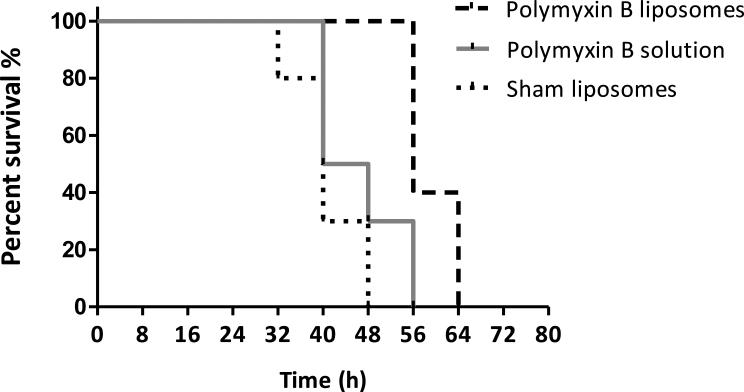
Therapy with liposomes for 24 h significantly prolonged the survival of animals infected with PA 9019 compared with treatment with PB solution and sham liposomes (P < 0.001). In contrast, survival was not prolonged with treatment of PB solution compared with sham liposomes. With sham liposomes treatment, the median survival was 40 h, whilst the median survival was 44 h with treatment of PB solution and 56 h with treatment of PB liposomes (Fig. 4). In all dead animals, the tissue bacterial burdens were considerably higher (>10 000×) than baseline, suggesting that pneumonia was likely the primary cause of death (data not shown). These observations were consistent with previous results of bacterial burden in lung tissues.
Fig. 4.
Survival of mice infected with Pseudomonas aeruginosa PA 9019 following treatment for 24 h for polymyxin B (PB). N = 10 in each group. Survival was significantly prolonged in the PB liposome group compared with the sham liposome group (P < 0.001).
4. Discussion
Pneumonia is the leading cause of infection-related mortality worldwide, and antibiotic resistance has become more prevalent in the past several decades. PB is increasingly used as the last resort to treat pulmonary infections due to MDR Gram-negative bacteria. However, PB was reported to have reduced efficacy in the treatment of pulmonary infections. Considering the high molecular weight, low lipophilicity and high plasma protein binding of PB [26], reduced treatment efficacy was postulated to be associated with a low drug exposure in ELF. Several attempts have been made to characterise the pharmacokinetics and pharmacodynamics of PB [22,26,27], however PB exposure in ELF is still unknown. In this study, major PB components distributed to the ELF in mice were quantified using a sensitive UPLC-MS/MS assay. Only limited PB exposure was found in ELF, which provided supportive evidence for our hypothesis. Furthermore, an efficacy study was performed in a validated murine neutropenic pneumonia model [21]. The bacterial burden in the lung tissues at 24 h was only found to be minimally reduced in mice treated with standard PB solution compared with no treatment. In addition, no survival benefits were observed in infected animals. Unsatisfactory antimicrobial activity of standard PB solution seen in this pneumonia model is consistent with previous clinical observations [14–16].
PK and pharmacodynamic (PD) relationships have been described for a number of drugs, often using serum concentrations as a surrogate for the concentrations at the actual site of infection. For pulmonary infections, concentrations of antibiotics in ELF are thought to reflect antibiotic activity more realistically [24]. This is the first report to explore the reason why standard PB solution is associated with reduced efficacy in the treatment of pulmonary infections. With limited drug exposure achieved at the infection site, standard PB solution was not found to be efficacious.
Intravenous liposomes have the potential for uptake by macrophages in the lungs and then release of PB into ELF, resulting in a higher drug exposure at the site of infection. We demonstrated that, compared with the standard formulation, liposome encapsulation increased PB distribution into the ELF. This preferential distribution may lead to improved treatment efficacy. The potential use of liposomes as a carrier system for PB delivery to the lungs has been explored by other investigators [28–31]. Most studies only reported enhanced in vitro susceptibility of liposomal PB in Gram-negative bacteria [29,31]. It was also demonstrated that direct delivery of liposomal PB to the lungs was efficacious in the treatment of pulmonary infections [28]. However, there was no detectable drug in the bloodstream, which might not be ideal in situations when bacteria disseminated systemically from the lungs. A preferred treatment option for pulmonary infections could achieve effective concentrations both in the ELF and systemically. Therefore, an i.v. liposomal PB formulation was developed in this study, which was found to have superior efficacy in a validated experimental pneumonia model against two P. aeruginosa isolates. Moreover, the improved efficacy of PB liposomes was confirmed by two clinically relevant endpoints, namely bacterial burden in lung tissues at 24 h and survival.
There are several limitations of this study. Only a limited number of bacterial strains were investigated; using additional isolates with a diverse background would enhance the robustness of the evaluation. Second, whilst the importance of protein binding on antimicrobial efficacy is well recognised, we did not specifically compare protein binding between the two formulations. Protein binding of PB in different mammalian species was previously found to be similar (data not shown), but since we did not expect a high protein content in ELF, the confounding from the bound PB was not thought to be highly relevant in the treatment of pneumonia. Third, PB is associated with considerable nephrotoxicity; however, we did not compare renal toxicity between the liposomal and standard formulations because the mouse model is not suitable for this purpose. Investigations on nephrotoxicity associated with the polymyxins are ongoing in our laboratory. Preliminary biodistribution studies suggested that the drug exposures achieved in renal tissues were lower with liposomes compared with the standard solution (data not shown). Finally, since the systemic drug exposure achieved by PB solution was lower than that expected with standard dosing in humans, clinical studies are warranted to verify the ELF drug exposure and efficacy in humans before this liposomal formulation can be used clinically.
In conclusion, we explored the reason for the lower efficacy of PB in the treatment of pulmonary infections. In addition, an i.v. liposomal PB formulation demonstrated superior treatment efficacy in a validated murine pneumonia model. These investigations are expected to shed more light on the potential of a novel drug delivery system that may improve the treatment of pulmonary infections.
Acknowledgments
Funding: This study was supported in part by the National Institutes of Health [R15AI089671-01]. The sponsor had no roles in the study design; collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in part at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), 9–12 September 2012, San Francisco, CA (abstract A-025).
Competing interests: None declared.
Ethical approval: The animal protocol was approved by the University of Houston (Houston, TX) Institutional Animal Care and Use Committee.
References
- 1.Balaji V, Jeremiah SS, Baliga PR. Polymyxins: antimicrobial susceptibility concerns and therapeutic options. Indian J Med Microbiol. 2011;29:230–42. doi: 10.4103/0255-0857.83905. [DOI] [PubMed] [Google Scholar]
- 2.Chen LF, Kaye D. Current use for old antibacterial agents: polymyxins, rifamycins, and aminoglycosides. Infect Dis Clin North Am. 2009;23:1053–75. doi: 10.1016/j.idc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159:1249–56. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- 4.Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gibert C. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996;275:866–9. [PubMed] [Google Scholar]
- 5.Tseng CC, Liu SF, Wang CC, Tu ML, Chung YH, Lin MC, et al. Impact of clinical severity index, infective pathogens, and initial empiric antibiotic use on hospital mortality in patients with ventilator-associated pneumonia. Am J Infect Control. 2012;40:648–52. doi: 10.1016/j.ajic.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Kwa A, Kasiakou SK, Tam VH, Falagas ME. Polymyxin B: similarities to and differences from colistin (polymyxin E). Expert Rev Anti Infect Ther. 2007;5:811–21. doi: 10.1586/14787210.5.5.811. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Z, Tam VH. Polymyxin B: a new strategy for multidrug-resistant Gram-negative organisms. Expert Opin Investig Drugs. 2008;17:661–8. doi: 10.1517/13543784.17.5.661. [DOI] [PubMed] [Google Scholar]
- 8.Shoji J, Hinoo H, Wakisaka Y, Koizumi K, Mayama M, Matsuura S. Isolation of two new polymyxin group antibiotics. (Studies on antibiotics from the genus Bacillus. XX). J Antibiot (Tokyo) 1977;30:1029–34. doi: 10.7164/antibiotics.30.1029. [DOI] [PubMed] [Google Scholar]
- 9.Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J. Isolation and structural characterization of polymyxin B components. J Chromatogr A. 2001;912:369–73. doi: 10.1016/s0021-9673(01)00585-4. [DOI] [PubMed] [Google Scholar]
- 10.He J, Ledesma KR, Lam WY, Figueroa DA, Lim TP, Chow DS, et al. Variability of polymyxin B major components in commercial formulations. Int J Antimicrob Agents. 2010;35:308–10. doi: 10.1016/j.ijantimicag.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J Antimicrob Chemother. 2011;66:2070–4. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- 12.Castanheira M, Sader HS, Jones RN. Antimicrobial susceptibility patterns of KPC-producing or CTX-M-producing Enterobacteriaceae. Microb Drug Resist. 2010;16:61–5. doi: 10.1089/mdr.2009.0031. [DOI] [PubMed] [Google Scholar]
- 13.Tam VH, Chang KT, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA, et al. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:1160–4. doi: 10.1128/AAC.01446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furtado GH, d'Azevedo PA, Santos AF, Gales AC, Pignatari AC, Medeiros EA. Intravenous polymyxin B for the treatment of nosocomial pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30:315–9. doi: 10.1016/j.ijantimicag.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Holloway KP, Rouphael NG, Wells JB, King MD, Blumberg HM. Polymyxin B and doxycycline use in patients with multidrug-resistant Acinetobacter baumannii infections in the intensive care unit. Ann Pharmacother. 2006;40:1939–45. doi: 10.1345/aph.1H353. [DOI] [PubMed] [Google Scholar]
- 16.Teng CB, Koh PT, Lye DC, Ang BS. Continuous versus intermittent infusion of polymyxin B in the treatment of infections caused by multidrug-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2008;31:80–2. doi: 10.1016/j.ijantimicag.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Allen TM. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs. 1998;56:747–56. doi: 10.2165/00003495-199856050-00001. [DOI] [PubMed] [Google Scholar]
- 18.Drulis-Kawa Z, Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int J Pharm. 2010;387:187–98. doi: 10.1016/j.ijpharm.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 19.Bakker-Woudenberg IA. Long-circulating sterically stabilized liposomes as carriers of agents for treatment of infection or for imaging infectious foci. Int J Antimicrob Agents. 2002;19:299–311. doi: 10.1016/s0924-8579(02)00021-3. [DOI] [PubMed] [Google Scholar]
- 20.Coune A. Liposomes as drug delivery system in the treatment of infectious diseases. Potential applications and clinical experience. Infection. 1988;16:141–7. doi: 10.1007/BF01644088. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Z, Ledesma KR, Singh R, Hou J, Prince RA, Tam VH. Quantitative assessment of combination antimicrobial therapy against multidrug-resistant bacteria in a murine pneumonia model. J Infect Dis. 2010;201:889–97. doi: 10.1086/651024. [DOI] [PubMed] [Google Scholar]
- 22.Tam VH, Cao H, Ledesma KR, Hu M. In vitro potency of various polymyxin B components. Antimicrob Agents Chemother. 2011;55:4490–1. doi: 10.1128/AAC.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing: seventeenth informational supplement. CLSI; Wayne, PA: 2007. Document M100-S17. [Google Scholar]
- 24.Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andes D, Craig WA. In vivo activities of amoxicillin and amoxicillin–clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother. 1998;42:2375–9. doi: 10.1128/aac.42.9.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwa AL, Lim TP, Low JG, Hou J, Kurup A, Prince RA, et al. Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol Infect Dis. 2008;60:163–7. doi: 10.1016/j.diagmicrobio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, et al. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis. 2008;47:1298–304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]
- 28.Omri A, Suntres ZE, Shek PN. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem Pharmacol. 2002;64:1407–13. doi: 10.1016/s0006-2952(02)01346-1. [DOI] [PubMed] [Google Scholar]
- 29.Alipour M, Halwani M, Omri A, Suntres ZE. Antimicrobial effectiveness of liposomal polymyxin B against resistant Gram-negative bacterial strains. Int J Pharm. 2008;355:293–8. doi: 10.1016/j.ijpharm.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Desai TR, Tyrrell GJ, Ng T, Finlay WH. In vitro evaluation of nebulization properties, antimicrobial activity, and regional airway surface liquid concentration of liposomal polymyxin B sulfate. Pharm Res. 2003;20:442–7. doi: 10.1023/a:1022664406840. [DOI] [PubMed] [Google Scholar]
- 31.McAllister SM, Alpar HO, Brown MR. Antimicrobial properties of liposomal polymyxin B. J Antimicrob Chemother. 1999;43:203–10. doi: 10.1093/jac/43.2.203. [DOI] [PubMed] [Google Scholar]