Abstract
Interleukin-12 (IL12) is a cytokine that is secreted by activated phagocytes and dendritic cells and that induces interferon-γ production by natural-killer and T lymphocytes. It consists of two subunits, p35 and p40, which are encoded by IL12A and IL12B, respectively. The first reported patient with a genetic cytokine disorder was a Pakistani child, who was homozygous for a large loss-of-function deletion (g.482+82_856–854del) in IL12B. This IL12-deficient child suffered from infections caused by bacille Calmette-Guérin (BCG) and Salmonella enteritidis. We herein report 12 additional patients from five other kindreds. In one kindred from India, the same large deletion that was described elsewhere (g.482+82_856–854del) was identified. In four kindreds from Saudi Arabia, a recessive loss-of-function frameshift insertion (g.315_316insA) was found. A conserved haplotype encompassing the IL12B gene suggested that a founder effect accounted for the recurrence of each mutation. The two founder mutational events—g.482+82_856–854del and g.315_316insA—were estimated to have occurred ∼700 and ∼1,100 years ago, respectively. Among a total of 13 patients with IL12 deficiency, 1 child had salmonellosis only and 12 suffered from clinical disease due to BCG or environmental nontuberculous mycobacteria. One patient also had clinical disease caused by virulent Mycobacterium tuberculosis, five patients had clinical disease caused by Salmonella serotypes, and one patient had clinical disease caused by Nocardia asteroides. The clinical outcome varies from case to case, since five patients (aged 2–11 years) died of overwhelming infection, whereas eight patients (aged 3–12 years) are still in good health and are not currently taking antibiotics. In conclusion, IL12 deficiency is not limited to a single kindred, shows significant variability of outcome, and should be considered in the genetic diagnosis of patients with mycobacteriosis and/or salmonellosis. To date, two founder IL12B mutations have been identified, accounting for the recurrence of a large deletion and a small insertion within populations from the Indian subcontinent and from the Arabian Peninsula, respectively.
Introduction
Patients with Mendelian susceptibility to mycobacterial disease (MIM 209950) (McKusick 1998) are vulnerable to poorly virulent mycobacterial species—such as bacille Calmette-Guérin (BCG) vaccines (Casanova et al. 1995, 1996) and environmental nontuberculous mycobacteria (NTM) (Levin et al. 1995; Frucht and Holland 1996). They are also vulnerable to the more virulent species Mycobacterium tuberculosis, the agent of tuberculosis (Jouanguy et al. 1997; Altare et al. 2001). Other infectious diseases occur rarely in these patients—with the exception of salmonellosis, which affects almost half of the patients. Most sporadic and familial cases are suggestive of autosomal recessive inheritance (Levin et al. 1995; Casanova et al. 1996); however, in some families, the syndrome segregates in an autosomal dominant (Jouanguy et al. 1999; Dupuis et al. 2001) or X-linked recessive (Frucht and Holland 1996; Frucht et al. 1999) pattern. Genetic heterogeneity is further suggested by histopathological and clinical phenotypic heterogeneity (Emile et al. 1997). Some patients die of overwhelming mycobacterial disease with lepromatous-like lesions in early childhood, whereas other patients develop, later in life, disseminated but curable infections with tuberculoid granulomas.
Five disease-causing autosomal genes (IFNGR1, IFNGR2, STAT1, IL12RB1, and IL12B) have been found, and a further degree of allelic heterogeneity accounts for the existence of nine defined disorders. Although genetically different, these disorders are physiologically related, as all result in impaired interferon-γ (IFNγ)–mediated immunity. IFNγ is a cytokine produced by natural-killer (NK) and T lymphocytes that binds to a heterodimeric ubiquitous receptor. Null recessive mutations in IFNGR1 are responsible for complete IFNγ-receptor ligand-binding–chain (IFNγR1) deficiency. These mutations abolish either receptor expression (Jouanguy et al. 1996; Newport et al. 1996; Pierre-Audigier et al. 1997; Altare et al. 1998b; Holland et al. 1998; Roesler et al. 1999; Cunningham et al. 2000) or the binding of surface-expressed receptors to IFNγ (Jouanguy et al. 2000; Allende et al. 2001). Partial recessive (Jouanguy et al. 1997) and dominant (Jouanguy et al. 1999; Villella et al. 2001) IFNγR1 deficiencies have also been described. Different recessive mutations in IFNGR2 are responsible for complete (Dorman and Holland 1998; Dorman et al. 2000) or partial (Döffinger et al. 2000) IFNγ signaling-chain (IFNγR2) deficiency. We also recently identified a dominant mutation in STAT1, resulting in a partial signal transducer and activator of transcription–1 (STAT1) deficiency and in impaired cellular responses to IFNγ (Dupuis et al. 2001).
Finally, two other genetic defects result in normal cellular responses to IFNγ but in abnormal interleukin-12 (IL12)–dependent production of IFNγ. Null recessive mutations have been identified in IL12RB1, which encodes the IL12-receptor β1 subunit (IL12Rβ1) that is expressed on NK and T cells (Altare et al. 1998a; de Jong et al. 1998; Verhagen et al. 2000; Aksu et al. 2001; Altare et al. 2001; Sakai et al. 2001). As do patients with partial IFNγR1, IFNγR2, and STAT1 deficiency, patients with complete IL12Rβ1 deficiency have a milder clinical phenotype than do patients with complete IFNγR1 and IFNγR2 deficiency, probably owing to residual IFNγ-mediated immunity (Dupuis et al. 2000). Whereas a number of patients with IL12Rβ1 deficiency have been reported, only one child has been found to carry a null homozygous large deletion in IL12B, which encodes the p40 subunit of IL12 (Altare et al. 1998c). IL12 is a heterodimeric cytokine (p70), comprising two subunits, p35 and p40, which is expressed by activated phagocytes and dendritic cells (Trinchieri 1998). To date, IL12 deficiency is the only human inherited cytokine disorder known. In this study, we investigated whether IL12 deficiency could be detected in other patients and, if so, whether it can be used to describe the pathogenic IL12B mutations involved and the associated clinical phenotypes.
Subjects and Methods
Subjects and Kindreds
Kindred A.—The parents are consanguineous, originating from Pakistan and currently living in the United Kingdom (fig. 1 and table 1). The proband (A.II.1) suffered from BCG and Salmonella enteritidis infectious diseases at the ages of 3 mo and 3.5 years, respectively. She has been diagnosed with IL12 deficiency and is now 10 years old and well, with no current treatment (Altare et al. 1998c).
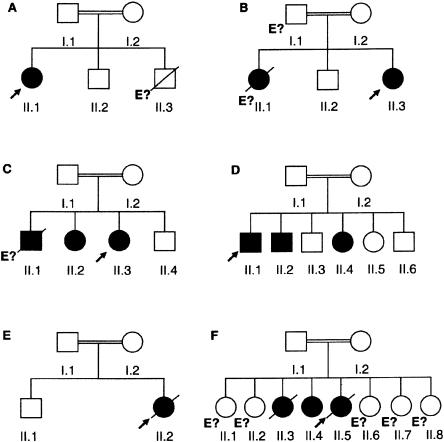
Figure 1.
Pedigrees of six families with IL12 deficiency caused by IL12B mutations. Each kindred is designated by a capital letter (A–F), each generation is designated by a roman numeral (I or II), and each individual is designated by an arabic numeral (1–8). Patients with mycobacteriosis and/or salmonellosis are represented by black symbols. The probands are indicated by arrows. “E?” indicates individuals for whom genetic analysis was not possible (e.g., B.II.1, who died of disseminated BCG infection at age 5 years).
Table 1.
Clinical Phenotype of Patients with IL12 Deficiency
|
Bacteriac |
||||||||
| Patient | Mutation | Place of Origina | Alive at Follow-Up? | Ageb (years) | BCG | NTM | Mtb | Other |
| A.II.1 | g.482+82_856–854del | Pakistan | Yes | 11 | D, R | … | … | S. enteritidis |
| B.II.1 | Not done | India | No | 5 | D, R | … | … | … |
| B.II.3 | g.482+82_856–854del | India | Yes | 10 | … | … | … | S. enteritidis |
| C.II.1 | Not done | Saudi Arabia | No | 2 | D, R | … | … | … |
| C.II.2 | g.315_316insA | Saudi Arabia | Yes | 10 | D | … | … | N. asteroides |
| C.II.3 | g.315_316insA | Saudi Arabia | Yes | 3 | D | … | … | … |
| D.II.1 | g.315_316insA | Saudi Arabia | Yes | 11 | L | … | … | … |
| D.II.2 | g.315_316insA | Saudi Arabia | Yes | 10 | L | … | … | … |
| D.II.4 | g.315_316insA | Saudi Arabia | Yes | 6 | L | … | L | S. paratyphi C |
| E.II.2 | g.315_316insA | Saudi Arabia | No | 2 | D, R | … | … | … |
| F.II.3 | g.315_316insA | Saudi Arabia | No | 11 | D | … | … | Salmonella |
| F.II.4 | g.315_316insA | Saudi Arabia | Yes | 12 | D | … | … | … |
| F.II.5 | g.315_316insA | Saudi Arabia | No | 5 | … | D, R | … | Salmonella group B |
Patient A.II.1 has been reported by Altare et al. (1998c), and patient B.II.1 has been reported by Murugasu et al. (1988).
At death or last follow-up, at the time of preparation of the manuscript of this report.
Mtb = M. tuberculosis. D = disseminated; R = recurrent; L = local. “Salmonella” denotes that serotype has not been determined.
Kindred B.—The parents are first cousins, originating from the Gujarat region of India and currently living in Singapore. Their first daughter (B.II.1) was inoculated with BCG at birth and died at age 5 years, of disseminated BCG infection (Murugasu et al. 1988). A lymph-node biopsy showed tuberculoid granulomas with multiple visible acid-fast bacilli. Her brother (B.II.2), who was also vaccinated with BCG, suffered no adverse effects and is now a healthy 15 year old. The youngest sister (B.II.3) was not vaccinated with BCG. At age 2–4 years, she suffered four episodes of disseminated S. enteritidis infection, all of which responded to antibiotics. She is now 9 years old and well, with no current treatment.
Kindred C.—The parents are first cousins, originating from and living in Saudi Arabia. Their first child (C.II.1) was inoculated with BCG at birth and died from disseminated BCG infection at age 2 years. Their second child (C.II.2) was vaccinated with BCG shortly after birth and developed disseminated BCG infection. Lymph-node biopsy showed mature granuloma formation and multiple acid-fast bacilli. This child responded very well to antimycobacterial drugs. She presented Nocardia asteroides pleurisy at age 8 years and again responded well to antibiotics. The patient is now 9 years of age, in good health, and not taking medication. The proband (C.II.3) was vaccinated with live BCG at birth and developed disseminated BCG infection 6 mo later. A lymph-node biopsy showed no mature granuloma formation and multiple acid-fast bacilli. She responded well to antimycobacterial therapy and is now a healthy 2-year-old. The last child (C.II.4) was not vaccinated with live BCG and is currently a healthy 1-year-old.
Kindred D.—The parents are first cousins, originating from and living in Saudi Arabia. The proband (D.II.1) was vaccinated with BCG at birth and presented axillary BCG adenitis at age 14 mo. He was treated with antibiotics and by surgical excision. His brother (D.II.2) was vaccinated with live BCG at birth and presented axillary BCG adenitis at age 3 mo. Both responded well to antibiotics. The proband and his brother are now 10 and 9 years old, respectively, and are healthy, with no current treatment. Another sibling (D.II.4) was vaccinated with live BCG at birth and presented axillary BCG adenitis 12 mo later. She was successfully treated with antibiotics and by surgical excision. At age 15 mo, she presented with Salmonella type C gastroenteritis. At age 2 years 6 mo, she developed occipital lymphadenitis due to M. tuberculosis. She was successfully treated with antibiotics but suffered a relapse at age 4 years. She is now 5 years old and well, with no antibiotic treatment. The three other siblings were vaccinated with BCG, with no adverse effect, and are currently healthy at ages 6, 3, and 1 years.
Kindred E.—The parents are first cousins, originating from and living in Saudi Arabia. The proband (E.II.2) was vaccinated with live BCG at birth and presented with disseminated BCG infection 8 mo later. After surgical excision, she was treated with antibiotics and INFγ. She did not respond to treatment and later died of disseminated infection at age 2 years. Her adenitis biopsy showed tuberculoid lymphadenitis with poorly formed granuloma and numerous visible acid-fast bacilli. Her brother (E.II.1) was vaccinated with live BCG, with no adverse effect.
Kindred F.—The parents are first cousins, originating from and living in Saudi Arabia. The third sister (F.II.3) was vaccinated with BCG at birth and presented with disseminated BCG infection 3 mo later. She also suffered from Salmonella adenitis. She was treated with antibiotics for a total of 18 mo. She remained well, with no treatment, for 8 years. At age 11 years, she developed meningoencephalitis. Unfortunately, this disease proved fatal, and the causal microorganism was not isolated. Her sister (F.II.4) was vaccinated with BCG at birth and presented with disseminated BCG infection a few months later. She was successfully treated with antibiotics. She is now 11 years old and well, with no antibiotic treatment. The proband (F.II.5) was not vaccinated with BCG at birth. She developed disseminated M. chelonae infection at age 3 years. She did not respond to treatment and died of disseminated infection at 5 year of age. She also presented Salmonella (group B) adenitis at age 3 years. Two other siblings were vaccinated with BCG, with no adverse effects, and are currently healthy at ages 16 and 13 years. The other three children were not vaccinated and are currently healthy at ages 5, 2, and 1 year. Our study was done in compliance with institutional requirements, and informed consent was obtained from each patient’s family.
Cell Culture
Epstein-Barr virus–immortalized lymphoblastoid (EBV-B) cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 (GibcoBRL) supplemented with 10% heat-inactivated pooled fetal-calf serum (GibcoBRL). They were incubated in 24-well plates at a concentration of 1×106 cells/ml with 10-7 M phorbol-12,13-dibutyrate (PDBu) (Sigma) for 18 h. EBV-B cell lines derived from a healthy individual and from a patient previously diagnosed with IL12 deficiency (Altare et al. 1998c) were used as a positive control (C+) and as a negative control (C−), respectively. Whole-blood cells were cultured in RPMI 1640 (GibcoBRL) in 24-well plates. The cells were infected with live BCG (M. bovis BCG, Pasteur substrain) either at a 20:1 multiplicity of infection alone or in the presence of IFNγ (5,000 IU/ml) or recombinant IL12p70 (20 ng/ml), for a period of 12 or 72 h.
Enzyme-Linked Immunosorbent Assay (ELISA)
Cell-culture supernatants were assayed for cytokines by ELISA, by use of matched antibody pairs according to the manufacturer’s suggestions. ELISA reagents for human IL12p70 and IL12p40 (R&D Systems), TNFα (CLB), and IFNγ (CLB) were used. Optical density was determined for each well, at 490 nm for IL12p70 and at 450 nm for IL12p40, TNFα, and IFNγ, by use of an automated MR5000 ELISA reader (Dynatech). The detection limits of the assays were 0.625 pg/ml for IL12p70, 31.2 pg/ml for IL12p40, 1.4 pg/ml for TNFα, and 5 pg/ml for IFNγ.
RNA Extraction and cDNA Synthesis
Total RNA was isolated from 2×107 EBV-B cell lines, with RNAzol (Biogenesis) and chloroform isoamylalcohol (Sigma). RNA was reverse transcribed by oligo (dT) with Superscript II reverse transcriptase (GibcoBRL), to produce the first-strand cDNA. In brief, 5 μg of total RNA suspended in RNase-free water was added to 20 μl of a reaction mixture consisting of 12.5 mM Tris-HCl, 0.75 mM MgCl2, 75 mM KCl, 5 mM DTT, and 0.5 mM each dNTP. After an initial incubation for 5 min at 42°C, 200 U of Superscript II reverse transcriptase was added. The reaction was continued for 60 min at 42°C and then was terminated by heating at 70°C for 10 min. The single-stranded cDNA was then stored at −20°C.
PCR Amplification
PCR amplification was performed with 1 μl of cDNA template and 50 μl of a reaction mixture consisting of 10 mM Tris-HCl, 2 mM MgCl2, 50 mM KCl, 0.2 mM each dNTP, and 1.25 U Taq DNA polymerase (Applied Biosystems). The primers used to amplify the coding regions of the IL12B and IL12A cDNAs (used at a concentration of 0.1 μM) were as follows: IL12B, sense (5′-ggC CCA gAg CAA gAT gTg TC-3′) and antisense (5′-Tgg gTC TAT TCC gTT gTg TC-3′); and IL12A, sense (5′-TCA CCg AgA AgC TgA TgT Ag-3′) and antisense (5′-Tgg ATg TAA TAg TCC CAT CC-3′). The IL12B and IL12A cDNA coding regions were amplified by PCR under the following conditions: initial denaturation at 94°C for 5 min; followed by 40 cycles at 94°C for 30 s, 55°C for 1 min, and 72°C for 90 s; and a final elongation step at 72°C for 10 min.
Genomic DNA Extraction and Amplification
Human genomic DNA was isolated from 2×107 EBV-B cells. The cells were lysed in extraction buffer (10 mM Tris, 0.1 M EDTA, 0.5% SDS, and 10 mg proteinase K/ml) overnight at 37°C and were extracted with phenol and chloroform, and the DNA was precipitated in ethanol. PCR amplification was performed using 100 ng genomic DNA as the template and 50 μl of a reaction mixture consisting of 10 mM Tris-HCl, 2 mM MgCl2, 50 mM KCl, 0.2 mM each dNTP, and 1.25 U Taq DNA polymerase (Applied Biosystems). The primers and conditions used for PCR amplification of the coding exons of IL12B, including the flanking intron sequences, are available on request.
Sequencing
Amplified PCR products were analyzed by gel electrophoresis in a 1% agarose gel and were purified by centrifugation through Sephadex G-50 Superfine resin (Amersham) on filter plates multiscreen MAHV-N45 (Millipore). A series of nested primers was used for sequencing (available on request). PCR products were sequenced by dideoxynucleotide termination with the BigDye terminator kit (Applied Biosystems). Sequences were analyzed on an ABI Prism 377 apparatus (Applied Biosystems).
Northern Blotting
Total RNA (10 μg) was separated by electrophoresis in a denaturing 1% agarose gel containing 4.5% formaldehyde at 55 V for 3 h, was transferred to a nitrocellulose membrane (Dupont-NEN), and was probed with [32P]-labeled IL12B or GAPDH probes consisting of 1-kb fragments of wild-type cDNA PCR products. The probes were produced by random priming. The primers used to amplify IL12B were sense (5′-ggC CCA gAg CAA gAT gTg TC-3′) and antisense (5′-Tgg gTC TAT TCC gTT gTg TC-3′). Transfer and hybridization were performed as described elsewhere (Jouanguy et al. 1996).
Southern Blotting
Genomic DNA (20 μg) was digested with BamHI (GibcoBRL), was run on a 0.6% agarose gel at 55 V for 4 h, was blotted onto a nitrocellulose membrane (Dupont-NEN), and was hybridized with a [32P]-labeled IL12B probe consisting of a 1-kb fragment of a wild-type cDNA PCR product. The primers used to amplify IL12B were sense (5′-ggC CCA gAg CAA gAT gTg TC-3′) and antisense (5′-Tgg gTC TAT TCC gTT gTg TC-3′). Transfer and hybridization were performed as described elsewhere (Altare et al. 1998c).
Polymorphic Marker Genotyping
Polymorphic CA repeats located in a 10,567-Mb interval around IL12B are available from Généthon. They were amplified, [33P]-labeled, and subjected to gel electrophoresis as described elsewhere (Jouanguy et al. 1996). Three single-nucleotide polymorphisms (SNPs) in IL12B (−1356G/T, 364+92A/G, and 1146 C/A) were genotyped by sequencing.
Dating of Mutations
We estimated the age of the two founder mutations (g.482+82_856-854del and g.315_316insA) by a likelihood method that we recently developed (E.G. and L.A., unpublished data). This method assumes that the affected individuals are descended from a common ancestor in whom the mutation was introduced n generations ago. The method estimates n on the basis of the size of the haplotype shared by patients on each side of the disease locus, D, by a maximum-likelihood approach. The likelihood expression is based on two functions: (1) the probability that no crossing-over occurred during n generations between D and a marker x located at a recombination fraction θx from D, a probability denoted as S(x)=(1-θx)n, and (2) the probability that a crossing-over occurred in the xth interval between markers x-1 and x located at recombination fractions θx-1 and θx (with θx-1<θx), a probability denoted as S(x-1)-S(x). The method also takes into account marker-allele frequencies, allowing for the possibility that recombination occurs not in the xth interval but in the (x-1)th or the (x-2)th interval, with a chromosome carrying the same allele or alleles as the ancestral haplotype at the (x-1)th or (x-2)th marker. Marker-allele frequencies were estimated from a sample of 32 subjects of Indian origin (g.482+82_856–854del mutation) and 25 subjects of Arabian origin (g.315_316insA mutation). The 95% confidence interval (95%CI) of n was obtained by a Bayesian approach, as proposed by Piccolo et al. (1996). Large simulation studies have shown that this method provides reliable estimates of n and have highlighted the importance of taking into account marker-allele frequencies in the likelihood expression (E.G. and L.A., unpublished data). Since the genetic distances available for closely linked markers are generally not very accurate, rates of recombination between markers were computed using both the overall genetic length of the haplotype and the physical distances between markers. Since the genetic and physical distances between D5S410 and D5S422, the two extreme markers of the haplotype, are 7.1 cM (Généthon Map) (Dib et al. 1996) and 10.567 Mb, respectively, as calculated by BLAST human-genome searches, the correspondence between genetic and physical distances over the whole region was estimated to be 0.672 cM for 1 Mb. Recombination fractions between the different markers and IL12B were then computed from genetic distances, by means of the Kosambi mapping function.
Results
Impaired Secretion of IL12 in EBV-B Cells
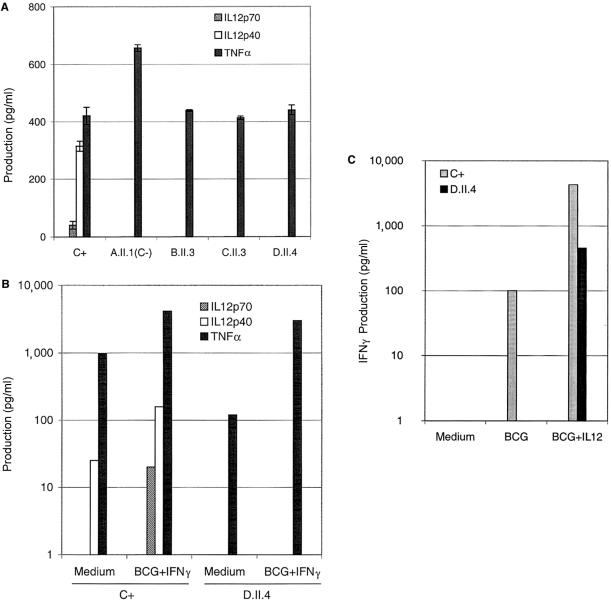
PDBu-activated EBV-B cell lines are known to secrete detectable levels of IL12p40 and IL12p70 (Trinchieri 1998). We therefore tested cells derived from 70 patients with idiopathic BCG or NTM disease, for IL12 secretion. Three activated cell lines (from patients B.II.3, C.II.3, and D.II.4) secreted no IL12p40 and no IL12p70 into the supernatant, as detected by ELISA (fig. 2A). Both IL12p70 and IL12p40 were detected in the supernatant of PDBu-activated control B cells (i.e., C+) (fig. 2A). As expected, neither IL12p40 nor IL12p70 was detected in the supernatant of PDBu-activated EBV-B cells from a child previously reported to have IL12p40 deficiency (i.e., C−; A.II.1) who carried a large homozygous loss-of-function deletion (g.482+82_856–854del) in IL12B (Altare et al. 1998c). Inducible TNFα levels confirmed that EBV-B cells from the two controls and from patients B.II.3, C.II.3, and D.II.4 were fully activated by PDBu (fig. 2A). Defective IL12 production was observed in EBV-B cells derived from three affected siblings from kindreds C (C.II.2) and D (D.II.1 and D.II.2) (data not shown). EBV-B cells were not available from affected siblings B.II.1 and C.II.1. These results suggest that at least six patients, from kindreds B–D, had IL12 deficiency.
Figure 2.
Impaired production of IL12. A, Production of IL12p70, IL12p40, and TNFα, by PDBu-stimulated EBV-B cells from a healthy positive control (C+) and a negative control (C−; A.II.1) (both of whom had previously been reported by Altare et al. [1998c]) and from patients (B.II.3, C.II.3, and D.II.4). Supernatants were harvested after 18 h of activation, for cytokine quantification by ELISA. The results are the mean ± SDs of two experiments. B, Production of IL12p70, IL12p40, and TNFα, by whole-blood cells from a healthy positive control (C+) and from patient D.II.4, either unstimulated (Medium) or stimulated with BCG plus IFNγ. Supernatants were harvested after 12 h of activation. C, Production of IFNγ by whole-blood cells from healthy positive control (C+) and from patient D.II.4, either unstimulated (Medium) or stimulated with BCG alone or with BCG plus recombinant IL12p70. The supernatants were harvested after 72 h of activation, for cytokine quantification by ELISA.
Impaired Secretion of IL12 in Blood Cells
To validate the results obtained with EBV-B cells, we measured the production of IL12p40 and IL12p70 by whole-blood cells from patient D.II.4; the cells were either unstimulated or stimulated with live BCG plus IFNγ (fig. 2B). Neither IL12p70 nor IL12p40 was detected by ELISA. In contrast, both IL12p70 and IL12p40 were detected in the control-cell supernatant (i.e., C+) (fig. 2B). Inducible TNFα levels confirmed that blood cells from the control and patient D.II.4 were fully activated (fig. 2B). Similar results were obtained if cells were stimulated with BCG alone (not shown). In a previous experimental study, we had shown that neither IL12p40 nor IL12p70 was detectable in the supernatant of similarly activated blood cells derived from patient A.II.1 (i.e., C−) (Altare et al. 1998c). We therefore measured IFNγ production by blood cells from a control (i.e., C+) and from patient D.II.4, the cells being either unstimulated or stimulated by live BCG or BCG plus IL12p70 (fig. 2C). The addition of recombinant exogenous IL12p70 led to an increase in IFNγ production in the patient’s blood cells, to almost normal levels, in response to BCG challenge (fig. 2C). This experiment shows that the lack of detectable IL12 accounts for the poor production of IFNγ.
A Large Deletion in IL12B
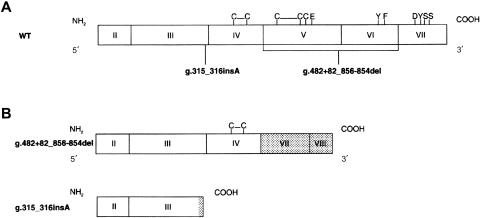
A cDNA was synthesized from mRNA derived from the EBV-B cells of patient B.II.3. This cDNA was used to amplify and sequence the IL12A and IL12B coding regions. A large frameshift deletion of 373 nt was detected between positions 482 and 856 in IL12B (not shown). The sequence of the coding region of the IL12A gene displayed no mutation. Genomic DNA was used as the template for PCR amplification of the eight IL12B exons and flanking intron regions. A homozygous 4.6-kb frameshift deletion mutation encompassing coding exons V and VI was identified and designated “482+82_856–854del” (fig. 3) (Dunnen and Antonarakis 2000). This deletion is identical to that found in the Pakistani patient (A.II.1) who elsewhere had been reported to have IL12p40 deficiency (Altare et al. 1998c) and was not found in 174 unrelated healthy controls from the Indian subcontinent (59 of whom were from the Gujarat region). This indicates that the frequency of this deletion can be estimated (with a P >.95) to be <0.009. By northern blot analysis, IL12B transcripts were not detected in B.II.3 and A.II.1 (i.e., C−) EBV-B cells, in contrast to those of the positive control (not shown). This mutation results in the loss of 167 of the original 328 amino acids and in the addition of 45 new amino acids to the COOH-terminal region of the mature polypeptide. In a previous experimental study, we had demonstrated that the g.482+82_856-854del mutation is a loss-of-function mutation, by transfecting a B cell line from the patient with the mutant and a wild-type IL12B allele (Altare et al. 1998c). As in kindred A (Altare et al. 1998c), we showed by Southern blot analysis that patient B.II.3 was homozygous—and that her healthy brother and mother were heterozygous—for the deletion, strongly suggesting that the deletion is recessive in kindred B (not shown). DNA from the father and from the first, deceased child (B.II.1) was not available for analysis.
Figure 3.
IL12B mutant alleles. A, Schematic representation of structural organization of coding region of wild-type (WT) IL12B gene. Exons I and VIII are not translated. Intrachain disulfite bounds and amino acids essential for dimerization with IL12p35 are indicated by the single-letter amino acid code. The positions of the two mutations found in IL12-deficient patients are also indicated. B, Schematic representation of coding region of two mutant IL12B alleles. Gray regions correspond to stretches of new amino acids resulting from the frameshift mutation.
A Small Insertion in IL12B
Sequencing of the coding region of the IL12B cDNA from patients C.II.3 and D.II.4 revealed the insertion of an adenosine between positions 315 and 316 (not shown). No mutation was observed in the sequence of the coding region of IL12A. Genomic amplification and sequencing of the IL12B exon III confirmed that the mutation (designated “g.315_316insA”) was homozygous (fig. 3). This mutation was not found in 115 Arabian healthy unrelated controls analyzed (73 of whom were from the Arabian Peninsula). This indicates that the frequency of this insertion can be estimated (with a P >0.95) to be <0.012. The frameshift results in a premature stop codon at nucleotide positions 342–344. By northern blot analysis, IL12B transcripts were not detected in PDBu-stimulated EBV-B cells from patients C.II.3 and D.II.4. IL12B transcripts were detected in PDBu-stimulated EBV-B cells from a control (i.e., C+) (not shown). This mutation results in the loss of 223 of the original 328 amino acids and in the addition of 9 new C-terminal amino acids. The encoded protein lacks all the amino acids shown by crystallography to be essential for p40 dimerization with p35 (fig. 3) (Yoon et al. 2000). Healthy relatives were found to be either heterozygous for the g.315_316insA mutant allele or homozygous for a wild-type allele. These data strongly suggest that the g.315_316insA mutation accounts for the lack of detectable p40 and p70 and is a loss-of-function recessive mutation.
IL12 Deficiency in Other Kindreds
We next sequenced IL12B exons in 16 patients selected from 4 kindreds from the Indian subcontinent and from 10 kindreds from the Arabian Peninsula, for whom EBV-B cells were not available. We identified four patients (E.II.2, F.II.3, F.II.4, and F.II.5) from two new kindreds (E and F) from Saudi Arabia that carried the homozygous mutation g.315_316insA (fig. 1). No mutations were found in the other patients. Parents and healthy siblings from kindreds C–F were heterozygous for g.315_316insA (fig. 1). DNA from the first deceased sibling (B.II.1) of kindred B and from the healthy siblings (F.II.1, F.II.2, F.II.6, F.II.7, and F.II.8) of kindred F were not available for analysis. No other mutations were found in the coding region of IL12B. In total, nine patients from four kindreds (C–F) from Saudi Arabia were found to be homozygous for an identical loss-of-function IL12B frameshift insertion (g.315_316insA) (fig. 1).
A Founder Effect Accounts for the Recurrence of These Mutations
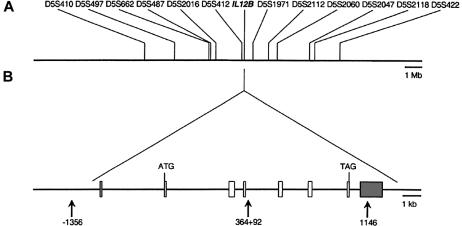
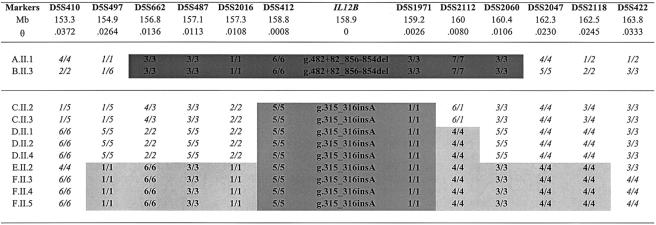
Polymorphic markers encompassing the IL12B gene on 5q31.1-5q33, including flanking dinucleotide repeats (D5S410, D5497, SD5S662, D5S487, D5S2016, D5S412, D5S1971, D5S2112, D5S2060, D5S2047, D5S2118, and D5S422) and intragenic SNPs (−1356 G/T, 364+92 A/G, and 1146 C/A) were genotyped in all patients (fig. 4). A 3.6-Mb homozygous haplotype defined by the three intragenic SNPs (not shown) and by seven microsatellites (D5S662–D52060) is shared by all affected siblings from kindreds A and B (who are homozygous for the g.482+82_856–854del mutation) (fig. 5). Patients from kindreds C–F (homozygous for the g.315_316insA mutation) share a 0.516-Mb common homozygous haplotype defined by the three intragenic SNPs (not shown) and two microsatellites (D5S412 and D5S1971) (fig. 5). These data strongly suggest that each mutation results from a founder event. We developed a new mutation-dating method to date both the closest ancestor common to all patients from kindreds A and B and the closest ancestor to all patients from kindreds C–F (E.G. and L.A., unpublished data). Indeed, methods described elsewhere (Guo and Xiong 1997; McPeek and Strahs 1999; Slatkin and Rannala 2000) do not apply to very small numbers of kindreds. For the g.482+82_856–854del mutation, the estimated number of generations since the appearance of the common ancestor is 29 (95%CI 9–115). If it is assumed that a generation is ∼24 years, then the g.482+82_856–854del mutation in IL12B is ∼700 years old (95%CI 216–2,760). For the g.315_316insA mutation, the estimated number of generations is 47 (95%CI 22–110), indicating that this mutation in IL12B is ∼1,100 years old (95%CI 528–2,640).
Figure 4.
High-resolution map of IL12B locus. A, Physical map of IL12B region on chromosome 5q33. The physical distances (in Mb) between IL12B and microsatellite markers were obtained from BLAST. B, Genomic organization of human IL12B gene and location of intragenic SNPs. White boxes indicate coding exons; gray boxes indicate untranslated exons. Translation initiation and termination codons are indicated. Arrows indicate positions of intragenic SNPs (−1356, 364+92, and 1146).
Figure 5.
Haplotypes encompassing IL12B in patients with IL12 deficiency. Markers encompassing IL12B, with physical distance (Mb) and recombination rate (θ), are shown. Physical distance was obtained from BLAST; the recombination rate was estimated by use of the correspondence of .672 cM for 1 Mb, over the whole region, and use of the Kosambi mapping function (see the “Dating of Mutations” subsection). The ancestral haplotypes shared by kindreds A and B, from the Indian subcontinent, is indicated by dark-gray shading; the ancestral haplotype shared by kindreds C–F, from Saudi Arabia, is indicated by intermediate-gray shading; and the ancestral haplotype shared by a subset of kindreds is indicated by light-gray shading.
Discussion
We report herein 12 patients from five new kindreds with complete IL12 deficiency associated with IL12B mutations. Our study illustrates that, although rare, this human cytokine gene disorder is not limited to the single patient who has been described elsewhere (Altare et al. 1998c). Twelve of the 13 patients with IL12 deficiency were found to suffer from mycobacteriosis, and 5 were found to suffer from salmonellosis, which was the sole clinical manifestation in 1 child. Despite an overall better clinical outcome than that had by patients with complete IFNγR deficiency, interfamilial heterogeneity was observed. In one kindred from India, we found a large deletion that was previously reported in a kindred from Pakistan (Altare et al. 1998c), whereas, in four kindreds from Saudi Arabia, we identified a new recessive loss-of-function IL12B small insertion. Each rare mutation resulted from a founder effect in either the Indian subcontinent (large deletion) or the Arabian Peninsula (small insertion), and, by a new method, we were able to date those original mutation events to ∼700 and ∼1,100 years ago, respectively.
This study confirms that IL12 deficiency is principally associated with infectious diseases caused by Mycobacteria (table 1). All but one of the patients reported here have had at least one episode of mycobacterial infection. All children inoculated with live BCG vaccine developed clinical infection, as did the previously reported IL12-deficient patient (Altare et al. 1998c). One patient who was not inoculated with BCG developed NTM infection (a fast-growing species, M. chelonae). One child with BCG infection subsequently developed M. tuberculosis adenitis. Most patients with IL12Rβ1 deficiency are susceptible to BCG (Altare et al. 1998a; de Jong et al. 1998; Verhagen et al. 2000), and two children who were apparently resistant to BCG developed M. chelonae (Aksu et al. 2001) and M. tuberculosis (Altare et al. 2001) infections. Another NTM, M. avium, which was detected in IL12Rβ1-deficient patients not previously inoculated with BCG (de Jong et al. 1998; Verhagen et al. 2000; Sakai et al. 2001), has not, to date, been diagnosed in the series of IL12-deficient patients whom we studied. Experimental studies have shown that IL12 is essential for protective immunity against Mycobacteria in mice. Animals with disrupted IL12B genes are highly susceptible to BCG (Cooper et al. 1997; Wakeham et al 1998), M. avium (Doherty and Sher 1998; Silva et al. 2001), and M. tuberculosis (Magram et al. 1996). Our results extend these observations, highlighting the crucial role that IL12 plays in antimycobacterial immunity in mice and humans. Clinically, IL12 deficiency should be considered in the genetic diagnosis of patients with BCG or NTM severe diseases. Further studies are required to investigate the role that rare and frequent IL12B mutations play in susceptibility to tuberculosis in the general population (Abel and Casanova 2000).
Together with our previous report (Altare et al. 1998c), this study suggests that the course of mycobacterial infection in patients with complete IL12 deficiency is less severe than that in patients with complete IFNγR deficiency (Jouanguy et al. 1996; Newport et al. 1996; Pierre-Audigier et al. 1997; Dorman and Holland 1998; Roesler et al. 1999; Cunningham et al. 2000; Jouanguy et al. 2000; Allende et al. 2001). Only 5 of the 13 IL12-deficient patients (aged 2–11 years) died of infection; the 8 survivors (aged 3–12 years) responded well to antibiotics and are now well and not currently taking medication (i.e., for 2–9 years have not undergone treatment). Granuloma formation is preserved but often multibacillary in patients with IL12 deficiency, whereas patients with complete IFNγR deficiency are not able to form mature granulomas. However, there is considerable interfamilial heterogeneity in terms of outcome. Nine patients have presented disseminated mycobacterial infection, which has proved fatal in four cases, whereas, in three children from the same kindred, BCG infection was clinically limited to the draining lymph nodes. Accordingly, the clinical outcome of IL12Rβ1 deficiency also differs greatly between patients, with two deaths reported (at age 5–8 years) and 10 survivors (aged 7–31 years) well and not currently taking medication (Altare et al. 1998a; de Jong et al. 1998; Verhagen et al. 2000; Aksu et al. 2001; Altare et al. 2001; Sakai et al. 2001).
Five patients have presented clinical Salmonella infection, as did the IL12-deficient patients described by Altare et al. (1998c) and 6 of 12 IL12Rβ1-deficient patients reported elsewhere (Altare et al. 1998a; de Jong et al. 1998; Verhagen et al. 2000). This is a well-known feature of the syndrome of Mendelian susceptibility to mycobacterial disease (Levin et al. 1995; Casanova et al. 1996). Other severe infections caused by Listeria monocytogenes (Roesler et al. 1999), Histoplasma capsulatum (Jouanguy et al. 1999), and viruses (Dorman et al. 1999; Cunningham et al. 2000; Uzel et al. 2000), which elsewhere have been reported in patients with IFNγR deficiency, were not diagnosed in patients with IL12 or IL12R deficiency; however, we report, for the first time, a case of clinical N. asteroides infection in a patient with an impaired IL12-IFNγ axis. It is difficult to determine whether there is a causal relationship between impaired IL12-mediated immunity and nocardiosis. Such a relationship is probable, however, given the considerable biochemical and phylogenetic similarity between Mycobacterium and Nocardia, which form, together with Corynebacterium, a monophyletic taxon of mycolic acid–containing acid-fast gram-positive bacteria (Embley and Stackebrandt 1994).
A novel heterodimeric cytokine, interleukin-23 (IL23), has recently been described (Oppmann et al. 2000). This cytokine consists of the p40 subunit of IL12 and a novel p19 subunit, encoded by a gene mapping to 12q13. IL23 is produced by activated mature dendritic cells and induces IFNγ production by memory T lymphocytes in humans and in mice. The patients with IL12p40 deficiency described herein therefore probably suffer not only from IL12 deficiency but also from IL23 deficiency. Further studies with hIL23-specific antibodies are necessary to investigate whether this is indeed the case. If IL23 deficiency is established in these patients, it will be important to discriminate between the specific contributions of IL12 and IL23 deficiencies to the development of mycobacterial disease in patients with IL12B mutations. IL12p35-deficient mice have a phenotype milder than do IL12p40-deficient mice, in terms of vulnerability to Leishmania major (Mattner et al. 1996) and to Cryptococcus neoformans (Decken et al. 1998), suggesting that IL23 may perhaps contribute to protection against mycobacteria. The identification of IL12p35-deficient patients with a milder phenotype than that of IL12p40-deficient patients would support this hypothesis.
Only two IL12B mutant alleles accounted for IL12 deficiency in the six kindreds identified. A large deletion was found in patients from the Indian subcontinent, and a small insertion was found in patients from the Arabian Peninsula. The Pakistani child and the Indian child, both originating from the northern region of the Indian subcontinent, presented a large deletion (g.482+82_856–854del) encompassing exons V and VI (Altare et al. 1998c). A 3.6-Mb homozygous haplotype encompassing IL12B is shared by the two patients, implying that a founder effect is involved. The g.315_316insA mutation, which was found in four unrelated kindreds from Saudi Arabia, has not previously been described and the 0.516-Mb homozygous haplotype shared by the four kindreds is also strongly suggestive of a founder effect. These are the first two examples of founder effects in genetic defects of the IL12-IFNγ axis, which predispose the individual to mycobacterial disease. The closest common ancestor carrying the founder allele in each region was dated by a new method (E.G. and L.A., unpublished data), which was developed for use with very small numbers of kindreds. The common ancestor of patients carrying the Indian deletion was estimated to have lived ∼700 years ago, whereas the common ancestor of patients carrying the Saudi Arabian insertion was estimated to have lived ∼1,100 years ago. The two IL12B mutant alleles are probably rare (frequency < .01) in the respective populations, because they were not found in all healthy individuals genotyped. Novel homozygous cases are, however, likely to be identified in the future, particularly in more-specific regions (e.g., northern India–Pakistan and Saudi Arabia). A direct clinical implication of this study is that well-defined IL12B mutations should be sought as a matter of priority in patients with mycobacteriosis and salmonellosis who originate from either the Indian subcontinent or the Arabian Peninsula.
Acknowledgments
We would like to thank members of INSERM U550, for helpful discussions; C. Antignac (INSERM U423), V. Cormier-Daire (INSERM U393), J. El Baghdadi (Institut Pasteur, Morocco), B. Al Ramadi (Al Ain Hospital, United Arab Emirates), P. Frossard (Aga Khan University, Pakistan), R. Krishnamoorthy (INSERM U458), and N. Gerard (INSERM U458), for control DNA samples; and D. Recan, for the EBV transformation of B cells. C.P. would like to thank A. Fischer for his support. C.P. was supported by Aventis Pasteur-Mérieux, Gallia, and INSERM. C.F. was supported by the Association pour la recherche contre le cancer. This work was supported by Action Incitative Blanche, Fondation BNP-Paribas, Fondation pour la Recherche Médicale, Fondation Schlumberger, Institut Universitaire de France, and Programme National de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires.
Electronic-Database Information
The accession number and URLs for data in this article are as follows:
- BLAST, http://www.ncbi.nlm.nih.gov/BLAST/ (for localization of microsatellites markers from the chromosome 5 and IL12B sequences)
- Généthon Map, ftp://ftp.genethon.fr/pub/Gmap/Nature-1995/data/data_chrom5 (for microsatellites markers from the chromosome 5 region)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for familial atypical mycobacteriosis [MIM 209950])
References
- Abel L, Casanova JL (2000) Genetic predisposition to clinical tuberculosis: bridging the gap between simple and complex inheritance. Am J Hum Genet 67:274–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu G, Trpan C, Çavuolu C, Soydan S, Altare F, Casanova JL, Kutukculer N (2001) Mycobacterium fortuitum-chelonae complex infection in a child with complete interleukin-12 receptor β1 deficiency. Pediatr Infect Dis J 20:551–553 [DOI] [PubMed] [Google Scholar]
- Allende LM, Lopez-Goyanes A, Paz-Artal E, Corell A, Garcia-Perez MA, Varela P, Scarpellini A, Negreira, S, Palenque E, Arnaiz-Villena A (2001) A point mutation in a domain of gamma interferon receptor 1 provokes severe immunodeficiency. Clin Diagn Lab Immunol 8:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL (1998a) Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432–1435 [DOI] [PubMed] [Google Scholar]
- Altare F, Ensser A, Breiman A, Reichenbach J, El Baghdadi J, Fischer A, Emile JF, Gaillard JL, Casanova JL (2001) IL-12 deficiency in a patient with abdominal tuberculosis. J Infect Dis 184:231–236 [DOI] [PubMed] [Google Scholar]
- Altare F, Jouanguy E, Lamhamedi-Cherradi S, Fondaneche MC, Fizame C, Ribierre F, Merlin G, Dembic Z, Schreiber R, Lisowska-Grospierre B, Fischer A, Seboun E, Casanova JL (1998b) A causative relationship between mutant IFNgR1 alleles and impaired cellular response to IFNγ in a compound heterozygous child. Am J Hum Genet 62:723–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altare F, Lammas D, Revy P, Jouanguy E, Döffinger R, Lamhamedi S, Drysdale P, Scheel-Toellner D, Girdlestone J, Darbyshire P, Wadhwa M, Dockrell H, Salmon M, Fischer A, Durandy A, Casanova JL, Kumararatne DS (1998c) Inherited interleukin 12 deficiency in a child with bacille Calmette-Guérin and Salmonella enteritidis disseminated infection. J Clin Invest 102:2035–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JL, Blanche S, Emile JF, Jouanguy E, Lamhamedi S, Altare F, Stephan JL, Bernaudin F, Bordigoni P, Turck D, Lachaux A, Albertini M, Bourrillon A, Dommergues JP, Pocidalo MA, Le Deist F, Gaillard JL, Griscelli C, Fischer A (1996) Idiopathic disseminated bacillus Calmette-Guerin infection: a French national retrospective study. Pediatrics 98:774–778 [PubMed] [Google Scholar]
- Casanova JL, Jouanguy E, Lamhamedi S, Blanche S, Fischer A (1995) Immunological conditions of children with BCG disseminated infection. Lancet 346:581 [DOI] [PubMed] [Google Scholar]
- Cooper AM, Magram J, Ferrante J, Orme IM (1997) Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med 186:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JA, Kellner JD, Bridge PJ, Trevenen CL, Mcleod DR, Davies HD (2000) Disseminated bacille Calmette-Guérin infection in an infant with a novel deletion in the interferon-γ receptor gene. Int J Tuberc Lung Dis 4:791–794 [PubMed] [Google Scholar]
- Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G (1998) Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 66:4994–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH (1998) Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435–1438 [DOI] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Döffinger R, Jouanguy E, Dupuis S, Fondanèche MC, Stephan JL, Emile JF, Lamhamedi-Cherradi S, Altare F, Pallier A, Barcenas-Morales G, Meinl E, Krause C, Pestka S, Schreiber RD, Novelli F, Casanova JL (2000) Partial interferon-γ receptor signaling chain deficiency in a patient with bacille Calmette-Guérin and Mycobacterium abscessus infection. J Infect Dis 181:379–384 [DOI] [PubMed] [Google Scholar]
- Doherty TM, Sher A (1998) IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol 160:5428–5435 [PubMed] [Google Scholar]
- Dorman SE, Holland SM (1998) Mutation in the signal-transducing chain of the interferon-γ receptor and susceptibility to mycobacterial infection. J Clin Invest 101:2364–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman SE, Shaw S, Uzel G, Buckley R, Holland SM (2000) A novel IFNGR2 mutation associated with disseminated M. abscessus infection in a Qatari infant. Abstract presented at the Second Annual Meeting of the Association for Patient Oriented Research, Arlington, VA, March 11–13 [Google Scholar]
- Dorman SE, Uzel G, Roesler J, Bradley JS, Bastian J, Billman G, King S, Filie A, Schermerhorn J, Holland SM (1999) Viral infections in interferon-γ receptor deficiency. J Pediatr 135:640–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnen JT, Antonarakis SE (2000) Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15:7–12 [DOI] [PubMed] [Google Scholar]
- Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rozenzweig S, Harris J, Holand SM, Schreiber RD, Casanova JL (2001) Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293:300–303 [DOI] [PubMed] [Google Scholar]
- Dupuis S, Döffinger R, Picard C, Fieschi C, Altare F, Jouanguy E, Abel L, Casanova JL (2000) Human interferon-γ-mediated immunity is a genetically controlled continuous trait that determines the outcome of mycobacterial invasion. Immunol Rev 178:129–137 [DOI] [PubMed] [Google Scholar]
- Embley TM, Stackebrandt E (1994) The molecular phylogeny and systematics of the actinomycetes. Annu Rev Microbiol 48:257–289 [DOI] [PubMed] [Google Scholar]
- Emile JF, Patey N, Altare F, Lamhamedi S, Jouanguy E, Boman F, Quillard J, Lecomte-Houcke M, Verola O, Mousnier JF, Dijoud F, Blanche S, Fischer A, Brousse N, Casanova JL (1997) Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J Pathol 181:25–30 [DOI] [PubMed] [Google Scholar]
- Frucht DM, Holland SM (1996) Defective monocyte costimulation for IFN-γ production in familial disseminated Mycobacterium avium complex infection: abnormal IL-12 regulation. J Immunol 157:411–416 [PubMed] [Google Scholar]
- Frucht DM, Sandberg DI, Brown MR, Gerstberger SM, Holland SM (1999) IL-12-independent costimulation pathways for interferon-γ production in familial disseminated Mycobacterium avium complex infection. Clin Immunol 91:234–241 [DOI] [PubMed] [Google Scholar]
- Guo SW, Xiong M (1997) Estimating the age of mutant disease alleles based on linkage disequilibrium. Hum Hered 47:315–337 [DOI] [PubMed] [Google Scholar]
- Holland SM, Dorman SE, Kwon A, Pitha-Rowe IF, Frucht DM, Gerstberger SM, Noel GJ, Vesterhus P, Brown MR, Fleisher TA (1998) Abnormal regulation of interferon-γ, interleukin-12, and tumor necrosis factor-α in human interferon-γ receptor 1 deficiency. J Infect Dis 178:1095–1104 [DOI] [PubMed] [Google Scholar]
- Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova JL (1996) Interferon-γ–receptor deficiency in an infant with fatal bacille Calmette–Guérin infection. N Engl J Med 335:1956–1961 [DOI] [PubMed] [Google Scholar]
- Jouanguy E, Dupuis S, Pallier A, Döffinger R, Fondanèche MC, Fieschi C, Lamhamedi-Cherradi S, Altare F, Emile JF, Lutz P, Bordigoni P, Cokugras H, Akcakaya N, Landman-Parker J, Donnadieu J, Camcioglu Y, Casanova JL (2000) In a novel form of IFN-γ receptor 1 deficiency, cell surface receptors fail to bind IFN-γ. J Clin Invest 105:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondanèche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber RD, Levin M, Fischer A, Hivroz C, Casanova JL (1997) Partial interferon-γ receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guérin infection and a sibling with clinical tuberculosis. J Clin Invest 100:2658–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondanèche MC, Dupuis S, Döffinger R, Altare F, Girdlestone J, Emile JF, Ducoulombier H, Edgar D, Clarke J, Oxelius VA, Brai M, Novelli F, HeyneK, Fischer A, Holland SM, Kumararatne DS, Schreiber RD, Casanova JL (1999) A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet 21:370–378 [DOI] [PubMed] [Google Scholar]
- Levin M, Newport MJ, D'Souza S, Kalabalikis P, Brown IN, Lenicker HM, Agius PV, Davies EG, Thrasher A, Klein N, Blackwell JM (1995) Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet 345:79–83 [DOI] [PubMed] [Google Scholar]
- Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento UF, Faherty DA, Gately MK (1996) IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity 4:471–481 [DOI] [PubMed] [Google Scholar]
- Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately MK, Louis JA, Alber G (1996) Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol 26:1553–1559 [DOI] [PubMed] [Google Scholar]
- McKusick VA (1998) Mendelian inheritance in man: catalogs of human genes and genetic disorders, 12th ed. Johns Hopkins University Press, Baltimore [Google Scholar]
- McPeek MS, Strahs A (1999) Assessment of linkage disequilibrium by the decay of haplotype sharing, with application to fine-scale genetic mapping. Am J Hum Genet 65:858–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugasu B, Quah TC, Quak SH, Low PS, Wong HB (1988) Disseminated BCG infection-a case report. J Singapore Paediatr Soc 30:139–141 [PubMed] [Google Scholar]
- Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M (1996) A mutation in the interferon-γ–receptor gene and susceptibility to mycobacterial infection. N Engl J Med 335:1941–1949 [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, et al (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725 [DOI] [PubMed] [Google Scholar]
- Piccolo F, Jeanpierre M, Leturcq F, Dodé C, Azibi K, Toutain A, Merlini L, Jarre L, Navarro C, Krishnamoorthy R, Tomé FM, Urtizberea JA, Beckmann JS, Campbell KP, Kaplan JC (1996) A founder mutation in the γ-sarcoglycan gene of gypsies possibly predating their migration out of India. Hum Mol Genet 5:2019–2022 [DOI] [PubMed] [Google Scholar]
- Pierre-Audigier C, Jouanguy E, Lamhamedi S, Altare F, Rauzier J, Vincent V, Canioni D, Emile JF, Fischer A, Blanche S, Gaillard JL, Casanova JL (1997) Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon γ receptor deficiency. Clin Infect Dis 24:982–984 [DOI] [PubMed] [Google Scholar]
- Roesler J, Kofink B, Wendisch J, Heyden S, Paul D, Friedrich W, Casanova JL, Leupold W, Gahr M, Rosen-Wolff A (1999) Listeria monocytogenes and recurrent mycobacterial infections in a child with complete interferon-γ-receptor (IFNγR1) deficiency: mutational analysis and evaluation of therapeutic options. Exp Hematol 27:1368–1374 [DOI] [PubMed] [Google Scholar]
- Sakai T, Matsuoka M, Aoki M, Nosaka K, Mitsuya H (2001) Missense mutation of the interleukin-12 receptor β1 chain–encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood 97:2688–2694 [DOI] [PubMed] [Google Scholar]
- Silva RA, Flórido M, Appelberg R (2001) Interleukin-12 primes CD4+ T cells for interferon-γ production and protective immunity during Mycobacterium avium infection. Immunology 103:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin M, Rannala B (2000) Estimating allele age. Annu Rev Genomics Hum Genet 1:225–249 [DOI] [PubMed] [Google Scholar]
- Trinchieri G (1998) Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol 70:83–243 [DOI] [PubMed] [Google Scholar]
- Uzel G, Premkumar A, Malech HL, Holland SM (2000) Respiratory syncytial virus infection in patients with phagocyte defects. Pediatrics 106:835–837 [DOI] [PubMed] [Google Scholar]
- Verhagen CE, de Boer T, Smits HH, Verreck FA, Wierenga EA, Kurimoto M, Lammas DA, Kumararatne DS, Sanal O, Kroon FP, van Dissel JT, Sinigaglia F, Ottenhoff TH (2000) Residual type 1 immunity in patients genetically deficient for interleukin 12 receptor β1 (IL-12Rβ1): evidence for an IL-12Rβ1–independent pathway of IL-12 responsiveness in human T cells. J Exp Med 192:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Picard C, Jouanguy E, Dupuis S, Popko S,Abughali N, Meyerson H, Casanova JL, Hostoffer RW (2001) Recurrent Mycobacterium avium osteomyelitis associated with a novel dominant interferon gamma receptor mutation. Pediatrics 107:e47 [DOI] [PubMed] [Google Scholar]
- Wakeham J WJ, Magram J, Croitoru K, Harkness R, Dunn P, Zganiacz A, Xing Z (1998) Lack of both type 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette-Guérin in IL-12-deficient mice. J Immunol 160:6101–6111 [PubMed] [Google Scholar]
- Yoon C, Johnston SC, Tang J, Stahl M, Tobin JF, Somers WS (2000) Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J 19:3530–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]