Abstract
The prevalence of type 2 diabetes among Australian residents is 7.5%; however, prevalence rates up to six times higher have been reported for indigenous Australian communities. Epidemiological evidence implicates genetic factors in the susceptibility of indigenous Australians to type 2 diabetes and supports the hypothesis of the “thrifty genotype,” but, to date, the nature of the genetic predisposition is unknown. We have ascertained clinical details from a community of indigenous Australian descent in North Stradbroke Island, Queensland. In this population, the phenotype is characterized by severe insulin resistance. We have conducted a genomewide scan, at an average resolution of 10 cM, for type 2 diabetes–susceptibility genes in a large multigeneration pedigree from this community. Parametric linkage analysis undertaken using FASTLINK version 4.1p yielded a maximum two-point LOD score of +2.97 at marker D2S2345. Multipoint analysis yielded a peak LOD score of +3.9 <1 cM from marker D2S2345, with an 18-cM 3-LOD support interval. Secondary peak LOD scores were noted on chromosome 3 (+1.8 at recombination fraction [θ] 0.05, at marker D3S1311) and chromosome 8 (+1.77 at θ=0.0, at marker D8S549). These chromosomal regions are likely to harbor novel susceptibility genes for type 2 diabetes in the indigenous Australian population.
Introduction
Type 2 diabetes (MIM 125853) is characterized by hyperglycemia due to impaired insulin secretion, insulin resistance in muscle, and nonsuppressible hepatic glucose production (DeFronzo 1988). Genetic factors play an important role in the development of diabetes. The monogenic forms of diabetes account for ∼5% of cases and are caused by mutations in genes encoding insulin, the insulin receptor, glucokinase, and the transcription factors hepatocyte nuclear factor-1α, (HNF-1α), HNF-1β, HNF-4α, insulin promoter factor-1, and NeuroD1/BETA2 (Steiner et al. 1995; Taylor 1995; Bell et al. 1996; Yamagata et al. 1996a, 1996b; Horikawa et al. 1997; Stoffers et al. 1998; Malecki et al. 1999). Mutations in mitochondrial genes can cause diabetes, often in association with hearing loss (Maassen and Kadowaki 1996). There has been relatively little progress in identifying the genes responsible for the more common late-onset forms of type 2 diabetes. These late-onset forms do not have a simple Mendelian genetic basis and are thought to result from the joint action of and complex interaction between genetic and environmental factors.
Type 2 diabetes is a major public health problem. The overall prevalence of type 2 diabetes among Australians aged >25 years is 7.5% (Zimmet and Welborn 2001). Prevalence rates up to six times higher have been reported in indigenous Australian communities (Bastian 1979), with a lower average age at onset of type 2 diabetes. The epidemiological and pathophysiological characteristics of type 2 diabetes support the hypothesis of the “thrifty genotype” (Neel 1963), but, to date, the nature of the genetic predisposition is largely unknown. The high prevalence of type 2 diabetes and associated metabolic disturbances in indigenous Australians are major factors contributing to high morbidity and mortality in this group (O'Dea 1992). In the group 35–54 years of age, the prevalence of diabetes in indigenous Australians is 20%–25%. Complications including renal failure, blindness, and foot ulcers leading to amputation are common. The clustering of hyperglycemia with insulin resistance, elevated plasma triglyceride, low HDL cholesterol, hypertension, and central obesity predisposes to macrovascular disease (O'Dea et al. 1990; O'Dea 1991; Guest et al. 1993; Gault et al. 1996; Hayman 1997).
Epidemiological evidence strongly implicates genetic factors in the susceptibility of indigenous Australians to type 2 diabetes. One line of evidence for genetic influences underlying ethnic differences in diabetes prevalence is the fact that diabetes and insulin resistance are inversely related to the amount of European admixture present in a population. Among indigenous Australians in New South Wales, the prevalence of diabetes was twice as high in inland towns as in coastal towns where European admixture was common (Williams et al. 1987). In a survey in central Australia, glucose intolerance and 2-h insulin levels were inversely related to European admixture (O'Dea et al. 1993). Such genetic effects, however, must depend on interaction with environmental factors, such as weight gain and low physical activity, since type 2 diabetes has remained uncommon among indigenous Australians who are able to continue living as hunter-gatherers (O'Dea 1992).
There are complexities involved in the mapping of diabetes-susceptibility genes. Different ethnic groups have distinct population histories and, in each population, different genes are likely to be responsible for disease susceptibility. Research efforts to date have largely centered on ethnic groups in the United States, Finland, and Western Europe. The genetic analysis of the indigenous Australian population is likely to make a unique contribution to the understanding of diabetes susceptibility and resistance. The major aim of this research program is the identification of susceptibility genes for type 2 diabetes in Australian indigenous pedigrees. The clinical relevance of this includes (i) the development of preclinical screening for at-risk individuals and (ii) the discovery of novel drug targets that may prove amenable to new forms of therapy for type 2 diabetes, both in these populations and more generally.
Methods
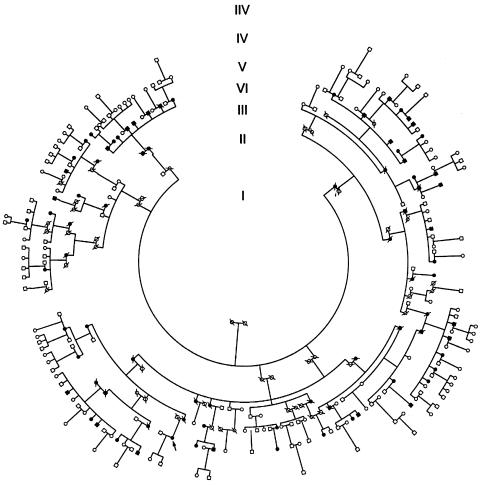
We have ascertained clinical details from a community of indigenous Australian descent in North Stradbroke Island, Queensland. We have conducted a genomewide scan, at an average resolution of 10 cM, for type 2 diabetes–susceptibility genes and have undertaken linkage and quantitative-trait analysis in a large multigeneration pedigree from this community (fig. 1). The pedigree consists of 232 living members, 56 of whom are affected with type 2 diabetes. To date, 138 individuals from the pedigree who are >18 years of age, including 49 who have diabetes, are participating in the study, with recruitment ongoing. The study protocol was approved by the Princess Alexandra Hospital Ethics Committee, and all participants provided informed consent before the study. These studies have been performed after full consultation with and with the approval of the community.
Figure 1.
Pedigree structure. Blackened symbols denote individuals with type II diabetes. Unblackened symbols denote unaffected individuals. The arrow indicates the proband.
Clinical Studies
The protocol included an interview that recorded age at onset of diabetes, history of treatment and complications, and measurements of height, weight, body-mass index (BMI), blood pressure, and waist and hip circumference. A fasting blood sample was taken for measurements of glucose, lipids, and C-peptide. The fasting plasma glucose and C-peptide concentrations were integrated by homeostasis-model assessment (HOMA) of beta-cell function and insulin sensitivity (Matthews et al. 1985; Levy et al. 1991). Beta-cell function and insulin sensitivity measured by HOMA have been shown to correlate with measures obtained by hyperglycemic and euglycemic clamp studies (Matthews et al. 1985; Levy et al. 1991). Individuals were considered “affected” for the linkage analysis if they met one of the following criteria (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 1997): (i) previously diagnosed type 2 diabetes being treated with medical therapy or (ii) fasting plasma glucose ⩾7 mmol/liter. Normoglycemia was defined as fasting plasma glucose <6.1 mmol/liter. Subjects with fasting plasma glucose ⩾6.1 mmol/liter and <7.0 mmol/liter were classified as having impaired fasting glucose. The subjects considered affected with type 2 diabetes had no history of ketosis, had been treated for ⩾3 mo by diet or by oral hypoglycemic agents, and had measurable C-peptide levels. The mean age of the subjects with diabetes was 54±12 years (±1 SD), the mean BMI was 32±7 kg/m2, the median HOMA insulin sensitivity was 16% (interquartile range 11%–29%), and the median HOMA beta-cell function was 166% (interquartile range 89%–237%).
Molecular Studies
DNA was prepared from peripheral lymphocytes. The subjects were genotyped with 474 autosomal markers, with an anticipated average heterozygosity of 0.74±0.11 (mean±SD), at the Australian Genome Research Facility. The mean sex-averaged distance between adjacent markers is 8.6±6.5 cM (range 0–34 cM). The pedigree structure was validated by genotypic profiling/haplotyping. Marker-error checking, marker-map evaluation, and allele-frequency estimation were undertaken as described elsewhere (Ewen et al. 2000). The genomewide scan was performed on 97 subjects, of whom 45 had type 2 diabetes. Fine mapping was performed on a total of 136 subjects, of whom 49 had type 2 diabetes.
Linkage Analysis
Two-point and multipoint parametric analyses were performed using FASTLINK version 4.1p (Cottingham et al. 1993; Schaffer et al. 1994) with an autosomal dominant, three-liability-class model and age-dependent penetrance factors. For ease of computation, the large pedigree was split into two. Simple counting estimates were used to calculate allelic frequencies within the pedigree. The genetic model used for the linkage analysis was an ad hoc model chosen to approximate the high cumulative incidence and recurrence risks of diabetes in this sample and was selected prior to these linkage analyses. We have not used a MOD (i.e., maximized-over-trait-model) approach. A dominant model was chosen, because of the density and pattern of disease in the pedigree. The penetrances in the three age classes were chosen to match the empiric Kaplan-Meier age-at-onset curves for the pedigree, bearing in mind that diagnosis in the earlier generations was delayed or absent. The three liability classes defined on the basis of age were as follows: class 1, age >65 years; class 2, age 45–65 years; and class 3, age <45 years. Phenocopy rates used in each liability class to accommodate potential heterogeneity were 0.2, 0.05, and 0.0001, respectively, and heterozygous and homozygous dominant penetrance factors were 0.99, 0.7, and 0.1, respectively. The disease-allele frequency is necessarily high, because of the high lifetime risk. Disease-gene frequency was set to define a 30% population prevalence, with a maximum sporadic (noninherited) frequency of 40%. This model leads to high baseline population rates of 20–30% and a sibling recurrence ratio of 1.8–2.5 and is consistent with prevalence data from the community (Shaw et al. 2000). The original analysis was performed with only overt diabetes classed as “affected.” A secondary analysis was carried out including impaired fasting glucose subjects in the “affected” classification.
Results
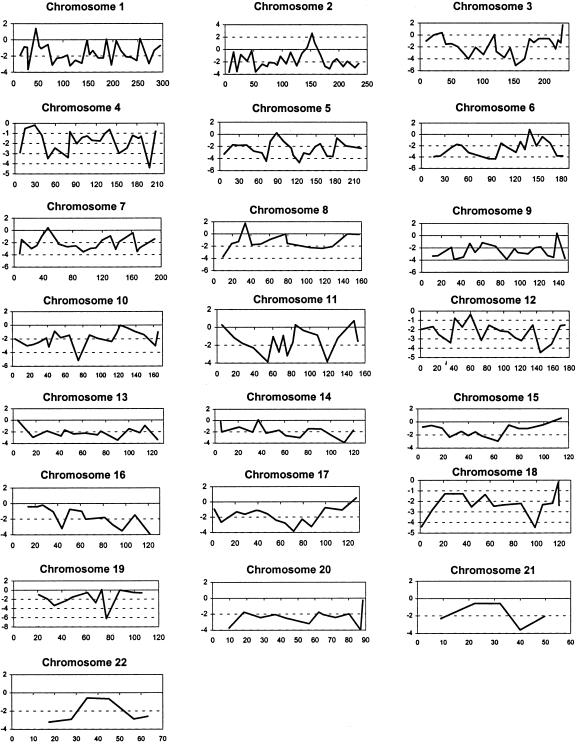
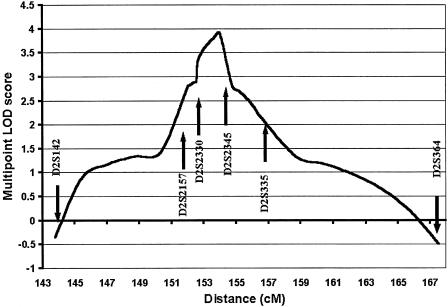
Peak two-point LOD scores from the genomewide scan are presented in figure 2. Suggestive linkage was indicated on chromosome 2, with a peak two-point LOD score of 2.61 at marker D2S2330 (recombination fraction [θ] 0.01). Fine mapping in the expanded data set was performed in this region, with a maximum two-point LOD score of +2.97 at D2S2345 (θ=0.01) (table 1). Multipoint analysis yielded a peak multipoint LOD score of +3.9, <1 cM from marker D2S2345, with a 3-LOD support interval of 18 cM (fig. 3). Secondary peak two-point LOD scores on the genomewide scan were noted on chromosome 8p (LOD score +1.77 and θ=0 at D8S549) and chromosome 3q (LOD score +1.8 and θ=0.05 at D3S1311). Two-point analysis of the genomewide scan data, when individuals with impaired fasting glucose were considered to be “affected,” did not remove these peaks, with peak two-point LOD scores of +1.79 at D2S2330 (θ=0.05), +1.51 at D3S1311 (θ=0.05) and +1.81 at D8S549 (θ=0). Analysis of the fine-mapping data in the chromosome 2 region gave a peak two-point LOD score of +1.96 at D2S2345 (θ=0.05) and a peak multipoint LOD score of +2.72 at marker D2S2345.
Figure 2.
Graphs of two-point LOD scores at θ=0.01, for each autosome from the genomewide scan. The x-axes represent distance in centimorgans, and the y-axes represent two-point LOD scores.
Table 1.
Peak Two-Point LOD Scores for Chromosome 2q Markers
| Marker | LOD Score | θ | Locationa(Mb) |
| D2S2313 | −.11 | .1 | 156.4 |
| D2S142 | .57 | .1 | 166.3 |
| D2S2157 | .51 | .05 | 175.1 |
| D2S2330 | 2.61 | .01 | 175.5 |
| D2S2345 | 2.97 | .01 | 177.2 |
| D2S335 | 1.27 | .05 | 182.5 |
| D2S364 | −.12 | .1 | 192.9 |
Location is according to Genethon Database and is taken from the Genome Browser Oct 2000 freeze.
Figure 3.
Graph of multipoint LOD scores for chromosome 2q. Marker positions are sex-averaged and are calculated on the basis of The Genetic Location Database.
Discussion
The genetic analysis of type 2 diabetes is challenging because of the disease's etiological complexity and genetic heterogeneity. Positive linkages with type 2 diabetes have been identified in a number of populations. Hanis et al. (1996) reported linkage with type 2 diabetes in Mexican-American affected sib pairs, at marker D2S125, in a region termed NIDDM1 (MIM 601283). The evidence for linkage was associated with a common G→A polymorphism (UCSNP-43) within the calpain-10 gene (CAPN10 [MIM 605286]) (Horikawa et al. 2000). Pima Indians with normal glucose tolerance who have a G/G genotype at UCSNP-43 were found to have decreased rates of postabsorptive and insulin-stimulated glucose turnover, resulting from decreased rates of glucose oxidation, and G/G homozygotes were found to have reduced CAPN10 mRNA expression in skeletal muscle (Baier et al. 2000). Mahtani et al. (1996) described linkage of MODY3-linked markers with type 2 diabetes in a subset of families from an isolated population in western Finland, in the absence of mutations in HNF-1α. Those authors inferred the presence of a gene in this region (NIDDM2 [MIM 601467]) that affects susceptibility to adult-onset diabetes associated with low insulin secretion. Shaw et al. (1998) identified positive linkage with the NIDDM2 region in a large pedigree of admixed European/Pacific Islander descent. Elbein et al. (1999) conducted a genomewide scan in Utah families of northern European ancestry, and a locus on chromosome 1q21-23 met genomewide criteria for significant linkage. Sib-pair analyses in the Pima Indian population indicated a diabetes-susceptibility locus on chromosome 1 (LOD score +4.1 at D1S127) (Hanson et al. 1998). A number of studies of different populations have found evidence for linkage on 20q in the MODY1 region, in the absence of mutations in HNF-4α (Bowden et al. 1997; Ji et al. 1997; Zouali et al. 1997; Malecki et al. 1998; Ghosh et al. 1999). These data indicate that type 2 diabetes is a heterogeneous disorder, with different genetic regions implicated in different populations. There has been replication of positive results in the regions of the MODY1 and MODY3 genes and on chromosome 1q, adding weight to the hypothesis that novel diabetes-susceptibility genes relevant to a number of populations are located in these regions.
Our data represent the first full-genome linkage analysis performed in an indigenous Australian pedigree. Type 2 diabetes in the indigenous Australian population is characterized by severe and predominant insulin resistance (Guest et al. 1993). Indigenous Australians have a sixfold increased risk of diabetes compared with Australians of European descent, and this risk is modulated by European admixture (Williams et al. 1987; O'Dea et al. 1993). Our hypothesis is that the indigenous Australian population harbors unique susceptibility genes for type 2 diabetes; however, the pathways involved may also have relevance to other ethnic groups, in terms of etiology and therapeutic potential. The advantages of studying this population include the availability of large pedigrees arising from a limited number of founders, giving statistical power to the analysis and reducing potential genetic heterogeneity.
The strongest evidence for multipoint linkage was found on chromosome 2q24.3 at D2S2345, with a two-point LOD score of +2.97 and a multipoint LOD score of +3.9 <1 cM centromeric to D2S2345. D2S2330, which gave the original peak two-point LOD score in this region, has previously been implicated by Vionnet et al. (2000), who found linkage with a maximum-binomial-likelihood LOD score and maximized LOD score of +1.25 and +1.22, respectively, at marker D2S2330. This linkage was in French whites, with affected status including both overt diabetes and glucose intolerance with a BMI <27 kg/m2. This is in contrast with the diagnostic criteria used and the clinical features of affected subjects in the present study, who have BMI of 32±7 kg/m2. Duggirala et al. (2001) found suggestive linkage to fasting-specific insulin in nondiabetic Mexican Americans, with a LOD score of 2.7 at marker D2S142. D2S142 is ∼10 Mb centromeric to D2S2330. These data give further support to the location of a type 2 diabetes–susceptibility gene in this region, across a number of different ethnic groups. The region is located ∼66 Mb centromeric to marker D2S362, for which positive linkage has been demonstrated in Mexican American subjects (Ehm et al. 2000), and ∼87 Mb centromeric to the NIDDM1 locus (Hanis et al. 1996).
Candidate genes within the region of interest on 2q include the gene encoding growth-receptor–binding protein 14 (Grb14 [MIM 601524]). Grb14 belongs to the Grb7 family and binds to receptor tyrosine kinases and tyrosine-phosphorylated proteins. Grb10 and Grb14 have been shown to bind the insulin receptor and are negative regulators of insulin signalling. Overexpression of Grb14 in Chinese hamster ovary cells stably expressing the insulin receptor results in decreased insulin stimulation of glycogen synthesis, accompanied by decreased tyrosine phosphorylation of insulin substrate-1. This would result in dysregulation of the downstream signalling component of insulin-mediated pathways (Kasus-Jacobi et al. 1998; Hemming et al. 2001). Another candidate in the region is the gene encoding islet-specific glucose-6-phosphate catalytic subunit–related protein. The activity of the glucose-6-phosphatase catalytic subunit is elevated in islets isolated from ob/ob mice, leading to increased glucose cycling and reduced glucose-stimulated insulin secretion (Khan et al. 1995; Ebert et al. 1999). Close to D2S142 and ∼10 Mb from our peak LOD score are the genes for mitochondrial glycerol-3-phosphate dehydrogenase 2 (MIM 138430), which possibly has a role to play in glucose-stimulated insulin secretion (Ferrer et al. 1996), and the inwardly rectifying potassium channel KCNJ3 (MIM 601534), which has been implicated in the regulatory pathways of insulin secretion (Vionnet et al. 1997).
To identify additional chromosomal regions that are likely to harbor genes affecting susceptibility to type 2 diabetes, we adopted more-liberal criteria than those proposed by Lander and Kruglyak (1995) to identify regions with positive results. Following the recommendations of Rao and Province (2000) we considered a P value of <.0023 as “highly suggestive” evidence for linkage and a P value <.01 as “suggestive” evidence for linkage. These P values correspond to LOD scores of 1.75 and 1.18, respectively. These more liberal criteria maximize the detection of genomic regions to be considered for further investigation while allowing true negative results to be reported. On chromosomes 3q29 and 8p22 there was highly suggestive evidence for linkage, with LOD scores of +1.8 at θ=0.05 for marker D3S1311 and +1.77 for marker D8S549. Whereas the region on chromosome 8p22 has not previously been linked to type 2–diabetes susceptibility, several studies have found the region on chromosome 3q27-qter to have positive linkage to metabolic traits and type 2 diabetes (Hegele et al. 1999; Kissebah et al. 2000; Vionnet et al. 2000). Kissebah et al. (2000) reported linkage of six quantitative traits representative of the metabolic syndrome to 3q27, in 507 white nuclear families. These traits gave peak LOD scores of 2.37–3.54 between markers D3S2427 and D3S2418. An indication of linkage between type 2 diabetes and D3S2418 was also reported by Hegele et al. (1999), in a genomewide scan in Canadian Oji-Cree. Vionnet et al. (2000) also reported linkage to 3q27-qter in French nuclear families and sib pairs with a relatively early age at onset (age <46 years). These reports are all within 20 cM of our peak LOD score at D3S1311 and support the presence of a diabetes-susceptibility gene in this region. Fine mapping will be undertaken in both the 8p and 3q regions. Several candidate genes of potential interest have been identified in the region of these secondary peaks, including lipoprotein lipase on chromosome 8p, which has been associated with insulin resistance in Mexican Americans (Yang et al. 2001), and type 1 protein phosphatase inhibitor 2 (MIM 601792), which reversibly inhibits and facilitates the correct conformational arrangement of the catalytic subunits of type 1 protein phosphatase, an activator of glycogen synthase (Alessi et al. 1993).
In conclusion, on the basis of a genomewide scan, we have identified a region of significant positive linkage with type 2 diabetes on chromosome 2q. Several candidate genes have been identified in the region, and we plan to continue fine mapping, haplotype analysis, and sequence analysis, to identify the nature of the causative gene. The identification of genetic markers for type 2 diabetes is fundamental for understanding the cause of the disease, for identifying subjects at risk at a preclinical stage, and for the development of more-effective preventative and therapeutic strategies for the management of the condition.
Acknowledgments
We are grateful to the people who participated in the research program, which would not have been possible without the commitment and enthusiasm of the friends and staff of the Yulu-Burri-Ba Aboriginal Corporation for Community Health, North Stradbroke Island, Queensland. This work was funded by an NHMRC Project Grant, by the Princess Alexandra Hospital Research and Development Foundation, by the Lions Medical Research Foundation, by the Novo Nordisk Regional Diabetes Support Scheme, by Merck Research Laboratories, by the Diabetes Australia Research Trust, and by the Sylvia and Charles Viertel Charitable Foundation.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Genethon, http://www.genethon.fr/ (for marker locations)
- Genetic Location Database, The, http://cedar.genetics.soton.ac.uk/public_html/ldb.html (for marker and chromosome locations)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for type 2 diabetes [MIM 125853], NIDDM1 [MIM 601283], NIDDM2 [MIM 601467], CAPN10 [MIM 605286], Grb14 [MIM 601524], glycerol-3-phosphate dehydrogenase 2 [MIM 138430], KCNJ3 [MIM 601534], and type 1 protein phosphatase inhibitor 2 [MIM 601792])
References
- Alessi DR, Street AJ, Cohen P, Cohen PT (1993) Inhibitor-2 functions like a chaperone to fold three expressed isoforms of mammalian protein phosphatase-1 into a conformation with the specificity and regulatory properties of the native enzyme. Eur J Biochem 213:1055–1066 [DOI] [PubMed] [Google Scholar]
- Baier LJ, Permana PA, Yang X, Pratley RE, Hanson RL, Shen GQ, Mott D, Knowler WC, Cox NJ, Horikawa Y, Oda N, Bell GI, Bogardus C (2000) A calpain-10 gene polymorphism is associated with reduced muscle mRNA levels and insulin resistance. J Clin Invest 106:R69–R73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian P (1979) Coronary heart disease in tribal Aborigines—the West Kimberley survey. Aust N Z J Med 9:284–292 [DOI] [PubMed] [Google Scholar]
- Bell GI, Pilkis SJ, Weber IT, Polonsky KS (1996) Glucokinase mutations, insulin secretion, and diabetes mellitus. Annu Rev Physiol 58:171–186 [DOI] [PubMed] [Google Scholar]
- Bowden DW, Sale M, Howard TD, Qadri A, Spray BJ, Rothschild CB, Akots G, Rich SS, Freedman BI (1997) Linkage of genetic markers on human chromosomes 20 and 12 to NIDDM in Caucasian sib pairs with a history of diabetic nephropathy. Diabetes 46:882–886 [DOI] [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schaffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA (1988) Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687 [DOI] [PubMed] [Google Scholar]
- Duggirala R, Blangero J, Almasy L, Arya R, Dyer TD, Williams KL, Leach RJ, O'Connell P, Stern MP (2001) A major locus for fasting insulin concentrations and insulin resistance on chromosome 6q with strong pleiotropic effects on obesity-related phenotypes in nondiabetic Mexican Americans. Am J Hum Genet 68:1149–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, Bischof LJ, Streeper RS, Chapman SC, Svitek CA, Goldman JK, Mathews CE, Leiter EH, Hutton JC, O'Brien RM (1999) Structure and promoter activity of an islet-specific glucose-6-phosphatase catalytic subunit-related gene. Diabetes 48:543–551 [DOI] [PubMed] [Google Scholar]
- Ehm MG, Karnoub MC, Sakul H, Gottschalk K, Holt DC, Weber JL, Vaske D, Briley D, Briley L, Kopf J, McMillen P, Nguyen Q, Reisman M, Lai EH, Joslyn G, Shepherd NS, Bell C, Wagner MJ, Burns DK, American Diabetes Association GENNID Study Group (2000) Genomewide search for type 2 diabetes susceptibility genes in four American populations. Am J Hum Genet 66:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein SC, Hoffman MD, Teng K, Leppert MF, Hasstedt SJ (1999) A genome-wide search for type 2 diabetes susceptibility genes in Utah Caucasians. Diabetes 48:1175–1182 [DOI] [PubMed] [Google Scholar]
- Ewen KR, Bahlo M, Treloar SA, Levinson DF, Mowry B, Barlow JW, Foote SJ (2000) Identification and analysis of error types in high-throughput genotyping. Am J Hum Genet 67:727–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (1997) Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197 [DOI] [PubMed] [Google Scholar]
- Ferrer J, Aoki M, Behn P, Nestorowicz A, Riggs A, Permutt MA (1996) Mitochondrial glycerol-3-phosphate dehydrogenase. Cloning of an alternatively spliced human islet-cell cDNA, tissue distribution, physical mapping, and identification of a polymorphic genetic marker. Diabetes 45:262–266 [DOI] [PubMed] [Google Scholar]
- Gault A, O'Dea K, Rowley KG, McLeay T, Traianedes K (1996) Abnormal glucose tolerance and other coronary heart disease risk factors in an isolated aboriginal community in central Australia. Diabetes Care 19:1269–1273 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Watanabe RM, Hauser ER, Valle T, Magnuson VL, Erdos MR, Langefeld CD, et al (1999) Type 2 diabetes: evidence for linkage on chromosome 20 in 716 Finnish affected sib pairs. Proc Natl Acad Sci USA 96:2198–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest CS, O'Dea K, Hopper JL, Larkins RG (1993) Hyperinsulinaemia and obesity in aborigines of south-eastern Australia, with comparisons from rural and urban Europid populations. Diabetes Res Clin Pract 20:155–164 [DOI] [PubMed] [Google Scholar]
- Hanis CL, Boerwinkle E, Chakraborty R, Ellsworth DL, Concannon P, Stirling B, Morrison VA, et al (1996) A genome-wide search for human non-insulin-dependent (type 2) diabetes genes reveals a major susceptibility locus on chromosome 2. Nat Genet 13:161–166 [DOI] [PubMed] [Google Scholar]
- Hanson RL, Ehm MG, Pettitt DJ, Prochazka M, Thompson DB, Timberlake D, Foroud T, Kobes S, Baier L, Burns DK, Almasy L, Blangero J, Garvey WT, Bennett PH, Knowler WC (1998) An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet 63:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman N (1997) Cardiovascular disease amongst indigenous Australians. Aboriginal and Islander Health Worker Journal 21:15–17 [PubMed] [Google Scholar]
- Hegele RA, Sun F, Harris SB, Anderson C, Hanley AJ, Zinman B (1999) Genome-wide scanning for type 2 diabetes susceptibility in Canadian Oji-Cree, using 190 microsatellite markers. J Hum Genet 44:10–14 [DOI] [PubMed] [Google Scholar]
- Hemming R, Agatep R, Badiani K, Wyant K, Arthur G, Gietz RD, Triggs-Raine B (2001) Human growth factor receptor bound 14 binds the activated insulin receptor and alters the insulin-stimulated tyrosine phosphorylation levels of multiple proteins. Biochem Cell Biol 79:21–32 [PubMed] [Google Scholar]
- Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI (1997) Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet 17:384–385 [DOI] [PubMed] [Google Scholar]
- Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI (2000) Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet 26:163–175 [DOI] [PubMed] [Google Scholar]
- Ji L, Malecki M, Warram JH, Yang Y, Rich SS, Krolewski AS (1997) New susceptibility locus for NIDDM is localized to human chromosome 20q. Diabetes 46:876–881 [DOI] [PubMed] [Google Scholar]
- Kasus-Jacobi A, Perdereau D, Auzan C, Clauser E, Van Obberghen E, Mauvais-Jarvis F, Girard J, Burnol AF (1998) Identification of the rat adapter Grb14 as an inhibitor of insulin actions. J Biol Chem 273:26026–26035 [DOI] [PubMed] [Google Scholar]
- Khan A, Hong-Lie C, Landau BR (1995) Glucose-6-phosphatase activity in islets from ob/ob and lean mice and the effect of dexamethasone. Endocrinology 136:1934–1938 [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, James RG, Marks JA, Krakower GR, Jacob HJ, Weber J, Martin L, Blangero J, Comuzzie AG (2000) Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA 97:14478–14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Levy JC, Rudenski A, Burnett M, Knight R, Matthews DR, Turner RC (1991) Simple empirical assessment of beta-cell function by a constant infusion of glucose test in normal and type 2 (non-insulin-dependent) diabetic subjects. Diabetologia 34:488–499 [DOI] [PubMed] [Google Scholar]
- Maassen JA, Kadowaki T (1996) Maternally inherited diabetes and deafness: a new diabetes subtype. Diabetologia 39:375–382 [DOI] [PubMed] [Google Scholar]
- Mahtani MM, Widen E, Lehto M, Thomas J, McCarthy M, Brayer J, Bryant B, et al (1996) Mapping of a gene for type 2 diabetes associated with an insulin secretion defect by a genome scan in Finnish families. Nat Genet 14:90–94 [DOI] [PubMed] [Google Scholar]
- Malecki MT, Antonellis A, Casey P, Ji L, Wantman M, Warram JH, Krolewski AS (1998) Exclusion of the hepatocyte nuclear factor 4α as a candidate gene for late-onset NIDDM linked with chromosome 20q. Diabetes 47:970–972 [DOI] [PubMed] [Google Scholar]
- Malecki M, Jhala U, Antonellis A, Fields L, Doria A, Orban T, Saad M, Warram J, Montminy M, Krolewski A (1999) Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genetics 23:323–328 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Neel J (1963) Diabetes mellitus: a “thrifty genotype” rendered detrimental by progress? Am J Hum Genet 14:353–362 [PMC free article] [PubMed] [Google Scholar]
- O'Dea K (1991) Westernization and non-insulin-dependent diabetes in Australian Aborigines. Ethn Dis 1:171–187 [PubMed] [Google Scholar]
- ——— (1992) Diabetes in Australian aborigines: impact of the western diet and life style. J Intern Med 232:103–117 [DOI] [PubMed] [Google Scholar]
- O'Dea K, Lion RJ, Lee A, Traianedes K, Hopper JL, Rae C (1990) Diabetes, hyperinsulinemia, and hyperlipidemia in small aboriginal community in northern Australia. Diabetes Care 13:830–835 [DOI] [PubMed] [Google Scholar]
- O'Dea K, Patel M, Kubisch D, Hopper J, Traianedes K (1993) Obesity, diabetes, and hyperlipidemia in a central Australian aboriginal community with a long history of acculturation. Diabetes Care 16:1004–1010 [DOI] [PubMed] [Google Scholar]
- Rao DC, Province MA (2000) The future of path analysis, segregation analysis, and combined models for genetic dissection of complex traits. Hum Hered 50:34-42 [DOI] [PubMed] [Google Scholar]
- Schaffer AA, Gupta SK, Shriram K, Cottingham RW Jr (1994) Avoiding recomputation in linkage analysis. Hum Hered 44:225–237 [DOI] [PubMed] [Google Scholar]
- Shaw J, Kesting J, Marczak M, Watego D, Purdie D, Hayman N (2000) The “Australian Families against Diabetes” program: epidemiology of type 2 diabetes in an urban indigenous Australian community. Australasian Epidemiologist 7:21–25 [Google Scholar]
- Shaw JT, Lovelock PK, Kesting JB, Cardinal J, Duffy D, Wainwright B, Cameron DP (1998) Novel susceptibility gene for late-onset NIDDM is localized to human chromosome 12q. Diabetes 47:1793–1796 [DOI] [PubMed] [Google Scholar]
- Steiner D, Tage H, Nanjo K, Chan S, Rubenstein A (1995) Familial syndromes of hyperproinsulinaemia and hyperinsulinaemia with mild diabetes. In: Striver C, Beaudet A, Sly W, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 897–904 [Google Scholar]
- Stoffers DA, Stanojevic V, Habener JF (1998) Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J Clin Invest 102:232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S (1995) Diabetes mellitus. In: Striver C, Beaudet A, Sly W, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 843–896 [Google Scholar]
- Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Leprêtre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P (2000) Genomewide search for type 2 diabetes–susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2–diabetes locus on chromosome 1q21-q24. Am J Hum Genet 67:1470–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vionnet N, Hani EH, Lesage S, Philippi A, Hager J, Varret M, Stoffel M, Tanizawa Y, Chiu KC, Glaser B, Permutt MA, Passa P, Demenais F, Froguel P (1997) Genetics of NIDDM in France: studies with 19 candidate genes in affected sib pairs. Diabetes 46:1062–1068 [DOI] [PubMed] [Google Scholar]
- Williams DR, Moffitt PS, Fisher JS, Bashir HV (1987) Diabetes and glucose tolerance in New South Wales coastal Aborigines: possible effects of non-Aboriginal genetic admixture. Diabetologia 30:72–77 [DOI] [PubMed] [Google Scholar]
- Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI (1996a) Mutations in the hepatocyte nuclear factor-4α gene in maturity-onset diabetes of the young (MODY1). Nature 384:458–460 [DOI] [PubMed] [Google Scholar]
- Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, et al (1996b) Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 384:455–458 [DOI] [PubMed] [Google Scholar]
- Yang H, Taylor K, Quinines M, Bulnes-Enriquez I, Goodarzi M, Guo X, Pressman S, Saad M, Hsueh W, Rotter J (2001) Evidence of both linkage and association of the lipoproten lipase (LPL) gen to insulin resistance in Mexican American (MA) families with coronary artery disease (CAD). Diabetes 50 Suppl 2:A27 [Google Scholar]
- Zimmet P, Welborn T (2001) The final report of the Australian Diabetes and Obesity Study (AusDiab). Commonwealth Department of Health and Aged Care, Canberra [Google Scholar]
- Zouali H, Hani EH, Philippi A, Vionnet N, Beckmann JS, Demenais F, Froguel P (1997) A susceptibility locus for early-onset non-insulin dependent (type 2) diabetes mellitus maps to chromosome 20q, proximal to the phosphoenolpyruvate carboxykinase gene. Hum Mol Genet 6:1401–1408 [DOI] [PubMed] [Google Scholar]