Abstract
“Early-onset glaucoma” refers to genetically heterogeneous conditions for which glaucoma manifests at age 5–40 years and for which only a small subset is molecularly characterized. We studied the role of MYOC, CYP1B1, and PITX2 in a population (n=60) affected with juvenile or early-onset glaucoma from the greater Toronto area. By a combination of single-strand conformation polymorphism and direct cycle sequencing, MYOC mutations were detected in 8 (13.3%) of the 60 individuals, CYP1B1 mutations were detected in 3 (5%) of the 60 individuals, and no PITX2 mutations were detected. The range of phenotypic expression associated with MYOC and CYP1B1 mutations was greater than expected. MYOC mutations included cases of juvenile glaucoma with or without pigmentary glaucoma and mixed-mechanism glaucoma. CYP1B1 mutations involved cases of juvenile open-angle glaucoma, as well as cases of congenital glaucoma. The study of a family with autosomal dominant glaucoma showed the segregation of both MYOC and CYP1B1 mutations with disease; however, in this family, the mean age at onset of carriers of the MYOC mutation alone was 51 years (range 48–64 years), whereas carriers of both the MYOC and CYP1B1 mutations had an average age at onset of 27 years (range 23–38 years) (P=.001). This work emphasizes the genetic heterogeneity of juvenile glaucoma and suggests, for the first time, that (1) congenital glaucoma and juvenile glaucoma are allelic variants and (2) the spectrum of expression of MYOC and CYP1B1 mutations is greater than expected. We also propose that CYP1B1 may act as a modifier of MYOC expression and that these two genes may interact through a common pathway.
Introduction
Glaucoma is a genetically heterogeneous cause of blindness that affects all age groups and all populations. When glaucoma manifests before the age of 40 years, it tends to be more aggressive, more resistant to medical therapy, and associated with more severe visual impairment (Ellis 1948; Johnson et al. 1993). Glaucoma in the first 40 years of life includes congenital/infantile glaucoma, which manifests at the age of <5 years (DeLuise and Anderson 1983); juvenile open-angle glaucoma (JOAG), in which the age at onset is 5–40 years; and variants that are associated with other changes in the anterior segment of the eye, such as pigment dispersion and Axenfeld-Rieger syndrome.
Juvenile glaucoma and congenital glaucoma (CG) are genetically heterogeneous, and only a small subset are molecularly characterized, with most mutations identified in MYOC (Fingert et al. 1999) and CYP1B1 (Bejjani et al. 1998). MYOC (MIM 601652), which is located on chromosome 1q25 at the GLC1A locus (MIM 137750) (Sheffield et al. 1993), was the first open-angle–glaucoma gene to be characterized and was associated with JOAG and primary adult-onset open-angle glaucoma (POAG). MYOC encodes a 504-amino-acid glycoprotein, which contains an olfactomedin domain (residues 246–501) where the majority (42/46 [91.3%]) of the mutations documented have been identified. The biological interactions of mutant myocilin protein and its role in the pathophysiology of glaucoma are still unclear. In normal eyes, MYOC mRNA is expressed in the iris, ciliary body, and trabecular meshwork (Fingert et al. 1998; Kubota et al. 1998; Huang et al. 2000), as well as in retinal photoreceptor cells (Kubota et al. 1997) and optic nerve head—specifically, the astrocytes (Noda et al. 2000). Perfusing the trabecular meshwork with mutant recombinant protein results in an increase in outflow resistance (Fautsch et al. 2000). Recent studies estimate that MYOC mutations are found in 3.4%–5% of sporadic POAG (Alward et al. 1998a; Fingert et al. 1999). In a small series, Shimizu et al. (2000) identified mutations in as many as 33% of familial cases of JOAG. The variability of GLC1A-related phenotypes is significant and includes age at onset, severity, rate of progression, and intraocular pressure (IOP) (Alward et al. 1998a; Fingert et al. 1999). This variability, which can be inter- or intrafamilial, is influenced by factors not yet identified, some of which are likely to be genetic.
Other genes, such as CYP1B1 and PITX2, may contribute to the underlying pathogenesis of JOAG. CYP1B1 (MIM 601771), located on chromosome 2p21 at the GLC3A locus (MIM 231300), encodes a 543-amino-acid dioxin inducible member of the cytochrome p450 gene superfamily, subfamily I. Mutations in this gene are associated with autosomal recessive CG (Stoilov et al. 1997; Bejjani et al. 1998; Stoilov et al. 1998; Plášilová et al. 1999; Bejjani et al. 2000; Martin et al. 2000). In ethnically mixed populations, mutations are found in 20%–30% of patients with CG (Héon et al. 2000; Kakiuchi-Matsumoto et al. 2001), whereas, in consanguineous populations, this increases to 85% (Stoilov et al. 1997; Bejjani et al. 1998; Plášilová et al. 1999).
Phenotypic variability is also documented for mutations of CYP1B1 but was always associated with CG. Although these mutations commonly manifest at birth or infancy, some family members of probands have been identified with glaucoma manifesting later in childhood, early adulthood, or with incomplete penetrance (Bejjani et al. 2000; Martin et al. 2000). Recently, we reported CYP1B1 mutations in Peters anomaly, a developmental anomaly of the anterior segment (Vincent et al. 2001), suggesting a role for this gene in ocular development and beyond CG.
The mRNA expression of CYP1B1 is demonstrated in the fetal and adult eye, specifically in the iris, trabecular meshwork, and ciliary body (Sutter et al. 1994; Shimada et al. 1996; Stoilov et al. 1997), as well as in many other sites throughout the body. A major hurdle in determining this enzyme’s role in the pathogenesis of CG is the identification and analysis of its natural substrate. CYP1B1 is highly efficient in 4-hydroxylation of 17-β-estradiol (Hayes et al. 1996), but other metabolic activities involve exogenous substrates, such as procarcinogens (Shimada et al. 1996; Crespi et al. 1997; Luch et al. 1998).
PITX2 (MIM 601542) is another gene involved with early-onset glaucoma and located on chromosome 4q25. It encodes a pairlike homeobox transcription factor and is expressed in developing eye, tooth, umbilicus, and pituitary gland (Semina et al. 1996). The spectrum of phenotypic expression of PITX2 mutations is very broad. This includes a risk factor for the development of glaucoma with either iris hypoplasia, Axenfeld-Rieger syndrome, or Peters anomaly (Héon et al. 1995; Alward et al. 1998b; Kulak et al. 1998; Doward et al. 1999), anomalies of development of the anterior segment of the eye. Functional assays of mutant PITX2 protein confirm a mutation-specific decrease in DNA binding and altered transactivation properties (Kozlowski and Walter 2000; Priston et al. 2001). The role that PITX2 plays in a juvenile glaucoma population with no evidence of anterior-segment anomaly has not been studied. We studied the role that MYOC, CYP1B1, and PITX2 play in a population with juvenile glaucoma from the greater Toronto area.
Methods
Patient Recruitment
The project was approved by the Toronto Hospital human subjects review committee and the Hospital for Sick Children research ethics board. After giving informed consent in accordance with the Declaration of Helsinki, patients were recruited through the eye clinics of these two hospitals and referring centers. This primary patient population was affected with juvenile glaucoma and included patients with glaucoma diagnosed after the age of 5 years but before the age of 40 years. Glaucoma was defined as raised IOP (>22 mmHg), evidence of visual-field (VF) loss, and/or optic nerve head cupping characteristic of glaucoma. No patient had any other associated ocular abnormalities or systemic disease. Most patients had a normal angle on gonioscopy, but a few had mixed-mechanism glaucoma, defined as an angle that was occludable for >180 degrees; some patients with anatomically normal and open-angle glaucoma were also included.
Patients with CG were excluded from the primary population that we studied. Patients were considered to have CG if IOP was raised in the first five years of life, with clinical findings consistent with prenatal or infantile onset of glaucoma (such as breaks in Descemet’s membrane, enlarged cornea, or buphthalmos). Also excluded were patients with secondary glaucoma due to trauma, uveitis, steroid use, or anterior-segment developmental anomalies.
Participants had a full eye examination and completed a standardized questionnaire. For older patients, clinical notes were reviewed with the referring ophthalmologist. Anterior-segment photos were taken when possible. When necessary, chloral hydrate sedation or general anesthesia was used for the examination of younger children and for the measurement of their IOP. Blood samples (20 ml) were collected for DNA extraction by protocols described elsewhere (Miller et al. 1988).
Mutational Analysis of MYOC
The coding regions of MYOC, exons 1–3, were amplified by PCR, through the use of primers and conditions described elsewhere (Alward et al. 1998a). Mutation screening used a combination of SSCP analysis and direct cycle sequencing.
SSCP analysis of MYOC.—After PCR in 20 ml total volume, 3 ml of PCR product was heat denatured in SSCP loading dye, was snap cooled, and was loaded on a 13% nondenaturing polyacrylamide gel. Samples were tested on a Hoefer SE260 Mighty Small II (Hoefer Pharmacia Biotech) at temperatures of 10°C and 24°C. Samples with abnormal mobility band shifts were sequenced using the corresponding fragment as template.
Sequencing of MYOC.—Direct sequencing used amplified genomic DNA. Primers were tailed with M13 universal primer (5′-gtaaaacgacggccagt-3′) or M13 reverse primer (5′-cacaggaaacagctatgac-3′). Amplicons were purified using QIAquick PCR Purification Kit (Qiagen), according to the manufacturer’s protocol. Column-purified amplicons were sequenced using Cy5.5-labeled M13 universal or M13 reverse primers and the Thermo Sequenase Cycle Sequencing Core Kit (Visible Genetic). Products were tested on a MicroGene Blaster automated DNA sequencing unit (Visible Genetic), as described elsewhere (Héon et al. 1999).
To authenticate the Gly399Val mutation, a control population of 140 individuals was screened for this sequence change by restriction-enzyme digestion of exon 3 with BanI (incubated at 37°C for 2 h), followed by electrophoresis on a 2% agarose gel containing ethidium bromide and by visualization under UV light. This control population included individuals known not to have any anterior-segment anomaly, glaucoma, or risk of glaucoma, and 40 of these were ethnically matched to the East Indian family with this mutation.
Mutational Analysis of CYP1B1
CYP1B1 consists of three exons, of which only exons 2 and 3 code for the protein. The coding sequence of exons 2 and 3 was amplified by PCR, through use of primers described elsewhere (Bejjani et al. 1998; Stoilov et al. 1998). Additional primers were designed from the mRNA sequence (GenBank accession number U56438) as follows: reverse, 5′-catgattcacagaccactgg-3′; and forward, 5′-ccagctcgattcttggacaa-3′ (Sutter et al. 1994). SSCP analysis and direct sequencing were undertaken in the same fashion for CYP1B1 as for MYOC. For the Arg368His mutation in exon 2, the control population (n=140) was screened by SSCP analysis. For the Lys345Phe sequence change, 100 controls were screened for the exon 2 by SSCP following restriction-enzyme digestion of amplicon 2B with RsaI incubated at 37°C for 2 h.
Mutational Analysis of PITX2
The coding regions of PITX2, exons 1–4, were amplified by PCR, through use of primers described elsewhere (Semina et al. 1996), and were screened for mutations by SSCP and direct sequencing, as described elsewhere (Kozlowski and Walter 2000). Fragment 4b was cut with EcoRI prior to SSCP analysis, to yield two fragments of lengths 283 and 250 bp.
Results
We screened 60 unrelated probands, (29 females and 31 males), with ages at diagnosis of 7–40 years (average 30.5 years). The mixed ethnicity of this population reflected that of the greater Toronto area, with predominantly English-Canadian, Western and Eastern European, and French-Canadian ancestry, as well as Afro-Caribbean, Chinese, Filipino, and North African. Fifty-three patients had JOAG alone, six also had pigmentary glaucoma, and one had mixed-mechanism glaucoma of juvenile onset.
Mutational Analysis of MYOC
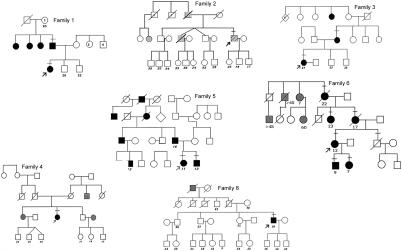
Screening of MYOC revealed eight different disease-associated mutations in 8 (13.3%) of the 60 individuals, all of whom had a positive family history (fig. 1 and table 2table 2). Polymorphic sequence changes (table 1) were detected in 9 (15%) of the 60 individuals; all except for Thr243Thr have been described elsewhere (Alward et al. 1998a). Six of the eight disease-causing mutations identified have been previously published, whereas two were novel—827C→A, (Thr377Lys) in case subject 6, and 1218 G→T, (Gly399Val) in case subject 7. Case subjects 6 and 7 both had strong family histories of glaucoma (figs. 1 and 2), which allowed us to confirm the segregation of the sequence change with the disease phenotype. Participants with glaucoma in the family of case subject 7 all had the novel MYOC Gly399Val mutation (fig. 2)—which was not present in 140 controls, including 40 individuals ethnically matched to this family. Participants with glaucoma in the family of case subject 6 (i.e., family 6) all carried the Thr377Lys mutation, which was not present in 100 controls (fig. 1). The genotype-phenotype correlation of case subjects with mutations and their respective family characteristics are summarized in table 2table 2.
Figure 1.
Pedigrees of probands with MYOC mutation, in families 1–6 and 8. Blackened symbols denote affected status for JOAG, grayed symbols denote affected status for POAG, unblackened symbols denote unaffected or unknown clinical status for glaucoma, and hatched symbols denote pigmentary glaucoma. Slash marks through symbols denote deceased individuals, and bars above symbols indicate that the individuals who had genetic testing. Arrow indicates the proband. Numbers below the symbols indicate current age (in years), for pedigrees 1–4 and 8; age (in years) at onset, for pedigree 5; and age (in years) at diagnosis, for pedigree 6.
Table 2.
MYOC Genotype-Phenotype Correlations[Note]
|
Age at Diagnosis(years) |
IOP at Diagnosis(mmHg) |
||||||
| Case Subject (Mutation) | Ethnic Background | Phenotype | Family History | Proband [Family] | Previously Published Mean [Range] | Proband [Family] | Previously Published Mean [Range] |
| 1 (Gly252Arg) | Chinese | JOAG | AD JOAG | 29 [29–38] | NDa | 20–21 [20–44] | ND |
| 2 (Thr293Lys) | Dutch | Pigmentary glaucoma | AD POAG | 31 [51–71], for POAG | NDb | ND | ND |
| 3 (Gly367Arg) | Italian/French | JOAG | AD JOAG | 22 [22–30] | 28 [8–54]c36.7 d | 28 | 38 |
| 4 (Gln368Stop) | English | JOAG | AD POAG | 39 | 59 [36–77]e | 30 | 30 [21–56] |
| 5 (Pro370Leu) | Greek | JOAG | AD JOAG | 10 [9–19] | 12 [5–27]f,g,h | High 20s to 30s [high 20s to 30s] | 45 [25–66] |
| 6 (Thr377Lys) | Irish/Scottish | JOAG | AD JOAG, POAG | 7 [7–60] | 37 [20–60], for Thr377Metb,i | 25 [25–35] | 31 [20–50], for Thr377Met |
| 7 (Gly399Val) | Guyanese/East Indian | Mixed-mechanism JOAG | AD JOAG, POAG, mixed mechanism | 28 [23–28] | New mutation | High 20s to 30s [high 20s to 30s] | New |
| 8 (Ala445Val) | French-Canadian | OHTN | POAG | 38 [NA] | NDb | OHTN | ND |
Note.— AD = autosomal dominant; OHTN = ocular hypertension; ND = not documented; NA = not available.
Rozsa et al. (1998).
Alward et al. (1998a).
Suzuki et al. (1997).
Taniguchi et al. (2000).
Stone et al. (1997).
Adam et al. (1997).
Stoilova et al. (1998).
Taniguchi et al. (1999).
Simms et al. (1999).
Table 2.
MYOC Genotype-Phenotype Correlations
|
Age at Diagnosis(years) |
IOP at Diagnosis(mmHg) |
||||||
| Case Subject (Mutation) | Ethnic Background | Phenotype | Family History | Proband [Family] | Previously Published Mean [Range] | Proband [Family] | Previously Published Mean [Range] |
| 1 (Gly252Arg) | Chinese | JOAG | AD JOAG | 29 [29–38] | NDa | 20–21 [20–44] | ND |
| 2 (Thr293Lys) | Dutch | Pigmentary glaucoma | AD POAG | 31 [51–71], for POAG | NDb | ND | ND |
| 3 (Gly367Arg) | Italian/French | JOAG | AD JOAG | 22 [22–30] | 28 [8–54]c36.7 d | 28 | 38 |
| 4 (Gln368Stop) | English | JOAG | AD POAG | 39 | 59 [36–77]e | 30 | 30 [21–56] |
| 5 (Pro370Leu) | Greek | JOAG | AD JOAG | 10 [9–19] | 12 [5–27]f,g,h | High 20s to 30s [high 20s to 30s] | 45 [25–66] |
| 6 (Thr377Lys) | Irish/Scottish | JOAG | AD JOAG, POAG | 7 [7–60] | 37 [20–60], for Thr377Metb,i | 25 [25–35] | 31 [20–50], for Thr377Met |
| 7 (Gly399Val) | Guyanese/East Indian | Mixed-mechanism JOAG | AD JOAG, POAG, mixed mechanism | 28 [23–28] | New mutation | High 20s to 30s [high 20s to 30s] | New |
| 8 (Ala445Val) | French-Canadian | OHTN | POAG | 38 [NA] | NDb | OHTN | ND |
| Note.— AD = autosomal dominant; OHTN = ocular hypertension; ND = not documented; NA = not available. | |||||||
| aRozsa et al. (1998). | |||||||
| bAlward et al. (1998a). | |||||||
| cSuzuki et al. (1997). | |||||||
| dTaniguchi et al. (2000). | |||||||
| eStone et al. (1997). | |||||||
| fAdam et al. (1997). | |||||||
| gStoilova et al. (1998). | |||||||
| hTaniguchi et al. (1999). | |||||||
| iSimms et al. (1999). | |||||||
| Table 3CYP1B1 Genotype-Phenotype Correlations | ||||||||
| Case Subject (Mutations 1/2) | Exon | Codon 432Genotype | EthnicBackground | FamilyHistory | Age at Diagnosis,for Proband [Family](years) | IOP atDiagnosis | PublishedPhenotype | Other Mutations Detected |
| 7 (Arg368His/wild type) | 3 | Val/Leua | East Indian/Guyanese | AD JOAG, POAG | 27 [23–38] | High 20s to 30sb | CG, incomplete penetrancee | MYOC Gly399Val |
| 9 (Arg368His/1546dup10 frameshift) | 3 | Within duplication | Italian/English/Scottish | JOAG, CG | 8 [2–8] | 26/21 [15–34]c | CG, incomplete penetrancee | Nil |
| 10 (Leu345Phe/wild type) | 2 | Val/Leua | African American | Nil | 36 | NDd | New | Nil |
Table 1.
Polymorphic Sequence Changes in MYOC
|
Published Frequency(%) |
||||
| Sequence Change | No. in StudyPopulation [%](n = 60) | Previously Published? | In Populationswith POAGa,b | In Control Populationsa,b |
| Promoter bp −83 | 1 [1.7%] | Yesa | 29.3b | 16.3b |
| Thr243Thr | 1 [1.7%] | No | Not published | Not published |
| Thr293Lys | 1 [1.7%] | Yesa | 17.3a | 17.7a |
| Tyr347Tyr | 5 [8.3%] | Yesa | 4.7b | 4.3b |
| Lys398Arg | 1 [1.7%] | Yesa | 1.2b | .8b |
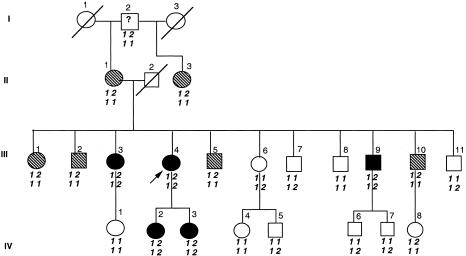
Figure 2.
Pedigree of family 7, with CYP1B1 (Arg368His) and MYOC (Gly399Val) mutations. Blackened symbols denote affected status for JOAG, unblackened symbols denote unaffected or unknown status, and hatched symbols denote affected status for POAG. Arrow indicates the proband. Numbers above the symbol indicate the identifier, whereas numbers (“1” for wild type and “2” for mutant allele) below the symbol indicate the genotype for MYOC (top) and for CYP1B1 (bottom).
Three mutations—Gly367Arg (case subject 3), Gln368Stop (case subject 4), and Pro370Leu (case subject 5)—were published elsewhere (Adam et al. 1997; Stone et al. 1997; Suzuki et al. 1997). The clinical manifestations observed here corresponded well with previously published findings in populations of mixed ethnicity. However, the phenotype associated with the Gly252Arg mutation was not previously described (Rozsa et al. 1998) and corresponded to a simple JOAG phenotype in this study (table 2table 2). The Thr377Lys change occurred in a mixed JOAG and POAG pedigree, with age at onset being 7–60 years and IOPs of 25–35 mmHg. This is similar to the Thr377Met phenotype, which involved the same codon (Alward et al. 1998a). The other three case subjects in our series (case subject 2, carrying Thr293Lys; case subject 7, carrying Gly399Val; and case subject 8, carrying Ala445Val) demonstrated varied clinical manifestations (table 2table 2). Case subject 2, (Thr293Lys) had pigment dispersion glaucoma at the age of 31 (table 2table 2). He had a family history of POAG with an average age at diagnosis of 51–71 years (fig. 1, pedigree 2) that was not confirmed clinically or molecularly by us, because family members were not available. Case subject 8 (Ala445Val) currently has no evidence of VF loss and is receiving medication because of previously documented raised IOP, asymmetrical discs (cup:disc [C:D] ratio 0.4 right eye, 0.6 left eye) and a family history of POAG (table 2table 2 and fig. 1, pedigree 8). This mutation was previously associated with glaucoma for which the clinical manifestations were not reported (Alward et al. 1998a).
Mutational Analysis of CYP1B1
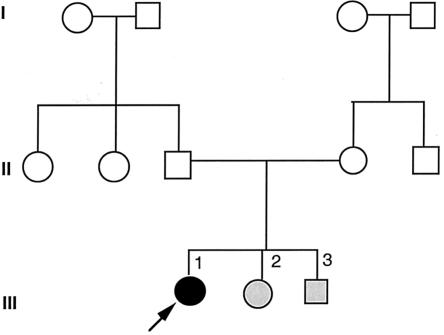
Screening of CYP1B1 detected three different mutations in three case subjects (figs. 2 and 3). The mutations and respective genotype-phenotype correlation are summarized in table 3table 3. These mutations were present in the heterozygous state (mutant/wild type [mt/wt]) in two case subjects (case subjects 7 and 10), and the third was a compound heterozygote (case subject 9). Two of these mutations, Arg368His and 1546dup10, have been described elsewhere (Bejjani et al. 2000), but the Leu345Phe change (4838C→T), within the I-helix of the heme-binding region, is here reported for the first time and was not present in 100 controls. For case subject 9, a diagnosis of JOAG was made at the age of 8 years, but case subject 9 had two younger siblings for whom CG was diagnosed (fig. 3 and table 4). All three children with glaucoma carried both CYP1B1 mutations, and the parents were each a carrier of one mutation. For case subject 10, a heterozygote for a novel missense mutation in exon 2D (Leu345Phe), a diagnosis of JOAG was made at the age of 36 years, because of characteristic glaucomatous-field loss. Case subject 10 was also heterozygous for the Val432Leu polymorphism.
Figure 3.
Pedigree of family 9, with CYP1B1 mutations (Arg368His and 1546dup10). Blackened symbols denote affected status for JOAG, whitened symbols denote unaffected or unknown status, and grayed symbols denote CG, rather than JOAG. Arrow indicates the proband.
Table 3.
CYP1B1 Genotype-Phenotype Correlations
| Case Subject (Mutations 1/2) | Exon | Codon 432Genotype | EthnicBackground | FamilyHistory | Age at Diagnosis,for Proband [Family](years) | IOP atDiagnosis | PublishedPhenotype | Other Mutations Detected |
| 7 (Arg368His/wild type) | 3 | Val/Leua | East Indian/Guyanese | AD JOAG, POAG | 27 [23–38] | High 20s to 30sb | CG, incomplete penetrancee | MYOC Gly399Val |
| 9 (Arg368His/1546dup10 frameshift) | 3 | Within duplication | Italian/English/Scottish | JOAG, CG | 8 [2–8] | 26/21 [15–34]c | CG, incomplete penetrancee | Nil |
| 10 (Leu345Phe/wild type) | 2 | Val/Leua | African American | Nil | 36 | NDd | New | Nil |
Table 4.
Clinical Features of Affected Individuals of Family 9
|
Age(years) |
Vertical C:D Ratio |
||||||||
| CaseSubjectand Eye | At Diagnosis | Currently | VisualAcuity atDiagnosis | CornealDiameter atDiagnosis(mm) | Angle or Age at Goniotomya | IOP at Diagnosis(mmHg) | At Diagnosis | Currently | Diagnosis |
| III.1: | 8 | 10 | JOAG | ||||||
| Right | 20/20 | 12.25 | Normal | 26 | .4 | .4 | |||
| Left | 20/20 | 12.50 | Normal | 21 | .2 | .2 | |||
| III.2: | 5 | 8 | 6 years | CG | |||||
| Right | 20/20 | 12.00 | 34 | .6 | .6 | ||||
| Left | 20/40 | 13.00 | 34 | .5 | .5 | ||||
| III.3: | 2.5 | 5 | 2.5 years | CG | |||||
| Right | 20/50 | 13.50 | 18 | .5 | .7 | ||||
| Left | 20/50 | 13.50 | 15 | .5 | .7 | ||||
Age at goniotomy is given when angle characteristics were not available.
Family 7
Case subject 7 had a MYOC mutation (Gly399Val) and a CYP1B1 mutation (Arg368His). Case subject 7 (fig. 2, proband III:4) had a strong family history of autosomal dominant glaucoma with variability in the age at onset (table 5). All participants with glaucoma carried the Gly399Val MYOC mutation. This novel missense MYOC mutation (1218G→T) resulted in substitution of a highly conserved glycine residue within the olfactomedin domain for a valine. The missense mutation in CYP1B1 exon 3 (7940G→A) resulted in a nonconservative substitution of arginine 368 by histidine. This mutation has been described elsewhere (Bejjani et al. 2000) in a Saudi Arabian population with CG with incomplete penetrance, and it was not found in 100 Saudi Arabian control chromosomes (Bejjani et al. 2000). We have detected this change in 1/140 (0.7%) control subjects. This was in an individual of Saudi Arabian descent with autosomal recessive retinitis pigmentosa but no glaucoma.
Table 5.
Clinical and Molecular Characteristics of Members of Family 7 Who Were Affected with Glaucoma[Note]
| CaseSubject | Diagnosis | Age atDiagnosis(years) | IOP atDiagnosis(OD/OS) | CurrentC:DRatioa(OD/OS) | Surgery | Drops | MYOCG399V | CYP1B1 R368H |
| I-2 | POAG suspected | 106b | ND | NDc | None | None | 399 | − |
| II-1 | MMG | 64 | 21 | .9/.9 | YLI | + | + | − |
| II:3 | MMG | 48 | ND | ND | YLI, Trab OU | None | + | − |
| III-1 | POAG | ND | ND | ND | Trab OU | None | + | − |
| III-2 | POAG | 48 | 28/29 | .9/total | ALT, Trab OU | None | + | − |
| III-3 | JOAG | 38 | 22 | .9/.9 | Trab OD, ALT OS | + | + | + |
| III-4 | MMG/JOAG | 27 | 25/30 | .95/total | Trab OU | + | + | + |
| III-5 | POAG | ∼50 | ND | ND | ND | ND | ND | ND |
| III-9 | JOAG | 23 | 33/30 | .8/.8 | Trab OU | + | + | + |
| III-10 | MMG | 49 | 25/28 | .4/.5 | None | + | + | − |
| IV-2 | JOAG | 23 | 27/28 | .7/.7 | Trab OS | + | + | + |
| IV-3 | JOAG suspected | 31b | 21/21 | .4/.6 | None | None | + | + |
Note.— OD = right eye; OS = left eye; OU = both eyes; MMG = mixed-mechanism glaucoma (>180 degrees of occludable angle); Trab = trabeculectomy; ALT = Argon laser trabeculoplasty; YLI = Yag laser iridotomy; ND = not documented.
Of the optic-nerve head.
For individuals suspected of having glaucoma, current age is given.
White nerve through cataract.
Individuals in this pedigree (fig. 2) carrying both the CYP1B1 and the MYOC mutations had JOAG with a mean age at onset of 27 years (range 23–38 years). Individuals with only the MYOC mutation had POAG with a mean age at onset of 51 years (range 48–64 years). By a two-tailed unpaired t-test analysis, the difference in age at onset between these two groups was statistically significant (P=.001). Individual IV:3, who has both mutations, is currently 28 years old and was treated for raised IOPs (28 mmHg) in the past. She currently has normal IOPs without medication, has suspicious asymmetric discs (C:D ratios of 0.4, for the right eye, and 0.6, for the left eye), has no evidence of visual-field loss, and is considered a strong glaucoma suspect. In addition to variable age at onset, several individuals in this pedigree have mixed-mechanism glaucoma (table 5).
Mutational Analysis of PITX2
Among the 60 individuals screened, no mutations were found in the coding regions of PITX2.
Discussion
We have shown that MYOC plays a role in 13.3% of the population with juvenile glaucoma that we studied. All case subjects with a MYOC mutation had a positive family history of autosomal dominant glaucoma, whether JOAG and/or POAG. The genotype-phenotype correlations of the patients that we studied and of patients with MYOC mutations who have been described elsewhere were very similar; for example, the Pro370Leu mutation has been described elsewhere, in pedigrees with very early onset (Adam et al. 1997; Stoilova et al. 1998; Taniguchi et al. 1999), and is here documented in a family in which the age at onset of glaucoma was 9–19 years. In support of the severity of this phenotype, an in vitro study of mutant recombinant myocilin proteins demonstrated complete insolubility of the Pro370Leu mutant (Zhou and Vollrath 1999). The novel Thr377Lys mutation has clinical features very similar to the Thr377Met mutation that has been described elsewhere (Alward et al. 1998a). These correlations may help optimize clinical management by providing early intervention in a phenotype known to be severe.
The clinical spectrum associated with the mutations identified is quite wide. For example, case subject 2 (who had the Thr293Lys MYOC) had glaucoma associated with pigment-dispersion syndrome diagnosed at the age of 31 years. Pigment dispersion and pigmentary glaucoma are genetically heterogeneous, with putative loci located on 7q35-q36 (Andersen et al. 1997) and 18q11 (Andersen et al. 1998), but no gene has been identified. The association of a MYOC mutation with pigmentary glaucoma is here reported for the first time. We also describe for the first time the association of MYOC mutations with mixed-mechanism glaucoma. Angle-closure glaucoma was thought to be a genetically distinct condition, and, at this time, no molecular information is available other than that some pedigrees have been reported to have angle-closure glaucoma (Talluto et al. 1998; Salmon 1999). The factor(s) underlying the significant variability of the expression of MYOC remain to be identified.
CYP1B1 mutations were present in 3 (5%) of the 60 individuals in the population that we studied, two of whom had a family history of glaucoma (table 3table 3). The spectrum of the CYP1B1-associated disease phenotype was also much broader than anticipated. The previously reported CYP1B1 mutations (Arg368His and 1546dup10) were supportive of autosomal recessive inheritance (Bejjani et al. 2000). Two affected individuals (case subjects 7 and 10) were heterozygotes (wt/mt) and had the leucine variant of the Val432Leu polymorphism. Although the entire coding sequence of CYP1B1 was analyzed in the patients whom we studied, it is possible that a second mutation exists in a promoter or other noncoding region of the other CYP1B1 allele that was not sequenced. No previous clinical manifestation of the heterozygous status has been described, and previously documented obligate carriers were not reported to have an increased incidence of glaucoma (Bejjani et al. 2000); however, the incidence of “heterozygote carriers” of CYP1B1 mutations in this group, 2/60 (3.3%), is higher than that in the 140 control individuals whom we studied (1/140 [0.7%]; P=.007). It is possible that the heterozygote state confers increased susceptibility to the development of glaucoma. In addition, polymorphisms such as the leucine variant of the Val432Leu polymorphism (8131G→C) may have functional implications. Studies have suggested that the leucine variant, compared with the valine 432 variant, has reduced activity in its ability to 4-hydroxylate 17-β-estradiol (Shimada et al. 1999, 2001; Hanna et al. 2000; Tang et al. 2000), although another study shows opposite results (Li et al. 2000).
The CYP1B1 missense mutation Arg368His, seen in case subjects 7 and 9, occurs at a CpG dinucleotide (7940G→A) within the J-helix of the heme-binding region. It was also reported in an Indian family (Panicker et al. 2001) and in one family from Saudi Arabia with autosomal recessive CG. In the latter family, this mutation showed incomplete penetrance, in that it occurred in the homozygous state in one unaffected individual of a sibship of four (Bejjani et al. 2000). Incomplete penetrance of CYP1B1 mutations was also described in 22 other pedigrees with four different mutations (Bejjani et al. 2000); however, not all patients were re-examined.
In family 7 (fig. 2), the combination of MYOC and CYP1B1 mutations appears to correlate with an earlier manifestation of the disease. Table 5 demonstrates that each affected family member with glaucoma who was tested (n=10) carried the MYOC mutation. This MYOC mutation, Gly399Val, is novel and occurs in the olfactomedin domain, where the majority of MYOC mutations occur (Fingert et al. 1999). Those members carrying both the MYOC and CYP1B1 mutations (n=5) are affected with juvenile glaucoma, whereas those with only the MYOC mutation (n=4) had POAG. The CYP1B1 mutation, Arg368His, may be a cause of a mild phenotype or a functional polymorphism or may modify the expression of MYOC. Whether the leucine variant of the Val432Leu polymorphism, which also segregated with the CYP1B1 mutation, has any functional implications warrants further investigations.
A recent study shows early menopause in women increases the risk for open-angle glaucoma, which suggests endogenous steroids may contribute to the pathogenesis of glaucoma (Hulsman et al. 2001). Myocilin is inducible by administration of dexamethasone, a steroid (Polansky et al. 1997). The interaction of CYP1B1 and MYOC has not been investigated. The exact substrate on which CYP1B1 interacts in the eye to cause glaucoma also remains to be identified. This substrate should have a role in the metabolism of 4-hydroxylation of 17-β-estradiol (Murray et al. 2001), an endogenously produced steroid. In vitro studies of mutant CYP1B1 protein result in decreased 4-hydroxylation of 17-β-estradiol (Stoilov et al. 2001). Metabolic impairment from the CYP1B1 heterozygous state (mt/wt) may further compromise the function of the mutant myocilin protein, with subsequent manifestation of the disease at an earlier age or influence the action of another glaucoma gene. This supports the recent work of Craig et al. that suggests that open-angle glaucoma may not really be a monogenic disease, at least not in all case subjects (Craig et al. 2001).
In addition to the potential modifier effect of CYP1B1 mutations on MYOC mutant phenotypes, the functional implications of CYP1B1 polymorphisms warrants further investigations. For example, the Val432Leu variant is demonstrated to alter the 4-hydroxylation of estradiol (Shimada et al. 1999, 2001; Hanna et al. 2000; Tang et al. 2000). The presence of such a Leu432 variation to those individuals with heterozygote CYP1B1 mutations may alter the metabolic activity of CYP1B1 enough to predispose to some changes of the anterior segment and to the development of glaucoma. Functional studies of mutant protein, in combination with these polymorphic variants, on estradiol substrates are required to test this hypothesis.
The functional implication of polymorphisms has been studied in a wide range of disorders and has been associated with increased susceptibility in several instances (Allikmets and the International ABCR Screening Consortium 2000; Aithal et al. 2001; Buchs et al. 2001; Niesler et al. 2001; Nikpoor et al. 2001; Wilkie et al. 2001). Additional evidence that other polymorphic variants of the cytochrome P450 family differ in their ability to bind to and metabolize pharmacological agents and endogenous hormones may explain the variability in responsiveness to medical treatment in glaucoma (Davies et al. 2001; Ingelman-Sundberg 2001; Jazwinska-Tarnawska et al. 2001; Kita et al. 2001; Shintani et al. 2001).
For case subject 9 (II.1 from family 9, who carried Arg368His and a frameshift mutation resulting from duplication of 10 bp), JOAG was diagnosed at age 8 years (fig. 3, pedigree 9). At diagnosis, she had raised IOP, asymmetrical discs, and corneal diameters within normal limits (Elstan 1997) (table 4). Both of her younger sibs (III.2 and III.3) had variable expression of CG, which was diagnosed at ages 5 and 2.5 years, respectively. The duplicated region included the Val432Leu polymorphism, thereby making it difficult to assess whether an additional variant was present. The molecular association of CG and JOAG has here been described for the first time. These findings, as well as recent evidence of CYP1B1 mutations in patients with Peters anomaly (Vincent et al. 2001), suggest that the role of CYP1B1 is not solely confined to the pathogenesis of CG but is implicated in other forms of glaucoma. This variability of the CYP1B1-related clinical manifestations and of age at onset, together with the incomplete penetrance, again suggests the influence of another genetic factor that will make the counseling of patients with early-onset glaucoma difficult.
Mutational analysis of case subjects with early-onset glaucoma has demonstrated (1) the strong variability of expression and allelic heterogeneity for MYOC and CYP1B1 mutations and (2) that congenital and juvenile glaucoma are allelic variants, at least in some cases. The range of the associated phenotypes, in addition to the incomplete penetrance described elsewhere, may have significant implications for the counseling of patients and families. Furthermore, this work suggests that MYOC and CYP1B1 may interact through a common pathway and that the inheritance of glaucoma may be multiallelic in some cases. We propose that MYOC function may be influenced by changes in CYP1B1 (including mutations and polymorphisms, such as Val432Leu). Molecular characterization of juvenile glaucoma was possible in 16% of cases and will be enhanced as more glaucoma genes are identified. This work not only emphasizes the genetic heterogeneity and complexity behind the pathogenesis of glaucoma but also opens new avenues for research.
Acknowledgments
This work was supported in part by the Glaucoma Research Society of Canada (E.H.); Royal Australian College of Ophthalmologists/OPSM Travelling Scholarship, University of Auckland Arthur Thomas Paterson Scholarship, and the Glaucoma Trust of New Zealand (A.L.V.). We are grateful for the participation of the patients and their families and for the referral of patients by Drs. G. Trope, M. Wolpert, F. Feldman, J. Christakis, L. Schonberger, and R. Buncic.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CYP1B1 mRNA sequence [accession number U56438])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MYOC [MIM 601652], GLC1A [MIM 137750], CYP1B1 [MIM 601771], GLC3A [MIM 231300], and PITX2 [MIM 601542])
References
- Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ (1997) Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet 6:2091–2097 [DOI] [PubMed] [Google Scholar]
- Aithal GP, Day CP, Leathart J, Daly AK, Hudson M (2001) Association of single nucleotide polymorphisms in the interleukin-4 gene and interleukin-4 receptor gene with Crohn's disease in a British population. Genes Immun 2:44–47 [DOI] [PubMed] [Google Scholar]
- Allikmets R, the International ABCR Screening Consortium (2000) Further evidence for an association of ABCR alleles with age-related macular degeneration. Am J Hum Genet 67:487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM (1998a) Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med 338:1022–1027 [DOI] [PubMed] [Google Scholar]
- Alward WL, Semina EV, Kalenak JW, Héon E, Sheth BP, Stone EM, Murray JC (1998b) Autosomal dominant iris hypoplasia is caused by a mutation in the Rieger syndrome (RIEG/PITX2) gene. Am J Ophthalmol 125:98–100 [DOI] [PubMed] [Google Scholar]
- Andersen JS, Parrish R, Greenfield D, DelBono EA, Haines JL, Wiggs JL (1998) A second locus for the pigment dispersion syndrome and pigmentary glaucoma maps to 18q11-q21. Am J Hum Genet Suppl 63:A279 [Google Scholar]
- Andersen JS, Pralea AM, DelBono EA, Haines JL, Gorin MB, Schuman JS, Mattox CG, Wiggs JL (1997) A gene responsible for the pigment dispersion syndrome maps to chromosome 7q35-q36. Arch Ophthalmol 115:384–388 [DOI] [PubMed] [Google Scholar]
- Bejjani B, Lewis R, Tomey K, Anderson K, Dueker D, Jabek M, Astle W, Otterund B, Leppert M, Lupsi J (1998) Mutations in CYP1B1, the gene for cytochrome P450B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet 62:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani B, Stockton D, Lewis R, Tomey K, Dueker D, Jabak M, Astle W, Lupski J (2000) Multiple CYP1B1 mutations and incomplete penetrance in an inbred population segregating primary congenital glaucoma suggest frequent de novo events and a dominant modifier locus. Hum Mol Genet 9:367–374 [DOI] [PubMed] [Google Scholar]
- Buchs N, di Giovine FS, Silvestri T, Vannier E, Duff GW, Miossec P (2001) IL-1B and IL-1Ra gene polymorphisms and disease severity in rheumatoid arthritis: interaction with their plasma levels. Genes Immun 2:222–228 [DOI] [PubMed] [Google Scholar]
- Craig JE, Baird PN, Healey DL, McNaught AI, McCartney PJ, Rait JL, Dickinson JL, Roe L, Fingert JH, Stone EM, Mackey DA (2001) Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology 108:1607–1620 [DOI] [PubMed] [Google Scholar]
- Crespi CL, Penman BW, Steimel DT, Smith T, Yang CS, Sutter TR (1997) Development of a human lymphoblastoid cell line constitutively expressing human CYP1B1 cDNA: substrate specificity with model substrates and promutagens. Mutagenesis 12:83–89 [DOI] [PubMed] [Google Scholar]
- Davies E, Holloway CD, Ingram MC, Friel EC, Inglis GC, Swan L, Hillis WS, Fraser R, Connell JM (2001) An influence of variation in the aldosterone synthase gene (CYP11B2) on corticosteroid responses to ACTH in normal human subjects. Clin Endocrinol (Oxf) 54:813–817 [DOI] [PubMed] [Google Scholar]
- DeLuise V, Anderson D (1983) Primary infantile glaucoma (congenital glaucoma). Surv Ophthal 28:1–19 [DOI] [PubMed] [Google Scholar]
- Doward W, Perveen R, Lloyd IC, Ridgway AE, Wilson L, Black GC (1999) A mutation in the RIEG1 gene associated with Peters' anomaly. J Med Genet 36:152–155 [PMC free article] [PubMed] [Google Scholar]
- Ellis O (1948) The etiology, symptomatology and treatment of juvenile glaucoma. Am J Ophthalmol 31:1589–1596 [DOI] [PubMed] [Google Scholar]
- Elstan J (1997) Developmental abnormalities of the anterior segment. In: Taylor D (ed) Paediatric ophthalmology. Blackwell Science, Oxford, pp 252–265 [Google Scholar]
- Fautsch MP, Bahler CK, Jewison DJ, Johnson DH (2000) Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci 41:4163–4168 [PubMed] [Google Scholar]
- Fingert JH, Héon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM (1999) Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet 8:899–905 [DOI] [PubMed] [Google Scholar]
- Fingert JH, Ying L, Swiderski RE, Nystuen AM, Arbour NC, Alward WL, Sheffield VC, Stone EM (1998) Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res 8:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF (2000) Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res 60:3440–3444 [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR (1996) 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA 93:9776–9781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héon E, Martin N, Billingsley G, Williams-Lyn D, Sutherland J, Levin A (2000) Molecular characterization of congenital glaucoma in the Greater Toronto Area. Invest Ophthalmol Vis Sci Suppl 41:S527 [Google Scholar]
- Héon E, Priston M, Schorderet D, Billingsley G, Girard P, Lubsen N, Munier F (1999) The γ-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héon E, Sheth BP, Kalenak JW, Sunden SL, Streb LM, Taylor CM, Alward WL, Sheffield VC, Stone EM (1995) Linkage of autosomal dominant iris hypoplasia to the region of the Rieger syndrome locus (4q25). Hum Mol Genet 4:1435–1439 [DOI] [PubMed] [Google Scholar]
- Huang W, Jaroszewski J, Ortego J, Escribano J, Coca-Prados M (2000) Expression of the TIGR gene in the iris, ciliary body, and trabecular meshwork of the human eye. Ophthalmic Genet 21:155–169 [PubMed] [Google Scholar]
- Hulsman CA, Westendorp IC, Ramrattan RS, Wolfs RC, Witteman JC, Vingerling JR, Hofman A, de Jong PT (2001) Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am J Epidemiol 154:138–144 [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M (2001) Genetic susceptibility to adverse effects of drugs and environmental toxicants: the role of the CYP family of enzymes. Mutat Res 482:11–19 [DOI] [PubMed] [Google Scholar]
- Jazwinska-Tarnawska E, Orzechowska-Juzwenko K, Niewinski P, Rzemislawska Z, Loboz-Grudzien K, Dmochowska-Perz M, Slawin J (2001) The influence of CYP2D6 polymorphism on the antiarrhythmic efficacy of propafenone in patients with paroxysmal atrial fibrillation during 3 months propafenone prophylactic treatment. Int J Clin Pharmacol Ther 39:288–292 [DOI] [PubMed] [Google Scholar]
- Johnson A, Drack A, Kwitek A, Cannon RL, Stone EM, Alward WL (1993) Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology 100:524–529 [DOI] [PubMed] [Google Scholar]
- Kakiuchi-Matsumoto T, Isashiki Y, Ohba N, Kimura K, Sonoda S, Unoki K (2001) Cytochrome P450 1B1 gene mutations in Japanese patients with primary congenital glaucoma. Am J Ophthalmol 131:345–350 [DOI] [PubMed] [Google Scholar]
- Kita T, Tanigawara Y, Aoyama N, Hohda T, Saijoh Y, Komada F, Sakaeda T, Okumura K, Sakai T, Kasuga M (2001) CYP2C19 genotype related effect of omeprazole on intragastric pH and antimicrobial stability. Pharm Res 18:615–621 [DOI] [PubMed] [Google Scholar]
- Kozlowski K, Walter MA (2000) Variation in residual PITX2 activity underlies the phenotypic spectrum of anterior segment developmental disorders. Hum Mol Genet 9:2131–2139 [DOI] [PubMed] [Google Scholar]
- Kubota R, Kudoh J, Mashima Y, Asakawa S, Minoshima S, Hejtmancik JF, Oguchi Y, Shimizu N (1998) Genomic organization of the human myocilin gene (MYOC) responsible for primary open angle glaucoma (GLC1A). Biochem Biophys Res Commun 242:396–400 [DOI] [PubMed] [Google Scholar]
- Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, Shimizu N (1997) A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics 41:360–369 [DOI] [PubMed] [Google Scholar]
- Kulak SC, Kozlowski K, Semina EV, Pearce WG, Walter MA (1998) Mutation in the RIEG1 gene in patients with iridogoniodysgenesis syndrome. Hum Mol Genet 7:1113–1117 [DOI] [PubMed] [Google Scholar]
- Li DN, Seidel A, Pritchard MP, Wolf CR, Friedberg T (2000) Polymorphisms in P450 CYP1B1 affect the conversion of estradiol to the potentially carcinogenic metabolite 4-hydroxyestradiol. Pharmacogenetics 10:343–353 [DOI] [PubMed] [Google Scholar]
- Luch A, Coffing SL, Tang YM, Schneider A, Soballa V, Greim H, Jefcoate CR, Seidel A, Greenlee WF, Baird WM, Doehmer J (1998) Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chem Res Toxicol 11:686–695 [DOI] [PubMed] [Google Scholar]
- Martin S, Sutherland J, Levin A, Klose R, Priston R, Héon E (2000) Molecular characterization of congenital glaucoma in a consanguineous Canadian community: a step towards preventing glaucoma-related blindness. J Med Genet 37:422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Dykes D, Polesky H (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Res 16:1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GI, Melvin WT, Greenlee WF, Burke MD (2001) Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol 41:297–316 [DOI] [PubMed] [Google Scholar]
- Niesler B, Flohr T, Nothen MM, Fischer C, Rietschel M, Franzek E, Albus M, Propping P, Rappold GA (2001) Association between the 5′ UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics 11:471–475 [DOI] [PubMed] [Google Scholar]
- Nikpoor B, Turecki G, Fournier C, Theroux P, Rouleau GA (2001) A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. Am Heart J 142:336–339 [DOI] [PubMed] [Google Scholar]
- Noda S, Mashima Y, Obazawa M, Kubota R, Oguchi Y, Kudoh J, Minoshima S, Shimizu N (2000) Myocilin expression in the astrocytes of the optic nerve head. Biochem Biophys Res Commun 276:1129–1135 [DOI] [PubMed] [Google Scholar]
- Panicker SG, Reddy ABM, Mandel AK, Ahmed N, Hasnain SE, Balasubramanian D (2001) The molecular genetic basis of primary congenital glaucoma in India. Invest Ophthalmol Vis Sci Suppl 42:S530 [Google Scholar]
- Plášilová M, Stoilov I, Sarfarazi M, Kádasi L, Feráková E, Ferák V (1999) Identification of a single ancestral CYP1B1 mutation in Slovak gypsies (Roms) affected with primary congenital glaucoma. J Med Genet 36:290–294 [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD (1997) Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211:126–139 [DOI] [PubMed] [Google Scholar]
- Priston M, Kozlowski K, Gill D, Letwin K, Buys Y, Levin AV, Walter MA, Héon E (2001) Functional analyses of two newly identified PITX2 mutants reveal a novel molecular mechanism for Axenfeld-Rieger syndrome. Hum Mol Genet 10:1631–1638 [DOI] [PubMed] [Google Scholar]
- Rozsa FW, Shimizu S, Lichter PR, Johnson AT, Othman MI, Scott K, Downs CA, Nguyen TD, Polansky J, Richards JE (1998) GLC1A mutations point to regions of potential functional importance on the TIGR/MYOC protein. Mol Vis 4:20 [PubMed] [Google Scholar]
- Salmon JF (1999) Predisposing factors for chronic angle-closure glaucoma. Prog Retin Eye Res 18:121–132 [DOI] [PubMed] [Google Scholar]
- Semina E, Reiter R, Leysens N, Alward W, Small K, Datson N (1996) Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 14:392–399 [DOI] [PubMed] [Google Scholar]
- Sheffield V, Stone E, Alward W (1993) Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet 4:47–50 [DOI] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR (1996) Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res 56:2979–2984 [PubMed] [Google Scholar]
- Shimada T, Watanabe J, Inoue K, Guengerich FP, Gillam EM (2001) Specificity of 17β-oestradiol and benzo[a]pyrene oxidation by polymorphic human cytochrome P4501B1 variants substituted at residues 48, 119 and 432. Xenobiotica 31:163–176 [DOI] [PubMed] [Google Scholar]
- Shimada T, Watanabe J, Kawajiri K, Sutter TR, Guengerich FP, Gillam EM, Inoue K (1999) Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis 20:1607–1613 [DOI] [PubMed] [Google Scholar]
- Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, Clarke MS, Schwartz AL, Downs CA, Vollrath D, Richards JE (2000) Age-dependent prevalence of mutations at the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol 130:165–177 [DOI] [PubMed] [Google Scholar]
- Shintani M, Ieiri I, Inoue K, Mamiya K, Ninomiya H, Tashiro N, Higuchi S, Otsubo K (2001) Genetic polymorphisms and functional characterization of the 5′-flanking region of the human CYP2C9 gene: in vitro and in vivo studies. Clin Pharmacol Ther 70:175–182 [DOI] [PubMed] [Google Scholar]
- Simms RM, Fingert JH, Craig JE, McNaught AI, Mackey DA (1999) Normal range of hearing associated with myocilin Thr377Met. Ophthalmic Genet 20:205–207 [DOI] [PubMed] [Google Scholar]
- Stoilov I, Akarsu A, Alozie I, Child A, Barsoom-Homsy M, Turacli M, Or M, Lewis R, Ozdemir N, Brice G, Aktan S, Chevrette L, Coca-Prados M, Sarfarazi M (1998) Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Hum Genet 62:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilov I, Akarsu AN, Sarfarazi M (1997) Identification of three truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet 6:641–647 [DOI] [PubMed] [Google Scholar]
- Stoilov IR, Costa VP, Vasconellos JPC, Mello MB, Betinjane AJ, Carani JCE, Oltrogge EV, Sarfarazi M (2001) Mutation screening of the CYP1B1 gene and phenotype-genotype correlation in primary congenital glaucoma cases from Brazil. Invest Ophthalmol Vis Sci Suppl 42:S530 [Google Scholar]
- Stoilova D, Child A, Brice G, Desai T, Barsoum-Homsy M, Ozdemir N, Chevrette L, Adam MF, Garchon HJ, Pitts Crick R, Sarfarazi M (1998) Novel TIGR/MYOC mutations in families with juvenile onset primary open angle glaucoma. J Med Genet 35:989–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC (1997) Identification of a gene that causes primary open angle glaucoma. Science 275:668–670 [DOI] [PubMed] [Google Scholar]
- Sutter T, Tang Y, Hayes C, Wo Y-Y, Jabs E, Li X, Yin H, Cody C, Grennlee W (1994) Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem 169:13092–13099 [PubMed] [Google Scholar]
- Suzuki Y, Shirato S, Taniguchi F, Ohara K, Nishimaki K, Ohta S (1997) Mutations in the TIGR gene in familial primary open-angle glaucoma in Japan. Am J Hum Genet 61:1202–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talluto D, Feith M, Allee S (1998) Simultaneous angle closure in twins. J Glaucoma 7:68–69 [PubMed] [Google Scholar]
- Tang YM, Green BL, Chen GF, Thompson PA, Lang NP, Shinde A, Lin DX, Tan W, Lyn-Cook BD, Hammons GJ, Kadlubar FF (2000) Human CYP1B1 Leu432Val gene polymorphism: ethnic distribution in African-Americans, Caucasians and Chinese; oestradiol hydroxylase activity; and distribution in prostate cancer cases and controls. Pharmacogenetics 10:761–766 [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Suzuki Y, Shirato S, Araie M (2000) The Gly367Arg mutation in the myocilin gene causes adult-onset primary open-angle glaucoma. Jpn J Ophthalmol 44:445–448 [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Suzuki Y, Shirato S, Ohta S (1999) Clinical phenotype of a Japanese family with primary open angle glaucoma caused by a Pro370Leu mutation in the MYOC/TIGR gene. Jpn J Ophthalmol 43:80–84 [DOI] [PubMed] [Google Scholar]
- Vincent AL, Billingsley G, Priston M, Williams-Lyn D, Sutherland J, Glaser T, Oliver E, Walter MA, Heathcote G, Levin A, Héon E (2001) Phenotypic heterogeneity of CYP1B1: mutations in a patient with Peters anomaly. J Med Genet 38:324–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie SE, Li Y, Deery EC, Newbold RJ, Garibaldi D, Bateman JB, Zhang H, Lin W, Zack DJ, Bhattacharya SS, Warren MJ, Hunt DM, Zhang K (2001) Identification and functional consequences of a new mutation (E155G) in the gene for GCAP1 that causes autosomal dominant cone dystrophy. Am J Hum Genet 69:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Vollrath D (1999) A cellular assay distinguishes normal and mutant TIGR/myocilin protein. Hum Mol Genet 8:2221–2228 [DOI] [PubMed] [Google Scholar]