Abstract
Vascular risk factors have been associated with cognitive decline, however, it remains unclear whether apolipoprotein E (APOE) genotype modifies this relationship. We aimed to further elucidate these relationships and extend previous findings by examining data from a more comprehensive cognitive assessment than used in prior studies. 1,436 participants from the prospective Framingham Offspring Cohort Study underwent health examination from 1991-1995, followed by a baseline neuropsychological assessment (1999-2003) and a repeat neuropsychological assessment approximately eight years later (2004-2009). Multivariate linear regression analyses were performed to examine the relationship between midlife vascular risk factors, presence of the APOE ε4 allele, and cognitive change. APOE genotype significantly modified the associations between both midlife hypertension and cardiovascular disease and decline in language abilities as well as midlife diabetes and decline in verbal memory, attention, and visuospatial abilities. Associations between increased midlife vascular risk burden and greater cognitive decline were observed among APOE ε4 carriers but not non-carriers. The present findings revealed a subgroup at increased risk for cognitive decline (APOE ε4 carriers with midlife exposure to vascular risk factors) and suggest that treatment of vascular risk factors during midlife may reduce the risk of cognitive impairment later in life, particularly among APOE ε4 carriers.
Keywords: Apolipoprotein E, Cognition, Vascular Risk, Aging, Diabetes, Hypertension, Cardiovascular Disease
Introduction
The presence of vascular risk factors in midlife is associated with greater cognitive decline[1] and the development of dementia later in life.[2, 3] Among nondemented older adults, some studies report that greater vascular risk burden is associated with cognitive decline restricted to a particular domain such as executive functioning[1] or language,[4] whereas other studies have found associations with poorer performance across multiple additional domains including processing speed, visuospatial abilities,[5] and memory.[6]
The presence of the apolipoprotein E (APOE) ɛ4 allele is a well-established susceptibility gene for Alzheimer’s disease (AD).[7] APOE genotype also influences susceptibility to atherosclerosis[8] and may increase risk of vascular dementia.[9] In terms of the role of the APOE ɛ4 allele as a risk factor for cognitive decline in normal aging, findings have been somewhat mixed. Some studies demonstrate poorer cognitive performance or greater decline among APOE ɛ4 carriers relative to non-carriers that is restricted to episodic memory[1] whereas other studies show poorer performance across a variety of additional abilities, including global cognitive functioning and executive functioning.[10] Further, several previously published reports show no association between APOE genotype and cognitive functioning in nondemented older adults,[11] particularly when those with preclinical AD are removed from the sample.[12]
There are few published reports examining whether APOE genotype influences the relationship between vascular risk burden and cognitive impairment, and even fewer have investigated these associations in a longitudinal context. Generally, findings across such studies have been inconsistent with some results demonstrating that the APOE ɛ4 allele in combination with vascular risk factors imparts increased risk of cognitive decline[6, 13, 14] whereas other studies have reported no such interaction.[1, 15] Notably, these studies have differed in terms of participant characteristics (e.g., mean age at baseline cognitive evaluation) and methodology (e.g., length of follow up, vascular risk factors assessed, cognitive measures administered). Further, in general, a caveat of existing longitudinal studies involves the limited nature of the cognitive domains and measures examined.
Given the potential significant public health impact of identification of risk factors for preclinical cognitive decline[16] as well as the limitations of existing studies examining the influence of vascular risk burden and APOE genotype on cognition in older adults, we aimed to further elucidate whether APOE genotype modifies the relationship between midlife vascular risk burden and later cognitive decline. In particular, we sought to extend findings of existing published reports by examining data from a more comprehensive cognitive assessment than used in previous studies. We hypothesized that APOE genotype would modify the relationship between vascular risk and cognitive decline across a variety of cognitive domains including memory, attention, executive functioning, visuospatial skills, and language. Specifically, we expected that the association between midlife vascular risk exposure and later cognitive decline would be stronger among APOE ε4 carriers relative to non-carriers. Further, given the possibility that vascular risk factors may interact with genetic risk to lead to cognitive decline through multiple divergent and convergent physiologic pathways, we examined each vascular risk factor and cognitive domain individually to determine whether these individual risk factors show differential relationships with the various cognitive domains.
Materials and Methods
Participants
The Framingham Heart Study is a community-based, prospective study that was initiated in 1948 to identify risk factors for cardiovascular disease. To date, the study has followed three generations of participants including (1) the Original Cohort; (2) the Offspring Cohort, which includes biological children of the Original cohort and Offspring spouses, who have been followed since 1971; and (3) the Third Generation Cohort, which includes children from members of the Offspring Cohort, who have been followed since 2000. Participants in the current study were members of the Offspring Cohort who have undergone health examinations approximately every four years since 1971.[17] Of the 3,799 participants from the Offspring Cohort who participated in the fifth examination (Exam 5) between 1991 and 1995, 1,602 individuals also underwent two neuropsychological assessments at least five years apart. The first assessment was administered between 1999 and 2003 and the second assessment, was conducted between 2004 and 2009. Of the 1,558 participants aged 45 to 84 at the first neuropsychological assessment, individuals were excluded from the present study for the following reasons: documented clinical stroke, dementia, or other neurological disorders (e.g., multiple sclerosis, head trauma) at the first assessment (n = 38); neurological conditions at the second assessment (n = 17); missing APOE genotype data (n = 28); APOE ε2/ε4 genotype given the ambiguity associated with the presence of both an allele imparting increased risk (ε4) as well as an allele with a possible protective impact (n = 31); and missing covariate data (n = 8). Individuals with mild cognitive impairment at the first assessment were not excluded. These inclusion and exclusion criteria resulted in a final sample for the present analyses of 1,436 participants.
Vascular Risk Factors
Vascular risk factors were assessed at Exam 5. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive medications;[18] history of cardiovascular disease was based on history of coronary heart disease (includes myocardial infarction, angina pectoris, and coronary insufficiency), cardiac failure, and intermittent claudication;[19] diabetes was defined as fasting glucose ≥ 7 mmol/L or use of an anti-diabetic therapy; and cigarette smokers were identified based on current smoking status (yes/no) at Exam 5.
Neuropsychological Assessment
Standardized neuropsychological measures administered at both assessments included measures of verbal memory (Wechsler Memory Scale [WMS] Logical Memory-delayed recall [LM-DR]), visual memory (WMS Visual Reproduction-delayed recall [VR-DR]), attention (Halstead-Reitan [HR] Trail Making Test Part A [Trails A]), executive functioning (HR Trail Making Test Part B [Trails B]), visuospatial skills (Hooper Visual Organization Test [VOT]), and language (Boston Naming Test 30-item version [BNT]). For the WMS measures, savings scores, which assess degree of memory retention, were calculated and expressed in percent using the following formulas: LM savings = ([LM Story A delayed recall/LM Story A immediate recall] X 100) and VR savings = ([VR delayed recall/VR immediate recall] X 100). For the Trail Making Test, a difference score (i.e., Trails B – Trails A) was calculated. This derived score has been argued to reflect a purer measure of the executive functioning abilities required to complete Trails B given that it is assumed to subtract out the simple sequencing, visual scanning, and psychomotor demands common to both Trails A and B.[20] The Center for Epidemiological Studies Depression Scale (CES-D) was administered during Exam 7, which was the examination closest to the baseline neuropsychological assessment.
Statistical Analyses
Multivariate linear regression analyses were performed to examine associations among vascular risk factors, APOE genotype, and annualized change in neuropsychological performance, adjusting for age at first neuropsychological assessment, sex, education, and time from Exam 5 to the first neuropsychological assessment. Educational attainment was obtained by self-report and studied as a three-category variable (no high school degree, high school degree, college degree). Annualized raw change on neuropsychological measures was calculated with the following formula: ([score at the second neuropsychological assessment - score at the first neuropsychological assessment]/time between the first and second neuropsychological assessments in years). The signs of all Trail Making Test variables were reversed during calculation of annual rate of change in order to be consistent with the other neuropsychological measures (i.e., negative rate of change indicates decline). Results (i.e., β values) are adjusted differences in mean decline, presented in standard deviation units. Interaction terms were included in models to examine the modifying effect of APOE genotype on the relationship between vascular risk factor exposure at Exam 5 and longitudinal change in neuropsychological performance. Secondary analyses were performed (a) additionally adjusting for depressive symptoms (based on CES-D score), and (b) excluding the 116 participants who reported significant depressive symptoms (i.e., scored a 16 or higher on the CES-D) at Exam 7. Significance levels of 0.10 were used for tests of interaction; levels of 0.05 were used for all other tests. All analyses were performed using Statistical Analyses System software version 9.2 (SAS Institute, Cary, NC).
Results
Demographic variables and risk factor characteristics
Table 1 presents baseline characteristics of the sample participants as well as attendees of Exam 5 who were not included in the current sample. In the study sample, midlife vascular risk factors were assessed at a mean age of 54 years. The first and last neuropsychological evaluations were performed at mean ages of 62 and 70 years, respectively. During the period of time between the two neuropsychological evaluations, 21 participants sustained a stroke and 13 developed dementia. Twenty percent of participants were APOE ε4 carriers. Attendees of examination 5 who were not in our study sample were somewhat older (mean age of 56 versus 54 years at Exam 5), completed less education (30% versus 40% had earned a college degree), and had greater vascular risk burden (significantly higher systolic blood pressure and rates of hypertension, CVD, diabetes, and smoking). Table 2 shows the mean raw scores and standard deviations for the various neuropsychological measures from the baseline and follow-up examinations as well as the annual rate of change.
Table 1.
Demographic and Risk Factor Characteristics of Participants in the Present Study Sample Compared to Participants who Attended Exam 5 but did Not Complete the Neuropsychological Assessment and Were Not Included in the Present Sample.
| Variable | Study Sample | Not in Sample | p | |
|---|---|---|---|---|
| N | 1,436 | 2,363 | ||
| Age at examination 5 (years; mean ± SE) | 54 ± 9 | 56 ± 11 | <0.001 | |
| Sex (% women) | 54 | 52 | .218 | |
| Education (%) | ||||
| No HS Degree | 3 | 7 | <0.001 | |
| HS Degree | 57 | 62 | ||
| College Degree | 40 | 30 | ||
| APOE ε4 carrier (%) | 20 | 21 | 0.480 | |
| MMSE score (mean ± SE)a | 29±1 | 29±2 | <0.001 | |
| Systolic blood pressure (mean ± SE)a | 124±18 | 128±19 | <0.001 | |
| Hypertension (≥ JNC 7 Stage I; %)a | 28 | 39 | <0.001 | |
| History of CVD (%)a | 5 | 11 | <0.001 | |
| Diabetes mellitus (%)a | 5 | 9 | <0.001 | |
| Smoker (%)a | 15 | 22 | <0.001 | |
Abbreviations: SE = standard error; NP = neuropsychological; HS = high school; APOE = apolipoprotein E; MMSE = Mini-Mental State Examination; CES-D = Center for Epidemiological Studies Depression Scale; JNC 7 = Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; CVD = cardiovascular disease.
At Exam 5
Table 2.
Neuropsychological Performance at Baseline and Follow up Assessments and Rate of Cognitive Change
| Neuropsychological Measure | Baseline score (mean ± SD) | Follow up score (mean ± SD) | Annual rate of change (mean ± SD) |
|---|---|---|---|
| WMS LM delayed recall | 11 ± 4 | 11 ± 4 | 0.07 ± 0.56 |
| WMS LM savings | 92 ± 19 | 91 ± 22 | -0.2 ± 4.0 |
| WMS VR delayed recall | 8 ± 3 | 8 ± 3 | -0.07 ± 0.46 |
| WMS VR savings | 91 ± 23 | 88 ± 25 | -0.4 ± 4.9 |
| WAIS Similarities | 17 ± 3 | 17 ± 4 | -0.03 ± 0.49 |
| Halstead-Reitan Trail Making Part A (minutes) | 0.5 ± 0.2 | 0.6 ± 0.3 | -0.005 ± 0.042* |
| Halstead-Reitan Trail Making Part B (minutes) | 1.4 ± 0.9 | 1.7 ± 1.4 | -0.06 ± 0.19* |
| Trail Making B-A (minutes) | 0.8 ± 0.8 | 1.2 ± 1.3 | -0.05 ± 0.19* |
| Hooper Visual Organization Test | 25 ± 3 | 25 ± 3 | -0.06 ± 0.35 |
| Boston Naming Test | 28 ± 2 | 27 ± 3 | -0.04 ± 0.30 |
Abbreviations: SD = standard deviation; WMS = Wechsler Memory Scale; LM = Logical Memory; VR = Visual Reproduction; WAIS = Wechsler Adult Intelligence Scale.
The signs of the three Trail Making Test variables were reversed during calculation of annual rate of change in order to be consistent with the other neuropsychological measures (i.e., negative rate of change indicates decline)
Main effect of midlife vascular risk factors on later cognitive decline
Across all participants (collapsed across APOE genotype), midlife vascular risk factors were associated with a more marked decline in various aspects of cognitive functioning approximately 15 years after the vascular risk assessment (and eight years after the initial neuropsychological assessment; Table 3). Hypertension in midlife was significantly associated with greater decline on measures of executive functioning (Trails B-A: -0.17 ± 0.06, p < 0.01), visual attention (Trails A: -0.19 ± 0.06, p < 0.01), and visuospatial skills (VOT: -0.15 ± 0.06, p < 0.05). History of cardiovascular disease at midlife was significantly associated with a more pronounced decline in attention (Trails A: -0.35 ± 0.13, p < 0.01) and language (BNT: -0.26 ± 0.12, p < 0.05). Midlife diabetes was significantly associated with greater decline on measures of language (BNT: -0.25 ± 0.12, p < 0.05). Smoking in midlife was significantly associated with a more marked decline in visual memory (VR delayed recall: -0.17 ± 0.07, p < 0.05). Findings were not changed after controlling for or excluding for depressive symptoms based on the CES-D.
Table 3.
Main effect of APOE genotype on cognitive decline and moderating effect of APOE genotype on the association between midlife vascular risk factors and later cognitive decline
| APOE ɛ4 | HTN | CVD | DM | SMK | ||
|---|---|---|---|---|---|---|
| WMS LM delayed recall | ||||||
| β ± SE | -0.17 ± 0.07* | -0.03 ± 0.06 | -0.06 ± 0.13 | -0.13 ± 0.12 | -0.04 ± 0.07 | |
| Interaction with APOE | n/a | NS | NS | NS | NS | |
| WMS LM savings | ||||||
| β ± SE | 0.00 ± 0.07 | 0.02 ± 0.06 | 0.21 ± 0.13 | -0.13 ± 0.13 | -0.01 ± 0.08 | |
| Interaction with APOE | n/a | NS | NS | 0.083 | NS | |
| WMS VR delayed recall | ||||||
| β ± SE | -0.10 ± 0.07 | -0.02 ± 0.06 | -0.08 ± 0.12 | -0.23 ± 0.12 | -0.17 ± 0.07* | |
| Interaction with APOE | n/a | NS | NS | NS | NS | |
| WMS VR savings | ||||||
| β ± SE | -0.03 ± 0.07 | 0.05 ± 0.06 | 0.11 ± 0.13 | -0.21 ± 0.13 | -0.02 ± 0.08 | |
| Interaction with APOE | n/a | NS | NS | NS | NS | |
| WAIS Similarities | ||||||
| β ± SE | -0.01 ± 0.07 | 0.11 ± 0.06 | -0.03 ± 0.13 | 0.05 ± 0.12 | -0.09 ± 0.07 | |
| Interaction with APOE | n/a | NS | NS | NS | NS | |
| Trail Making Part A | ||||||
| β ± SE | 0.03 ± 0.06 | -0.19 ± 0.06** | -0.35 ± 0.13** | -0.10 ± 0.12 | 0.02 ± 0.08 | |
| Interaction with APOE | n/a | NS | NS | 0.022 | NS | |
| Trail Making Test B-A | ||||||
| β ± SE | -0.08 ± 0.07 | -0.17 ± 0.06** | -0.10 ± 0.13 | 0.13 ± 0.13 | 0.01 ± 0.08 | |
| Interaction with APOE | n/a | NS | NS | NS | NS | |
| Hooper Visual Organization Test | ||||||
| β ± SE | -0.04 ± 0.07 | -0.15 ± 0.06* | -0.20 ± 0.13 | 0.02 ± 0.13 | 0.09 ± 0.08 | |
| Interaction with APOE | n/a | NS | NS | 0.048 | NS | |
| Boston Naming Test | ||||||
| β ± SE | 0.02 ± 0.07 | -0.06 ± 0.06 | -0.26 ± 0.12* | -0.25 ± 0.12* | 0.07 ± 0.07 | |
| Interaction with APOE | n/a | 0.029 | 0.032 | NS | NS | |
Abbreviations: HTN = hypertension; CVD = cardiovascular disease; DM = diabetes mellitus; SMK = smoking; n/a = not applicable; NS = not statistically significant (i.e., p > 0.10).
p-value for interaction with APOE genotype listed if p < 0.10
0.01 < p < 0.05
0.001 < p < 0.01
Main effect of APOE genotype on cognitive decline
Across all participants (independent of midlife vascular risk exposure), the presence of the APOE ε4 allele was significantly associated with a more marked decline in verbal memory (LM delayed recall: -0.17 ± 0.07, p < 0.05; Table 3). APOE genotype was not associated with decline on any other cognitive measure (p values > 0.05). Results were not changed when controlling for or excluding for depressive symptoms based on the CES-D.
Moderating effect of APOE genotype on association between midlife vascular risk factors and later cognitive decline
There were several significant interactions whereby APOE genotype modified the relationship between midlife vascular risk factors and later cognitive change (Table 3). Specifically, APOE genotype significantly modified the associations between both midlife hypertension and midlife cardiovascular disease and decline in language abilities. Further, APOE genotype modified the relationship between midlife diabetes and cognitive decline across a variety of cognitive domains including verbal memory, attention, and visuospatial abilities (Figure 1). Among APOE ε4 carriers, diabetes was associated with a similar rate of decline across the various cognitive domains (β values ranged from -0.52 to -0.74). Significant associations between increased midlife vascular risk burden and greater cognitive decline were observed among APOE ε4 carriers but not among non-carriers (Table 4). Consistent with the findings for main effects of vascular risk factors and APOE genotype, results were not changed when either adjusting for or excluding for depressive symptoms.
Figure 1. Annual change in cognitive performance by APOE genotype and diabetes status.
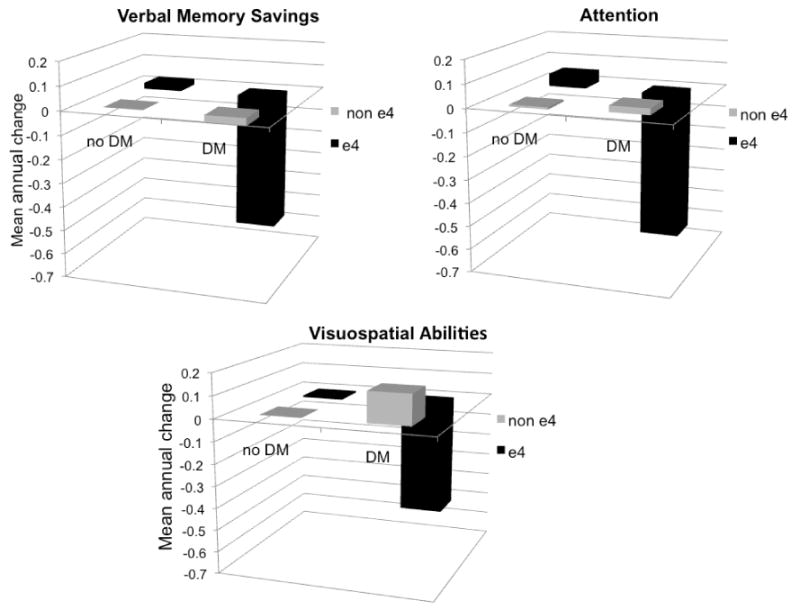
Mean annual change in cognitive performance presented in standard deviation units and adjusted for age at first neuropsychological assessment, sex, education, and time from Exam 5 (when diabetes status was assessed) to the first neuropsychological assessment. APOE genotype significantly modified the relationship between diabetes and change in performance for all three cognitive variables (p ≤ 0.083 for all interactions). Cognitive abilities were assessed with the following measures: Wechsler Memory Scale Logical Memory savings score (verbal memory savings), Halstead-Reitan Trail Making Test Part A (attention), and Hooper Visual Organization Test (visuospatial abilities). DM = diabetes mellitus.
Table 4.
Association between midlife vascular risk factors and later cognitive decline stratified by APOE ɛ4 status (for those interactions where p < 0.10)
| HTN | CVD | DM | ||
|---|---|---|---|---|
| WMS LM savings | ||||
| APOE ɛ4- | -0.01 ± 0.13 | |||
| APOE ɛ4+ | -0.61 ± 0.33 | |||
| Halstead-Reitan Trail Making Part A | ||||
| APOE ɛ4- | 0.05 ± 0.13 | |||
| APOE ɛ4+ | -0.74 ± 0.33* | |||
| Hooper Visual Organization Test | ||||
| APOE ɛ4- | 0.15 ± 0.14 | |||
| APOE ɛ4+ | -0.52 ± 0.31 | |||
| Boston Naming Test | ||||
| APOE ɛ4- | 0.01 ± 0.06 | -0.13 ± 0.13 | ||
| APOE ɛ4+ | -0.28 ± 0.17 | -0.66 ± 0.34* | ||
All values represent β ± SE.
0.01 < p < 0.05
Discussion
In a sample of 1,436 participants the presence of the APOE ε4 allele exacerbated cognitive decline associated with midlife exposure to vascular risk factors. APOE genotype significantly modified the associations between both midlife hypertension and cardiovascular disease and decline in language abilities as well as midlife diabetes and decline in verbal memory, attention, and visuospatial abilities.
The present findings add to the growing body of evidence demonstrating the impact of APOE genotype on the link between vascular risk burden and cognitive impairment. These findings identify a subgroup of individuals at increased risk for cognitive decline (i.e., APOE ε4 carriers with midlife exposure to vascular risk factors) and highlight the importance of considering the influence of genetic factors when examining the associations between cognition and vascular risk burden given that they may modify these relationships. These findings extend results from previous longitudinal studies, which examined very few cognitive domains,[1, 6, 13-15] by demonstrating associations between vascular risk factors and cognition across all domains assessed including memory, executive functioning, attention, visuospatial abilities, and language. Results were unchanged after controlling for or excluding for depression suggesting that depression is not influencing the present findings. Further, these findings suggest that these relationships are more complex than many prior studies imply and that they differ depending on the particular vascular risk factor and cognitive domain examined as well as genetic influences. The complexity of these relationships may explain, in part, the disparate results across previous studies.
ApoE plays a role in the development, remodeling, and regeneration of the central nervous system through the transport and redistribution of lipids among cells for normal cholesterol homeostasis,[21] repair of injured neurons,[22] maintenance of synaptodendritic connections,[22] and removal of toxins.[23] The various isoforms (i.e., apoE2, apoE3, apoE4) differ in their abilities to carry out these tasks. ApoE4, in particular, has been linked to various neuropathological processes including increased amyloid β (Aβ) deposition[24] and, in a pathway independent of Aβ, inefficient responses to central nervous system stressors (e.g., ischemia, inflammation).[25]
The literature examining the relationship between the various apoE isoforms and susceptibility to stroke is complex and somewhat mixed.[26] Nonetheless, multiple studies have reported that APOE ɛ4 carriers are at increased risk for atherosclerosis and stroke.[27-29] Given the role of apoE in lipid metabolism, atherosclerosis, stroke, Aβ clearance and deposition, and response to central nervous system stressors, there are multiple potential pathways by which apoE4 may lead to decline in brain functioning and cognition in the context of aging.
Even among relatively healthy older adults free of clinical dementia or stroke, both vascular neuropathology and AD-related neuropathology are relatively common and may coexist.[30, 31] When both pathologies are present it is generally unclear whether their relationship is one of mere co-existence, interaction, or causality. As such, it is often challenging to determine whether cognitive dysfunction is primarily related to the impact of vascular risk factors on the brain, underlying AD-related neuropathology,[32] or a combination. Although we show that presence of the APOE ε4 allele coupled with vascular risk factors may impart greater risk for cognitive decline in the context of aging, it is difficult to disentangle the underlying mechanism(s) of this effect given that the APOE ε4 allele has been associated with both vascular and AD-related neuropathological processes.
We observed main effects of midlife vascular risk exposure on later decline in executive functioning, however, we did not see differential decline in this particular domain as some previous studies have reported.[1] It should be noted that the present study and the study by Debette and colleagues, which demonstrated that midlife vascular risk exposure was associated with later decline in executive functioning but not memory, differed in terms of methodology and sample characteristics. For instance, in contrast to the study by Debette and colleagues, we examined additional cognitive domains (Debette and colleagues restricted their analyses to two cognitive domains: memory and executive functioning) and required a minimum time interval between the two neuropsychological evaluations (i.e., five years). Despite these differences, the two studies observed several similar effects (e.g., significant association between midlife hypertension and later decline in executive functioning). The results reported by Debette and colleagues corroborate previous studies linking midlife vascular risk factors and cognitive decline.[6, 15] The present results extend these findings and suggest that, even among those with relatively low vascular risk burden, genetic risk for AD significantly interacts with vascular risk factors to exacerbate cognitive decline.
APOE genotype modified associations between hypertension, cardiovascular disease, and diabetes, and cognitive decline, however, its influences appeared to be particularly salient for individuals with diabetes. Although the pathophysiology underlying these relationships remain unclear, neuropathological studies have demonstrated that older adults with diabetes who are also APOE ε4 carriers show increased risk of cerebral amyloid angiopathy, neuritic plaques, and neurofibrillary tangles whereas those with only diabetes show increased risk for only large infarcts.[33] Various mechanisms have been proposed to explain this modifying effect.[34] One such mechanism relates to increased Aβ plaque deposition among individuals with diabetes. Insulin-degrading enzyme (IDE) degrades Aβ but may be not be available to do so when insulin levels are increased thus requiring greater use of IDE for insulin regulation[35] and, therefore, resulting in higher levels of AD pathology.[33] A second proposed mechanism relates to neuronal damage related to oxidative stress and accumulation of advanced glycation endproducts associated with hyperglycaemia. Given that apoE4 is associated with impaired neuronal repair mechanisms, diabetes may result in greater neuronal damage in APOE ε4 carriers.[34] Taken together, it is possible that the presence of both diabetes and the APOE ε4 allele results in increased neurodegenerative and vascular pathology thereby affecting a wide range of cognitive abilities.
Vascular risk factors are highly correlated with one another making it difficult to disentangle the separate effects of each[1]. Further, despite potential differences among them, vascular risk factors share downstream effects related to endothelial dysfunction, atherosclerosis, and stroke.[36] However, although speculative, it is possible cardiovascular disease and hypertension, which interacted with APOE genotype to result in greater decline in language functioning but not any other cognitive ability, interact with genetic risk to exert their influence on cognitive decline through distinct mechanisms than diabetes.
Importantly, regardless of the precise mechanisms by which vascular risk factors and the APOE ε4 allele negatively affect cognition and the brain, many midlife vascular risk factors such as diabetes and hypertension are modifiable and should be treated.[15] A better understanding of the earliest cognitive changes in those individuals who are at increased risk for developing dementia is directly relevant to early and accurate identification.[16] Given the present dearth of effective therapies to treat vascular and neurodegenerative cognitive impairment, approaches designed to identify early and control vascular risk factors in order to maintain cerebrovascular health into later ages hold great promise.[30]
There are limitations that need to be considered when interpreting the present findings and that should be addressed in future studies. These include a lack of neuroimaging or neuropathological data, which precludes our ability to definitively link our findings to possible underlying physiologic mechanisms. In addition, the participants were predominantly Caucasian which may limit the generalizability of our results to the older adult population at large. Also, many participants who attended Exam 5 were excluded from the present study for reasons listed above (e.g., they did not complete the neuropsychological assessment; they were not eligible for the study based on other exclusion and/or inclusion criteria). However, those included in the present study sample were younger, had completed more education, and had less vascular risk burden than those who were not included. Given that the present sample was younger and healthier than the total sample that attended Exam 5, the present results are more likely to underestimate rather than overestimate any effects. Further, there are other variables that may influence the relations among vascular risk factors, APOE genotype, and cognitive decline that were not assessed in the present study and should be considered in future investigations (e.g., medical conditions such as chronic kidney disease; alcohol consumption; physical activity levels).
Despite these limitations, the present study has several notable strengths including a longitudinal design, a large community-based sample, a comprehensive neuropsychological assessment, inclusion of several vascular risk factors, and the exclusion of those participants with a diagnosis of clinical dementia or stroke at baseline. Given the paucity of existing research, there is an incomplete understanding of the associations among vascular risk burden, genetic risk factors, and cognitive functioning. Future prospective longitudinal studies incorporating clinical, genetic, neuroimaging, and neuropathological data are needed given that vascular and degenerative pathologies are common in older age and often coexist, which may result in similar clinical phenotypes.[30] Such studies will further elucidate the contributions of different pathologies and help to characterize phenotypes of the preclinical and prodromal periods of different forms of dementia.
Acknowledgments
This research was made possible by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (NIH/NHLBI Contract #N01-HC-25195) and grants from the National Institutes of Health (K24 AG26431, T32 MH 19934-17, P30 AG013846, R01 AG12674, AG08122, AG16495, AG033193, and AG031287).
Grant Support: This research was made possible by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (NIH/NHLBI Contract #N01-HC-25195) and grants from the National Institutes of Health (K24 AG26431, T32 MH 19934-17, P30 AG013846, R01 AG12674, AG08122, AG16495, AG033193, and AG031287).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–8. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Mosley TH, Jr., Gottesman RF, et al. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80:1194–201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivipelto M, Ngandu T, Laatikainen T, et al. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 4.Nation DA, Wierenga CE, Delano-Wood L, et al. Elevated pulse pressure is associated with age-related decline in language ability. Journal of the International Neuropsychological Society : JINS. 2010;16:933–8. doi: 10.1017/S1355617710000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reijmer YD, van den Berg E, van Sonsbeek S, et al. Dementia risk score predicts cognitive impairment after a period of 15 years in a nondemented population. Dement Geriatr Cogn Disord. 2011;31:152–7. doi: 10.1159/000324437. [DOI] [PubMed] [Google Scholar]
- 6.Carmelli D, Swan GE, Reed T, et al. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology. 1998;50:1580–5. doi: 10.1212/wnl.50.6.1580. [DOI] [PubMed] [Google Scholar]
- 7.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science (New York, NY) 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 8.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Chuang YF, Hayden KM, Norton MC, et al. Association between APOE epsilon4 allele and vascular dementia: The Cache County study. Dement Geriatr Cogn Disord. 2010;29:248–53. doi: 10.1159/000285166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small BJ, Rosnick CB, Fratiglioni L, et al. Apolipoprotein E and cognitive performance: a meta-analysis. Psychology and aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- 11.Jorm AF, Mather KA, Butterworth P, et al. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Bondi MW, Salmon DP, Galasko D, et al. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology and aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- 13.Haan MN, Shemanski L, Jagust WJ, et al. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA : the journal of the American Medical Association. 1999;282:40–6. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 14.Kalmijn S, Feskens EJ, Launer LJ, et al. Cerebrovascular disease, the apolipoprotein e4 allele, and cognitive decline in a community-based study of elderly men. Stroke; a journal of cerebral circulation. 1996;27:2230–5. doi: 10.1161/01.str.27.12.2230. [DOI] [PubMed] [Google Scholar]
- 15.Knopman DS, Mosley TH, Catellier DJ, et al. Atherosclerosis Risk in Communities Study Brain MRIS. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2009;5:207–14. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 19.Wolf PA, D’Agostino RB, Belanger AJ, et al. Probability of stroke: a risk profile from the Framingham Study. Stroke; a journal of cerebral circulation. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 20.Heaton RK, Nelson LM, Thompson DS, et al. Neuropsychological findings in relapsing-remitting and chronic-progressive multiple sclerosis. J Consult Clin Psychol. 1985;53:103–10. doi: 10.1037//0022-006x.53.1.103. [DOI] [PubMed] [Google Scholar]
- 21.Pitas RE, Boyles JK, Lee SH, et al. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–60. [PubMed] [Google Scholar]
- 22.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science (New York, NY) 1988;240:622–30. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 23.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5644–51. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisniewski T, Castano EM, Golabek A, et al. Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994;145:1030–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Mahley RW, Huang Y. Apolipoprotein (apo) E4 and Alzheimer's disease: unique conformational and biophysical properties of apoE4 can modulate neuropathology. Acta Neurol Scand Suppl. 2006;185:8–14. doi: 10.1111/j.1600-0404.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 26.Jak AJ, Nation DA, Delano-Wood L, et al. Relationship between vascular risk factors and mild cognitive impairment. Cognitive Sciences. 2011;5:99–124. [Google Scholar]
- 27.Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol. 2004;3:227–35. doi: 10.1016/S1474-4422(04)00708-2. [DOI] [PubMed] [Google Scholar]
- 28.Kolovou G, Daskalova D, Mikhailidis DP. Apolipoprotein E polymorphism and atherosclerosis. Angiology. 2003;54:59–71. doi: 10.1177/000331970305400108. [DOI] [PubMed] [Google Scholar]
- 29.McCarron MO, Delong D, Alberts MJ. APOE genotype as a risk factor for ischemic cerebrovascular disease: a meta-analysis. Neurology. 1999;53:1308–11. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]
- 30.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider JA, Aggarwal NT, Barnes L, et al. The neuropathology of older persons with and without dementia from community versus clinic cohorts. Journal of Alzheimer's disease : JAD. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Annals of neurology. 2006;60:677–87. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging S. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 34.Dore GA, Elias MF, Robbins MA, et al. Presence of the APOE epsilon4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine-Syracuse Study. Diabetologia. 2009;52:2551–60. doi: 10.1007/s00125-009-1497-2. [DOI] [PubMed] [Google Scholar]
- 35.Messier C. Diabetes, Alzheimer's disease and apolipoprotein genotype. Exp Gerontol. 2003;38:941–6. doi: 10.1016/s0531-5565(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 36.Olsson Y, Brun A, Englund E. Fundamental pathological lesions in vascular dementia. Acta Neurol Scand Suppl. 1996;168:31–8. doi: 10.1111/j.1600-0404.1996.tb00370.x. [DOI] [PubMed] [Google Scholar]


