Abstract
This paper summarizes the discussions regarding animal paradigms for assessing perception at the seventh meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS). A breakout group at the meeting addressed candidate tests in animals that might best parallel the human paradigms selected previously in the CNTRICS program to assess two constructs in the domain of perception: gain control and visual integration. The perception breakout group evaluated the degree to which each of the nominated tasks met pre-specified criteria: comparability of tasks across multiple species; construct validity; neuroanatomical homology between species; and dynamic range across parametric variation.
Keywords: Prepulse inhibition of startle, Event related potentials, Mismatch negative, Visual integration
1. Overview of CNTRICS requirements for animal models and measures of perceptual processing in schizophrenia
Perception
The purpose of this overview is to summarize the discussion of the breakout group on perception from the recent meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS). Discussion addressed the relative strengths and weaknesses of current animal models of perception in the context of the two prior reports of CNTRICS consensus findings on this topic (Butler et al., 2012; Green et al., 2009). Within this meeting the aspects of perception reviewed were “gain control” and sensory integration of visual stimuli. Each nominated test was evaluated with respect to a standardized set of translational criteria including:
The extent to which comparable data exist across multiple species;
Construct validity;
Neuroanatomical homology between species; and
Dynamic range across parametric variation.
Gain control, as defined by the previous CNTRICS groups, refers to processes that enable sensory systems to adapt and optimize their response levels to take into account their immediate context, in order to make best use of a limited dynamic signaling range (Butler et al., 2012). The committee made a distinction between the regulation of responses within the immediate context, rather than evaluating mechanisms of long-term adaptation in such functions. After substantial discussion, the majority opinion was that only tasks requiring immediate regulation of a response owing to the immediate context were suitable as translational measures of gain control. This feature was seen as a key requirement for the definition of gain control. Three tasks were nominated and evaluated, including: (1) prepulse inhibition of startle (PPI); (2) event related potential (ERP) amplitude; and (3) mismatch negativity (MMN). Of these three paradigms, PPI and MMN were selected for inclusion into the battery. PPI was considered to be a “mature” task, at least in animals, although one that could benefit by further development in humans, especially in combination with imaging approaches (Butler et al., 2012). MMN was highlighted as an area still in need of some additional research and development. Despite differing opinions of the participants regarding the applicability of ERPs to this construct, ERPs were eventually rejected from inclusion in the battery owing to some members’ opinion that ERPs lack of specificity for the construct of gain control. More specifically, amplitude of the N1 (N100 in a human or N40 in a mouse) likely would be an excellent candidate due to the nearly perfect correspondence for parametric and pharmacological response properties between species, including gain control (Featherstone et al., 2012; Jutzeler et al., 2011; Maxwell et al., 2004a,b; Metzger et al., 2007; Rudnick et al., 2010; Siegel et al., 2005). Alternatively, P1 amplitude and gating deficits (as measured with P50 in humans and P20 in mice) have proven less reliable across both human and animal studies, reducing enthusiasm for this particular measure as a true endophenotype of schizophrenia or as a valid animal model for the disease (de Wilde et al., 2007a,b).
Integration is defined by CNTRICS as the processes linking the output of neurons – that individually code local (typically small) attributes of a scene – into a global (typically larger) complex structure more suitable for guidance of behavior (Butler et al., 2012). Two tasks were nominated and evaluated with respect to this construct, including: (1) coherent motion; and (2) contour integration. Discussion focused on unimodal, lower level sensory integration as a perceptual measure rather than either context dependent or integration of cross-modal stimuli. The group in general knew of no existing literature to support the use of these paradigms in the rodent. This lack of knowledge accurately reflected the state of the art as a comprehensive literature review since then has confirmed that very little has been published on the use of these paradigms within the rodent. Accordingly, no task can be included within a battery at this time. Nevertheless, the little work that is published suggests that these tests can be ported to the rodent, and within the session the group thought that touch-screen equipped operant boxes may serve as means to measure this construct within the rodent. Nevertheless, a major concern expressed by the group was the degree to which the visual cortex of the rodent would be analogous to the primate. As such, if these tasks of visual perception can be used in the rodent it will also be necessary to confirm which regions of the rodent visual cortex are activated to confirm the translational validity of the approach.
2. Gain control
2.1. Prepulse inhibition
Paradigm description
The inability to effectively attenuate the startle response when it is preceded by a weak prepulse stimulus has been well documented in schizophrenia patients, with observations dating as far back as 1978 (Braff et al., 1978). In the PPI paradigm, subjects are exposed to a weak prestimulus followed 30–300 ms later by a stronger startle stimulus that may be in the same or different modality (Hoffman and Ison, 1980). The response to the startle stimulus is measured as a whole-body movement in animals or electromyographic measure of the eyeblink in humans and compared to the startle response when no prestimulus is used. This difference provides a quantitative measure of inhibition that is thought to reflect a subject’s sensorimotor gating ability (Braff and Geyer, 1990). PPI of the startle reflex is a useful schizophrenia endophenotype for several reasons, as summarized previously (Green et al., 2009). The PPI deficit that is observed in schizophrenia patients correlates clinically to symptoms such as thought disorder and distractibility (Turetsky et al., 2007). In their 2007 review, Turetsky et al. set forth a battery of criteria by which to define an endophenotype. PPI satisfies these requirements by being reproducible, highly heritable, and easy to measure. Reliability has been demonstrated over intervals of several months, and PPI deficits in the unaffected siblings of schizophrenia probands are demonstrative of a genetic basis for the effect. For this reason, PPI emerged as a promising endophenotype for schizophrenia and is now widely accepted as one potential means of assessing the genetic basis of the disease (Powell et al., 2009).
Cross-species comparability
As with other measures using the startle response as a read-out, there is considerable evidence for the homology of PPI across species, at least within mammals.
One of the primary advantages of PPI is its ability to translate between mice, rats, and humans, since it is one of the few tests that is largely conserved across all vertebrate species (Geyer et al., 2002). PPI testing requires no training or special preparation of the subject, and most setups are entirely automated. The ability to test multiple animals at once makes the PPI paradigm relatively high throughput and therefore advantageous for basic science related to understanding disease pathophysiology and treatment development.
An extensive literature details the effects of drugs and drug–drug interactions on PPI in mice, rats, and humans, as summarized in previous CNTRICS reports (Butler et al., 2012; Green et al., 2009) and prior reviews (Braff et al., 2001; Geyer et al., 2001). Despite the strong evidence for genetic contributions to the PPI deficits seen in schizophrenia patients, it is also clear that the PPI attenuation seen in schizophrenia patients is partially “state-related.” Certainly, acute pharmacological agents modify PPI in humans as well as in animals (Braff et al., 2001). Further it appears that atypical antipsychotic treatments are associated with reduced PPI deficits in schizophrenia patients, although many studies report deficient PPI in patients treated with typical antipsychotics.
In keeping with evidence for developmental abnormalities in schizophrenia, a variety of pre- and post-natal manipulations in animals that have been considered potential models of the developmental etiologies that contribute to the schizophrenias have been found to produce deficits in PPI. These deficits typically emerge about the time of puberty in both rats and mice and can be reversed by treatment with antipsychotics (Powell et al., 2009). Furthermore, a number of genes have been linked to modulation of PPI using mutant and inbred mouse lines. Rodents provide ideal models in which to study genetic variations because PPI levels are known to differ from one strain to another in both rats and mice, but remain stable throughout multiple generations of the same strain (Turetsky et al., 2007). Powell et al. (2009) provide a thorough review of the myriad of mutations that have been linked to PPI deficits, including defects in the genes for proteins such as reelin, neural cell adhesion molecule, and proline dehydrogenase. Mutations in the Fabp7 gene that lead to alterations in the level of fatty acid binding protein found in the brains of schizophrenia patients also lead to a deficit in PPI (Watanabe et al., 2007). Additionally, the altered PPI levels seen in Huntington’s disease, fragile X syndrome, and DiGeorge syndrome suggest that the genes responsible for these phenotypes may also help to regulate PPI (Geyer et al., 2002).
Neuroanatomical homology
As discussed in the previous CNTRICS report on perception measures (Butler et al., 2012), another advantage of PPI is that it is amenable for study in functional MRI environments, using either blocked or single-trial designs. The recently demonstrated ability to conduct simultaneous fMRI and electrophysiological measures of startle behavior represents an approach that was recommended for further development by CNTRICS (Butler et al., 2012). Although still limited in extent, the human imaging studies of PPI already indicate that similar neuroanatomical substrates regulate startle in rodents (Swerdlow et al., 2001) and humans (Kumari et al., 2003). This is an area of research that warrants considerable further development to assess the parallels of the human anatomical contributions to the extensive understanding of the complex circuitry that is now known to mediate and modulate PPI in the rat.
Construct validity
The evidence for the validity of PPI as a measure of gain control is thoroughly discussed in the two preceding reports on this topic from the CNTRICS initiative (Butler et al., 2012; Green et al., 2009). In brief, PPI is an excellent example of how immediately preceding stimuli alter the apparent gain in the ability of a sensory stimulus to elicit a graded motor response. Accordingly, PPI is typically considered to provide an operational model of sensorimotor gating.
Dynamic range
One of the major reasons that PPI has been such an effective paradigm for explorations of pharmacological, genetic, and neurobiological phenomena is that it is exquisitely sensitive and predictable in response to parametric manipulations. Very well understood functions of PPI performance as related to manipulations of stimulus modality, timing, intensity, and quality are evident in the literature. Furthermore, these parametric features are very similar rodents and in humans (Swerdlow et al., 1994). Thus, investigators are able to adjust parameters to ensure that floor and ceiling effects are avoided and to demonstrate that the procedures utilized are consistent with previous reports. There is excellent stability and high test–retest reliability of PPI across time in both rodents and humans, facilitating the use of within-subjects and longitudinal designs.
Potential weaknesses
While PPI serves as a useful endophenotype for schizophrenia, it is important to point out that certain confounds and complexities may exist. First, as with most psychiatric endophenotypes, it is clear that deficits in PPI are not limited to patients with schizophrenia, being reported as well in bipolar mania, Huntington’s disorder, panic disorder, and others (Braff et al., 2001). Alterations in PPI may result from phenotypes that are not related to schizophrenia, such as hearing deficits. Some strains of mice, namely DBA/2 and C57BL/6, demonstrate high frequency hearing loss with age, rendering them potentially unable to hear the prestimulus very late in life (Geyer et al., 2002). Fortunately, cross-modal PPI paradigms can be used to test the ability of a visual stimulus to inhibit the startle response elicited by either an acoustic or tactile stimulus, enabling empirical tests of such potential confounds in either rats or mice (Brody and Geyer, 2004; Weber and Swerdlow, 2008). Additionally, PPI levels vary based on sex, both in humans and in rodents. Even within biological sex, it can be difficult to compare female subjects because PPI fluctuates throughout the menstrual cycle (Aasen et al., 2005).
2.2. Auditory event related potentials
Overview of electroencephalography (EEG)
EEG was the first physiological technique used to examine the brain by recording electric field potentials with the capability to reflect both the normal and abnormal electrical activity of the brain. EEG evolved into an indispensable method for studying cerebral information processing, particularly due to the introduction of source localization techniques and the decomposition of event-related activity into its frequency components (Winterer, 2011). Conventionally, EEG is recorded from the scalp using numerous electrodes affixed to specific scalp locations and is represented as changes in potential difference. The scalp EEG reflects the summated potentials from a large synchronously activated population of pyramidal cells in the cerebral cortex. These potentials are thought to originate primarily from excitatory and inhibitory neural electric activity, including action potential (AP) and postsynaptic potentials (Dietrich and Kanso, 2010).
Electroencephalography provides a method to investigate general function of the brain including its reaction to particular stimuli that will be represented as changes in the EEG, globally known as event-related potentials (ERP) or evoked potentials (EP). These event-related potentials are defined as the oscillatory brain responses that are triggered by the occurrence of particular stimuli (auditory, visual, or somatosensory). Significant voltage fluctuations are detectable resulting from evoked neural activity and allow one to measure distinct stages in neural information processing. Moreover, ERPs reflect sub-cortical and cortical information processing in real time and thus they provide a useful tool for examining cognitive mechanisms in both normal brain function and disorder-related impairments. Differential stages of information processing are mainly represented by the following ERP components: P50 (positive voltage deflection 50 ms post event); N100 (negative voltage deflection 100 ms post event); P200, P300, and the mismatch negativity. EEG recordings can be made from a variety of species including rodents, where the characteristic positive and negative deflections occur at approximately 40% the latency of equivalent human components (Connolly et al., 2004; Maxwell et al., 2004b; Siegel et al., 2003). Therefore, the P20, N40, P80 and P120 represent ERP deflections in mice analogous to the P50, N100, P200 and P300 in humans (Fig. 1) (Siegel et al., 2003).
Fig. 1.
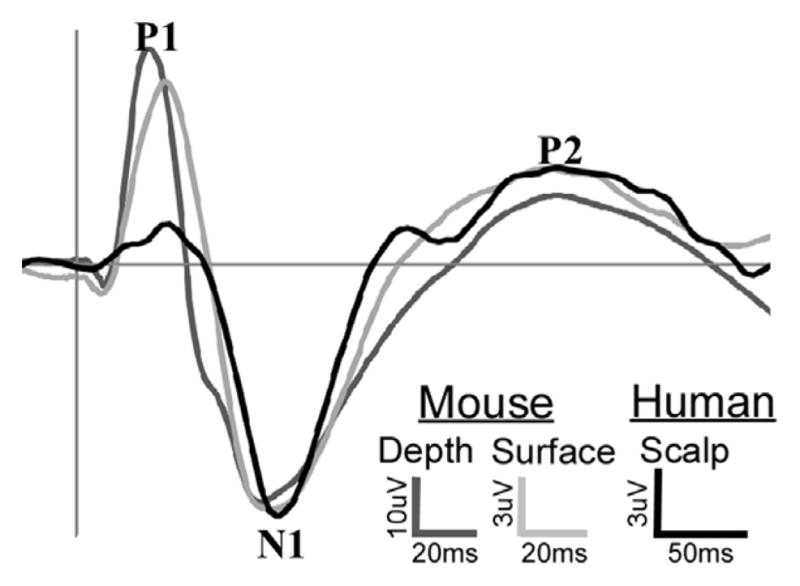
Representative grand average auditory event related potential recordings from human scalp, mouse surface and mouse depth electrodes. Note that the overall pattern of event related activity is consistent across species and locations, with a prominent P1, N1 and P2 components. These responses are termed “obligatory” as they occur in response to a simple tone or click in rodents, non-human primates or clinical populations. These components occur at 40% of the human latency in mouse. Thus, the P1 occurs at 20 ms in mouse and 50 ms in human, while the N1 and P2 occur at 40 and 80 ms in mouse and 100 and 200 ms in human respectively. Studies over the past decade demonstrate that each component in mouse shares psychometric and pharmacological response properties with the corresponding human ERP component, yielding excellent predictive validity (Amann et al., 2008; Connolly et al., 2004; Ehrlichman et al., 2009; Halene and Siegel, 2008; Maxwell et al., 2004a,b, 2006; Metzger et al., 2007; Phillips et al., 2007; Rudnick et al., 2008; Siegel et al., 2003, 2005).
2.3. Mismatch negativity
Paradigm description
Mismatch negativity (MMN) reflects the context-dependent information processing that is required to compare a deviant incoming stimulus with the neural representation already stored in the transient auditory memory (Bomba and Pang, 2004). When a string of tones with a specific regularity (sequence of homogenous tones) is presented, it is thought that the brain stores the features of this auditory stimulation in a short-duration neural memory trace (Ulanovsky et al., 2004). While this echoic memory is still active, each new auditory input is compared to the existing trace for a break of regularity (deviant tone), which generates a neuronal adaptation giving rise to the MMN (Naatanen, 2000). MMN is most frequently elicited in an auditory oddball paradigm. In this paradigm, a sequence of repetitive standard stimuli is randomly interrupted by a deviant oddball stimulus which may differ in stimulus characteristics such as pitch, intensity, or duration. Peaking between 100 and 225 ms, MMN is a difference wave between responses to frequent and deviant stimuli. Since the intensity of this peak is a function of deviation from the previous stimuli, the extent of deviation of the response, and difference in the stimuli, can be used as a measure of gain control. MMN is dependent on the preceding string of stimuli, and in this way it is highly context dependent. Rather than being a simple response to novelty, MMN is thought to be a response to deviation from the remembered pattern. Of particular importance, MMN is evoked irrespective of attention (e.g. present in comatose patients) (Fischer et al., 2000). In clinical neurosciences, MMN has been widely used in various applications, in particular in schizophrenia research, due to its good reproducibility and the ability to assess it without a task (Garrido et al., 2009). Schizophrenia patients display reduced mismatch negativity (MMN) (Davalos et al., 2003; Javitt et al., 1993, 2000; Light and Braff, 2005; Michie, 2001; Shelley et al., 1999; Umbricht et al., 2000).
Cross-species comparability
A novel auditory stimulus can differ from standard stimuli in terms of pitch or duration, and both methods for eliciting the MMN have been accomplished in rodents, highlighting that this paradigm can be translated into other species (Tikhonravov et al., 2008; Umbricht et al., 2005). One preclinical study demonstrated a duration-elicited MMN following the N40 potential in mice (Umbricht et al., 2005). While this study did not examine whether pharmacological manipulations could impair generation of the MMN, subsequent studies utilizing pitch-deviants have succeeded in modeling deficits associated with schizophrenia. Specifically, ketamine and MK-801 have been shown to reduce MMN amplitude in mice and rats, respectively (Ehrlichman et al., 2008; Tikhonravov et al., 2008). Similarly, disruptions in neuregulin 1, a protein implicated in both glutamate signaling and the pathophysiology of schizophrenia (Hahn et al., 2006), also impairs MMN in mice (Ehrlichman et al., 2009).
Neuroanatomical homology
Unfortunately, it is difficult to conclusively determine the regions involved in generating these signals since numerous patterns of activation can give rise to similar EEG profiles in both humans and rodents. Hence, at present it is not possible to say if comparable neuroanatomical regions are involved across species.
Dynamic range
Despite the lack of comparative neuroanatomical work, the construct validity, translatability, and dynamic range of MMN make it an ideal candidate for inclusion into a preclinical battery for research into perceptual abnormalities in schizophrenia.
Potential weaknesses
While inclusion is recommended, the task is not as developed as PPI, for example, and would benefit from additional research. For instance, while it is thought that this paradigm is dependent upon an echoic memory, this belief has not been confirmed empirically, and the neuroanatomical underpinnings remain only partially described.
3. Sensory integration of visual stimuli
Integration
Within the field of perceptual visual integration, two tasks were nominated for further development as preclinical tests for schizophrenia: coherent motion detection and contour integration. Both tasks have been relatively well developed in NHPs, however little more the proof of concept work has been performed within the rodent. Accordingly here we briefly review what is known about the tasks in primates, and then propose ways that these processes could be studied in the rodent. At this time sufficient data does not exist for the rodent versions of these tasks to evaluate them against the basic CNTRICS requirements.
3.1. Coherent motion
Paradigm description
In coherent motion detection, a series of dots, moving in random directions (noise), are displayed upon a computer screen, as detailed and illustrated in the prior CNTRICS report (Green et al., 2009). Embedded within the randomness, a portion of the dots moves in a uniform direction (signal) and the participant is required to determine the direction of motion. Schizophrenia patients demonstrate a lower level of accuracy than normal participants at comparable levels of difficulty (signal to noise), and also require a higher proportion of uniform motion (signal) to detect coherent motion in the first instance (Green et al., 2009). Conceptually, coherent motion detection and contour integration are very similar. In contour integration, a perceived contour (series of dots grouped in a manner to be perceived as a continuous shape/contour) is displayed upon a screen (signal) and then presented amongst a series of distracters (identical dots as those used in the signal, but displayed at random) representing varying degrees of noise (Green et al., 2009). As in visual integration, schizophrenia patients need a larger signal to detect the contour, and will perform worse than controls under equal conditions of difficulty (Green et al., 2009).
Cross-species comparability
These tasks do have many similarities, however numerous imaging studies in humans and cellular recordings or selective ablation studies in non-human primates (NHP) suggest that the two tasks are dependent upon distinct, but overlapping neuronal structures (Butler et al., 2012). The visual cortex is an extremely complex series of related structures with numerous inter-connections. Generally it is thought that contour integration occurs very early within the processing of the visual experience, and within the visual cortex (beginning in V1; Bauer and Heinze, 2002b; Kovacs, 1996). Moreover, the detection of these contours is thought to happen locally, primarily within the V1/V2 itself. However the V2 also displays longer connections with much of the rest of the visual cortex, and schizophrenia patients show differences in activity within area V2 during an fMRI study of visual integration (Silverstein et al., 2009). In contrast, coherent detection of motion is thought to occur within the V5, further downstream in the visual cortex and a later step in the processing of the visual experience (Maunsell and Newsome, 1987; Newsome and Pare, 1988; Rees et al., 2000). Furthermore, it is thought that coherent motion cannot occur with local filters alone, rather V5 must interact with other structures to properly perceive the global movement. While having many similarities, the requirements for these two visual experiences, and the underlying neurobiologies, are distinct. Thus, it is unclear if the changes observed in schizophrenia are the result of common or independent processes (for a comprehensive and recent review on the basic functions of the visual system see Graham, 2011) (Graham, 2011).
Neuroanatomical homology
Coherent motion detection is not a behavioral paradigm designed for the study of disease, or even cognition per se. Rather it has its foundation in the study of visual processing in normal humans (Morgan and Ward, 1980a,b). Classically, NHPs are trained to distinguish coherent motion, and will then perform the task while single cell recordings are made. The local cellular responses to specific kinds of stimuli could then be compared. In some instances, these regions were then lesioned to strengthen the argument that they were responsible for “processing” a specific type of stimuli. In this instance the medial temporal areas (MT; NHP), sometimes referred to as V5 (human), have been shown to have cells that specifically respond to coherent motion (Newsome et al., 1989, 1990) in a direction-selective manner.
Dynamic range
Lesions to V5 cause a substantial decrease in sensitivity for these types of stimuli, a greater amount of coherent motion was necessary for the monkey to recognize this (Newsome and Pare, 1988). However this effect was only transient. This finding led the authors to suggest that other components of the visual system could compensate, at least behaviorally, for these effects (it is important to note that this difference was seen behaviorally, no evidence has been presented to suggest that motion sensitive neurons “moved”). Yet recent findings suggest that while cells within the MT may be selective for motion, interactions between numerous brain regions may be necessary to perceive global motion effectively (Hedges et al., 2011a,b). Perhaps more importantly from a drug discovery perspective, there is reason to believe that sub-regions of the visual system have variable sensitivities to pharmacological manipulations. While more work in this area is required, a 2004 study in healthy volunteers (Carter et al., 2004) with the 5-HT2a/1a agonist psilocybin demonstrated that coherent motion could be disrupted while a lower level task (contrast sensitivity for drifting gratings) was not. These two tasks are thought to be dependent upon different portions of the visual cortex.
Potential limitations
As a preclinical model, coherent motion detection is not a mature task. Evidence suggests that NHP versions of the task could be adapted as a preclinical model that could be very accurately translated to the human. While little emphasis has been placed upon the development of translational challenge models, a wealth of evidence suggests the NHP coherent motion detection has excellent dynamic range, is likely dependent upon similar brain regions as human versions of the task, and has high construct validity. However, this approach would require the use of NHP preclinical model of schizophrenia. Unfortunately, ethical NHP models of schizophrenia, other than acute ketamine challenges remain largely unidentified, and it would be very difficult to pursue coherent motion detection in NHPs alone. Considering the immature state of the NHP work, it seems entirely reasonable to attempt to develop these tasks in the rodent. Notably, two separate groups have demonstrated that rodents can perform tasks of coherent motion detection (Douglas et al., 2006; Hupfeld and Hoffmann, 2006). Both groups use a swim maze, split with a divider, and a monitor at one end. On each side of the monitor a different pattern of moving dots is displayed. A hidden platform is located below one pattern while nothing is below the other pattern. Accordingly, animals (rats and mice) come to associate a specific image pattern (random or with coherent motion) with escape from the water. While such a swim maze task will not be high throughput, it enables evaluation of numerous disease models, serves as an important proof of concept that rodents process information in a similar manner as primates, and is certainly “higher” throughput than primate models. Moreover, it seems likely that such an assay could be adapted to a touch-screen approach (Bussey et al., 2012), potentially allowing dozens of animals to be tested within a single day and access to numerous disease models. An alternative approach would be to focus on changes in cellular activity that result from passive observation of stimuli. In a recent report, Kemp and Manahan-Vaughan (2012) measured longer-term depression in rodents that had been trained to drink while passively observing a computer-generated spatial environment. Conceivably, a similar approach could be used here, but with different classes of stimuli. Being that these are pre-attentive perceptual experiences, animals may not need to attend actively to the stimuli for a response to be evoked. While a path forward can be envisaged, a direct translational approach may also be hampered until the rodent visual cortex is described more accurately. Partial evidence suggests that it may function in a very similar manner as in humans (Douglas et al., 2006), however the sub-regions within the rodent cortex remain largely un-described and the presence of human analogs remains undetermined. It can be easily envisaged how detection of coherent motion can be made to work in a setting suitable for schizophrenia research as well as drug discovery. However at this point in time considerable methodological development is required to determine if rodent versions of the task engage similar brain regions as in the human and if similar manipulations will translate across species. While the task is promising it fails to meet basic CNTRICS requirements owing to a lack of available data at this time. Nonetheless the group agreed that this is a promising avenue for future additional research.
3.2. Contour integration
Similar to coherent detection of motion, contour integration has its origins in the study of vision, and how visual stimuli are combined to create a meaningful representation of the world we navigate within. Specifically, contour integration can be viewed as the ability to make a gestalt representation of the world, by combining small, related objects into a meaningful whole. Contour integration is heavily dependent upon the visual cortex, specifically it has been proposed that the ability to detect a contour amongst noise is a low-level process, likely occurring within the V1/V2. This conclusion suggests that contour integration and coherent motion are partially dissociable at the structural level (Altmann et al., 2003; Bauer and Heinze, 2002a; Kourtzi et al., 2003), although this dissociation has yet to be tested explicitly, and establishing such a dissociation would be necessary to justify the inclusion of coherent motion and contour integration into any proposed battery. A recent fMRI study indicated that abnormal functioning was seen in V2–V4 during a task of visual integration in schizophrenic individuals when compared to a control group (Silverstein et al., 2009). Accordingly, this result provides very strong evidence for perceptual impairments associated with schizophrenia at the very earliest levels of the visual experience. Moreover recreational ketamine users who had recently used ketamine were shown to have impaired contour integration, although this deficit was not observed several days later (Uhlhaas et al., 2007). While additional research is required, ketamine could be an effective means to model deficits associated with schizophrenia in contour integration in a normal population.
While NHPs could be used to develop a preclinical model for perceptual contour integration, the same obstacles would be present as for coherent perception of motion, substantially limiting the utility of such a model. Accordingly, in order to move forward with contour integration as a preclinical tool, there is a real need to develop models for the rodent. Unfortunately, to date the authors know of no attempt to measure visual integration within the rat or mouse, as such it is possible that this process may not occur in these species. However a single study has demonstrated that contour integration does occur within the primary visual cortex of the opossum (Oliveira et al., 2002). It has been proposed that “the opossum’s cerebral cortex is a valid model to study the original plan and general principles of cortical organizations in mammals” (Oliveira et al., 2002). Therefore it is very likely that contour integration will be observed within the rodent. Unfortunately contour integration has another similarly with coherent motion detection, which is that at this time it fails to meet the basic requirement for acceptance into a CNTRICS battery owing to a lack of available data on the task within the rodent. However as will coherent detection of motion a viable path can be envisioned for necessary task development.
As with coherent detection of motion, touch-screen-equipped operant boxes would likely be an ideal technology to develop contour integration in the rodent. It has already been demonstrated that to some degree rodents can generalize across shapes when performing a visual discrimination, a process in many ways similar to contour integration. Accordingly, if rats are trained to an exemplar image composed of Gabor elements, and with little interference, it should be possible to train them to “search” for this image amongst interference. While the touch-screen system would likely result in higher throughput, the swim maze variety as used by Hupfeld and Hoffmann (2006) or Douglas et al. (2006) would likely also be effective (Douglas et al., 2006; Hupfeld and Hoffmann, 2006). Failing this, it may also be possible to measure synaptic connectivity electrophysiologically within region V1 and change here as a potential predictor of change in contour integration within a clinical setting.
4. Final recommendations
Models of gain control and integration were nominated as crucial aspects of perception to be included within a preclinical battery for the study of cognition in schizophrenia. Within gain control, PPI, MMN, and ERPs were nominated as potential tests for inclusion into a final battery. The group recommended PPI as being a mature task ready for inclusion, with further development of its application in imaging environments being suggested. Similarly, MMN was considered to be relatively mature, although it was felt that MMN would benefit from additional research especially in rats and additional human populations. Within the area of integration both coherent motion and contour integration were proposed. While “proof-of-concept” studies have been performed suggesting that these processes can be measured in the rodent, viable pre-clinical models do not yet exist for coherent motion and contour integration. As such this was seen as an area that could immediately benefit from additional research in both assay development and in confirmation of cross-species structural homology.
Acknowledgments
The CNTRICS conference was supported by NIMH grant R13 MH078710.
References
- Aasen I, Kolli L, Kumari V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: implications for pharmacological and treatment studies. Journal of Psychopharmacology. 2005;19:39–45. doi: 10.1177/0269881105048890. [DOI] [PubMed] [Google Scholar]
- Altmann CF, Bulthoff HH, Kourtzi Z. Perceptual organization of local elements into global shapes in the human visual cortex. Current Biology: CB. 2003;13:342–349. doi: 10.1016/s0960-9822(03)00052-6. [DOI] [PubMed] [Google Scholar]
- Amann LC, Phillips JM, Halene TB, Siegel SJ. Male and female mice differ for baseline and nicotine-induced event related potentials. Behavioral Neuroscience. 2008;122:982–990. doi: 10.1037/a0012995. [DOI] [PubMed] [Google Scholar]
- Bauer R, Heinze S. Contour integration in striate cortex. Classic cell responses or cooperative selection? Experimental brain Research. Experimentelle Hirnforschung Experimentation cerebrale. 2002a;147:145–152. doi: 10.1007/s00221-002-1178-6. [DOI] [PubMed] [Google Scholar]
- Bauer R, Heinze S. Inter-cellular spike coincidences in visual detection tasks. Die Naturwissenschaften. 2002b;89:316–318. doi: 10.1007/s00114-002-0333-z. [DOI] [PubMed] [Google Scholar]
- Bomba MD, Pang EW. Cortical auditory evoked potentials in autism: a review. International Journal of Psychophysiology. 2004;53:161–169. doi: 10.1016/j.ijpsycho.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Archives of General Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brody SA, Geyer MA. Interactions of the mGluR5 gene with breeding and maternal factors on startle and prepulse inhibition in mice. Neurotoxicity Research. 2004;6:79–90. doi: 10.1007/BF03033300. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophrenia Bulletin. 2012;38:81–91. doi: 10.1093/schbul/sbr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Pettigrew JD, Burr DC, Alais D, Hasler F, Vollenweider FX. Psilocybin impairs high-level but not low-level motion perception. Neuroreport. 2004;15:1947–1951. doi: 10.1097/00001756-200408260-00023. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochemical Research. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Polk SD, Ross RG. Mismatch negativity in detection of interval duration deviation in schizophrenia. Neuroreport. 2003;14:1283–1286. doi: 10.1097/00001756-200307010-00019. [DOI] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH. Failure to find P50 suppression deficits in young first-episode patients with schizophrenia and clinically unaffected siblings. Schizophrenia Bulletin. 2007a;33:1319–1323. doi: 10.1093/schbul/sbm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH. A meta-analysis of P50 studies in patients with schizophrenia and relatives: differences in methodology between research groups. Schizophrenia Research. 2007b;97:137–151. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin. 2010;136:822–848. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Neve A, Quittenbaum JP, Alam NM, Prusky GT. Perception of visual motion coherence by rats and mice. Vision Research. 2006;46:2842–2847. doi: 10.1016/j.visres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Luminais SN, White SL, Rudnick ND, Ma N, Dow HC, Kreibich AS, Abel T, Brodkin ES, Hahn CG, Siegel SJ. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Research. 2009;1294:116–127. doi: 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. Journal of Cognitive Neuroscience. 2008;20:1403–1414. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Phillips JM, Thieu T, Ehrlichman RS, Halene TB, Leiser SC, Christian E, Johnson E, Lerman C, Siegel SJ. Nicotine receptor subtype-specific effects on auditory evoked oscillations and potentials. PLoS ONE. 2012;7:e39775. doi: 10.1371/journal.pone.0039775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Morlet D, Giard M. Mismatch negativity and N100 in comatose patients. Audiology & Neuro-otology. 2000;5:192–197. doi: 10.1159/000013880. [DOI] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Stephan KE, Friston KJ. The mismatch negativity: a review of underlying mechanisms. Clinical Neurophysiology. 2009;120:453–463. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Molecular Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Graham NV. Beyond multiple pattern analyzers modeled as linear filters (as classical V1 simple cells): useful additions of the last 25 years. Vision Research. 2011;51:1397–1430. doi: 10.1016/j.visres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophrenia Bulletin. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nature Medicine. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Halene TB, Siegel SJ. Antipsychotic-like properties of phosphodiesterase 4 inhibitors: evaluation of 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (RO-20-1724) with auditory event-related potentials and prepulse inhibition of startle. Journal of Pharmacology and Experimental Therapeutics. 2008;326:230–239. doi: 10.1124/jpet.108.138586. [DOI] [PubMed] [Google Scholar]
- Hedges JH, Gartshteyn Y, Kohn A, Rust NC, Shadlen MN, Newsome WT, Movshon JA. Dissociation of neuronal and psychophysical responses to local and global motion. Current Biology: CB. 2011a;21:2023–2028. doi: 10.1016/j.cub.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges JH, Stocker AA, Simoncelli EP. Optimal inference explains the perceptual coherence of visual motion stimuli. Journal of Vision. 2011b:11. doi: 10.1167/11.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological Review. 1980;87:175–189. [PubMed] [Google Scholar]
- Hupfeld D, Hoffmann KP. Motion perception in rats (Rattus norvegicus sp.): deficits in albino Wistar rats compared to pigmented Long-Evans rats. Behavioural Brain Research. 2006;170:29–33. doi: 10.1016/j.bbr.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG., Jr Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biological Psychiatry. 1993;33:513–519. doi: 10.1016/0006-3223(93)90005-x. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Jutzeler C, McMullen ME, Featherstone RF, Tatard-Leitman VM, Gandal MJ, Carlson GC, Siegel SJ. Electrophysiological deficits in schizophrenia: models and mechanisms. In: Uehara T, editor. Psychiatric Disorders. InTech; Rijeka: 2011. [Google Scholar]
- Kemp A, Manahan-Vaughan D. Passive spatial perception facilitates the expression of persistent hippocampal long-term depression. Cerebral Cortex. 2012;22:1614–1621. doi: 10.1093/cercor/bhr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Tolias AS, Altmann CF, Augath M, Logothetis NK. Integration of local features into global shapes: monkey and human FMRI studies. Neuron. 2003;37:333–346. doi: 10.1016/s0896-6273(02)01174-1. [DOI] [PubMed] [Google Scholar]
- Kovacs I. Gestalten of today: early processing of visual contours and surfaces. Behavioural Brain Research. 1996;82:1–11. doi: 10.1016/s0166-4328(97)81103-5. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, ffytche D, Soni W, Mitterschiffthaler MT, Vythelingum GN, Simmons A, Williams SC, Sharma T. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Research. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of General Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Newsome WT. Visual processing in monkey extrastriate cortex. Annual Review of Neuroscience. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Kanes SJ, Abel T, Siegel SJ. Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience. 2004a;129:101–107. doi: 10.1016/j.neuroscience.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Kelly MP, Kanes SJ, Abel T, Siegel SJ. Mice expressing constitutively active Gsalpha exhibit stimulus encoding deficits similar to those observed in schizophrenia patients. Neuroscience. 2006;141:1257–1264. doi: 10.1016/j.neuroscience.2006.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, Turetsky BI, Bilker WB, Lenox RH, Siegel SJ. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2004b;29:739–746. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, Liang Y, Siegel SJ. Effects of nicotine vary across two auditory evoked potentials in the mouse. Biological Psychiatry. 2007;61:23–30. doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Michie PT. What has MMN revealed about the auditory system in schizophrenia? International Journal of Psychophysiology. 2001;42:177–194. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Ward R. Conditions for motion flow in dynamic visual noise. Vision Research. 1980a;20:431–435. doi: 10.1016/0042-6989(80)90033-4. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Ward R. Interocular delay produces depth in subjectively moving noise patterns. The Quarterly Journal of Experimental Psychology. 1980b;32:387–395. doi: 10.1080/14640748008401833. [DOI] [PubMed] [Google Scholar]
- Naatanen R. Mismatch negativity (MMN): perspectives for application. International Journal of Psychophysiology. 2000;37:3–10. doi: 10.1016/s0167-8760(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Salzman CD, Movshon JA. Neuronal mechanisms of motion perception. Cold Spring Harbor Symposia on Quantitative Biology. 1990;55:697–705. doi: 10.1101/sqb.1990.055.01.065. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, Volchan E, Pessoa L, Pantoja JH, Joffily M, Souza-Neto D, Marques RF, Rocha-Miranda CE. Contour integration in the primary visual cortex of the opossum. Neuroreport. 2002;13:2001–2004. doi: 10.1097/00001756-200211150-00002. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Ehrlichman RS, Siegel SJ. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event-related potential and evoked gamma. Neuroscience. 2007;144:1314–1323. doi: 10.1016/j.neuroscience.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behavioural Brain Research. 2009;204:282–294. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain: A Journal of Neurology. 2000;123 (Pt 8):1624–1633. doi: 10.1093/brain/123.8.1624. [DOI] [PubMed] [Google Scholar]
- Rudnick ND, Koehler C, Picciotto MR, Siegel SJ. Role of beta2-containing nicotinic acetylcholine receptors in auditory event-related potentials. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1358-6. [DOI] [PubMed] [Google Scholar]
- Rudnick ND, Strasser AA, Phillips JM, Jepson C, Patterson F, Frey JM, Turetsky BI, Lerman C, Siegel SJ. Mouse model predicts effects of smoking and varenicline on event-related potentials in humans. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2010;12:589–597. doi: 10.1093/ntr/ntq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophrenia Research. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Maxwell CR, Majumdar S, Trief DF, Lerman C, Gur RE, Kanes SJ, Liang Y. Monoamine reuptake inhibition and nicotine receptor antagonism reduce amplitude and gating of auditory evoked potentials. Neuroscience. 2005;133:729–738. doi: 10.1016/j.neuroscience.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Berten S, Essex B, Kovacs I, Susmaras T, Little DM. An fMRI examination of visual integration in schizophrenia. Journal of Integrative Neuroscience. 2009;8:175–202. doi: 10.1142/s0219635209002113. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Archives of General Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tikhonravov D, Neuvonen T, Pertovaara A, Savioja K, Ruusuvirta T, Naatanen R, Carlson S. Effects of an NMDA-receptor antagonist MK-801 on an MMN-like response recorded in anesthetized rats. Brain Research. 2008;1203:97–102. doi: 10.1016/j.brainres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophrenia Bulletin. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Millard I, Muetzelfeldt L, Curran HV, Morgan CJ. Perceptual organization in ketamine users: preliminary evidence of deficits on night of drug use but not 3 days later. Journal of Psychopharmacology. 2007;21:347–352. doi: 10.1177/0269881107077739. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Farkas D, Nelken I. Multiple time scales of adaptation in auditory cortex neurons. Journal of Neuroscience. 2004;24:10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Archives of General Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotki D, Latanov A, Nitsch R, Lipp HP. Deviance-related electrophysiological activity in mice: is there mismatch negativity in mice? Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2005;116:353–363. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Toyota T, Owada Y, Hayashi T, Iwayama Y, Matsumata M, Ishitsuka Y, Nakaya A, Maekawa M, Ohnishi T, Arai R, Sakurai K, Yamada K, Kondo H, Hashimoto K, Osumi N, Yoshikawa T. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biology. 2007;5:e297. doi: 10.1371/journal.pbio.0050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Swerdlow NR. Rat strain differences in startle gating-disruptive effects of apomorphine occur with both acoustic and visual prepulses. Pharmacology, Biochemistry, and Behavior. 2008;88:306–311. doi: 10.1016/j.pbb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer GaMRW. Electrophysiology of schizophrenia. In: Harrison DRWaPJ., editor. Schizophrenia. 3. Wiley-Blackwell; Oxford, UK: 2011. pp. 311–333. [Google Scholar]


