Abstract
An intriguing discovery in recent years is that resting-state functional connectivity (RSFC) is associated with cognitive performance. The current study investigated whether RSFC within the reading network was correlated with Chinese adults’ reading abilities in their native language (L1, Chinese) and second language (L2, English). Results showed that RSFC within the reading network was positively correlated to reading abilities in L1 and L2, and RSFC between reading areas and the default network was negatively correlated to reading abilities in L1 and L2. Further conjunction and contrast analyses revealed that L1 and L2 shared similar RSFC correlates including connectivities between the areas for visual analysis (e.g., bilateral posterior fusiform gyrus, lateral occipital cortices, and right superior parietal lobules) and those for phonological processing (e.g., bilateral precentral gyri and postcentral gyrus, Wernicke’s area). These results indicate that RSFC is a potential neural marker for reading abilities in both L1 and L2, with important theoretical implications for reading in L1 and L2.
Keywords: functional connectivity, resting-state, reading ability, first and second languages, the reading network
Introduction
Although reading a familiar word takes less than one second and is a seemingly simple task for a typical adult, the brain has to integrate signals from many regions specialized for cognitive processing including visual, phonological, semantic, and other linguistic processing (Turkeltaub et al., 2002; Vigneau et al., 2006). Conventional functional MRI studies have mapped out several “critical reading areas” in the left hemisphere because of their consistent involvement in reading across different languages (both first and second languages [L1] and [L2], both alphabetic and logographic writings) (Bolger et al., 2005; Tan et al., 2005; Xue et al., 2006b). This reading network includes the left inferior frontal gyrus (IFG, including Broca's area)(Bokde et al., 2001; Costafreda et al., 2006; Gough et al., 2005; Roskies et al., 2001), the left superior temporal gyrus (STG, including Wernicke’s area)(Chang et al., 2010; Geschwind, 1970; Simos et al., 2000), the left temporo-occipital cortex (e.g., the left posterior fusiform gyrus [FFG], including the so-called “visual word form area” [VWFA]) (Cohen and Dehaene, 2004; Cohen et al., 2002; Dehaene et al., 2005), as well as some other regions around the left sylvian fissure (Bolger et al., 2005; Price, 2000; Tan et al., 2005).
Because reading takes coordination of the different areas in the reading network, brain imaging data have been further analyzed to examine functional connectivity (FC) between these areas. Previous studies have examined FC in the reading network with various reading tasks (e.g., reading aloud and semantic judgment) (Hampson et al., 2006; Seghier and Price, 2010; Wu et al., 2009), reading materials (e.g., words and pseudowords) (Mechelli et al., 2002), and types of brain data (e.g., fMRI and EEG) (Ligges et al., 2010; Schinkel et al., 2011). FC during reading has generally been found to be associated with reading (dis)ability. For example, Hampson et al. (2006) found that good readers showed stronger FC between BA39 (the left angular gyrus and part of the middle temporal gyrus) and Wernicke's area than did poor readers. Some studies found that readers with dyslexia showed decreased FC between left BA39 and other reading-related areas (Horwitz et al., 1998; Pugh et al., 2000). One recent study also revealed a disruption of FC between the VWFA and reading regions in children with dyslexia (van der Mark et al., 2011).
In addition to FC during reading, studies have also evaluated task-independent FC in the reading network at resting state. The low-frequency spontaneous BOLD fluctuations (≈ 0.01–0.1Hz), which used to be treated as noise in conventional analysis, have been suggested to reflect neuronal function (Biswal et al., 1995; Biswal et al., 1997; Damoiseaux et al., 2006; Fox and Raichle, 2007). These signals can be captured quickly with brief (5–10 min) fMRI scans. Temporal correlations of the signals between one region (the seed region) and other parts of the brain are calculated to index resting state functional connectivity (RSFC). This technique has been utilized to characterize motor (Biswal et al., 1995), visual (Nir et al., 2006), and attention (Fox et al., 2006) systems as well as the reading network (Koyama et al., 2010). It provides us with a new way to evaluate functional connectivity within the brain.
Using this approach, Hampson at al. (2002) first demonstrated RSFC between two classical reading areas, Broca's and Wernicke's areas. Their work was extended to more seed regions in subsequent studies. The topographical functional connectivity pattern in the left middle frontal, parietal, and temporal areas was revealed for the three subregions of Broca’s area (Xiang et al., 2010). Other reading areas also showed functional connectivity at resting state, for example, between the left FFG and left IFG and between the left STG and left IFG (Koyama et al., 2010; Turken and Dronkers, 2011). More recently, RSFC within the reading network (e.g., Wernicke's and Broca's areas) was observed in a large sample (970 subjects) from various countries (Tomasi and Volkow, 2012). Further research revealed that RSFC also existed between the reading area (VWFA) and the attention network (Vogel et al., 2012).
Although the above studies demonstrated the existence of RSFC within the reading network, only one study thus far has examined the relationship between RSFC and reading ability (Koyama et al., 2011). In their study of 25 children and 25 adults who were native English speakers, Koyama et al. found that English reading ability was positively correlated with RSFC among motor regions (the left precentral gyrus [PCG] and postcentral gyrus) and that between speech regions (Broca's and Wernicke's areas) in both children and adults. They further found that, for adults only, reading ability was negatively correlated with RSFC between the left FFG and the default network (Koyama et al., 2011).
The current study examined how RSFC within the reading network was associated with L1 (Chinese) and L2 (English) reading abilities in a group of native Chinese speakers. Researchers have long been interested in how the brain represents L1 and L2. A fundamental question is whether the neural substrates are shared or segregated for L1 and L2. Previous studies found both dissociated (Dehaene et al., 1997; Kim et al., 1997) and shared neural basis for L1 and L2 (Nakada et al., 2001; Perani and Abutalebi, 2005; Tan et al., 2003). The notions of “assimilation” and “accommodation” (Nelson et al., 2009) have recently been proposed to explain L1 and L2 processing, especially when they belong to different language systems. Assimilation means that the brain uses the L1 network to support L2 (thus, a shared neural basis) (Liu et al., 2007a) and accommodation means that the brain’s reading network adapts to the features of L2 (e.g., a new writing system that could not be assimilated because it is based on a distinct neural mechanism) (Nelson et al., 2009). In this study, we examined both shared and divergent RSFC correlates of reading abilities in Chinese (L1) and English (L2).
Method
Subjects
Forty three Chinese students (age range: 19–24 years, mean age = 21, SD = 1.4, 23 female and 20 male) from Beijing Normal University participated in our experiment. All were native Chinese speakers, learning English as their L2 (since elementary school) and passed the college entrance examination of Chinese and English. They had normal or corrected-to-normal vision, with no previous history of neurological or psychiatric diseases and were strongly right-handed as judged by Snyder and Harris’s handedness inventory (Snyder and Harris, 1993). Informed written consent was obtained from the subjects before the experiment. This study was approved by the IRB of the National Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University.
Behavioral assessment
English reading ability was assessed using the Sight Word Efficiency subtest in the Test of Word Reading Efficiency (TOWRE-SWE), a nationally normed measure of word reading accuracy and fluency in the U.S. for individuals from 6 to 24 years of age (Torgesen et al., 1999). Reading score was indexed by the number of printed words that were accurately read within 45 seconds. Test items were arranged in order of difficulty from easy to more difficult items. There are two equivalent forms (A and B) in the test, each with 104 items. Both forms were administered in the current study and their scores were averaged.
Chinese character reading was measured by the Chinese Character Reading Efficiency Test (CCRET). This test was developed in the format of TOWRE-SWE. There were also 104 items in the CCRET selected from the Chinese character psycholinguistic norms (Liu et al., 2007b) with word frequency ranging from 4 to 5636 (mean=196), number of strokes ranging from 2 to 14 (mean =7.3), and number of units ranging from 1 to 5 (mean= 2.4). Reading score was indexed by the number of printed Chinese characters that were accurately read in 45 seconds. Test items were arranged in order of difficulty from easy to more difficult items. Both tests (TOWRE-SWE and CCRET) have been used in our previous study that examined the structure of the left FFG and L1 and L2 reading in Chinese subjects (Zhang et al., 2013). The correlation between reading scores of L1 and L2 was significant (r = .50, p < .001).
A non-verbal reasoning (or intelligence) test, Raven’s Advanced Progressive Matrices (RAPM), was also used in the current study to test whether RSFC in the reading-related ROIs was specifically related to reading skills or rather generally related to basic cognitive abilities. RAPM has been widely used in previous studies (see Zhu et al., 2010, for a detailed description of this test as used in the current study). Table 1 shows subjects’ basic demographic information and cognitive scores.
Table 1.
Characteristics of the subjects
| Characteristics | Mean(SD) | Range |
|---|---|---|
| Age (years) | 21(1.4) | 19–24 |
| Gender (F/M) | 22/20 | |
| Handedness | All right-handed | |
| Raven’ Progressive Matrices | ||
| Score | 27.9(3.8) | 20–35 |
| Time (minutes) | 31.4(7.6) | 13–40 |
| Reading score of L1 | 85.5(13.0) | 51–104 |
| Reading score of L2 | 72.6(9.2) | 54–93 |
Note: Standard deviations are shown in parentheses.
MRI data acquisition
Data were acquired with a 3.0 T Siemens MRI scanner in the MRI Center of Beijing Normal University. A single-shot T2*-weighted gradient-echo EPI sequence was used for a brief scan (8 min) which comprised 240 continuous echo planar imaging functional volumes with the following parameters: TR/TE/θ = 2000 ms/25 ms/90°, FOV = 192 × 192 mm, matrix = 64 × 64, and slice thickness = 3 mm. During the scan, subjects laid supine on the scanner bed. Foam pads were used to minimize head motion. Subjects were instructed to close their eyes, keep their head still, think about nothing in particular, and just relax. All subjects orally reported having their eyes closed and being awake during the scan. Anatomical MRI was acquired using a T1-weighted, three-dimensional, gradient-echo pulse-sequence (MPRAGE) with TR/TE/θ = 2530 ms/3.09 ms/10°, FOV = 256 × 256 mm, matrix = 256 × 256, and slice thickness = 1 mm. Two hundred and eight sagittal slices were acquired to provide high-resolution structural images of the whole brain.
Data preprocessing
Image preprocessing was carried out using tools from the FMRIB’s software library (http://www.fmrib.ox.ac.uk/fsl) version 4.1.8. The first five volumes in each time series were automatically discarded by the scanner to allow for T1 equilibrium effects. The remaining images went through further preprocessing steps including motion correction (MCFLIRT) (Jenkinson et al., 2002), spatial smoothing (Gaussian kernel of full width half maximum 5 mm), mean-based intensity normalization of all volumes by the same factor, and temporal bandpass filtering (0.01–0.1 Hz). Finally, a 2-step registration procedure was used whereby EPI images were first registered to the MPRAGE structural images using 12-degrees-of-freedom linear transformations with FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001), and then into the standard MNI152 brain template (Montreal Neurological Institute) with 2-mm3 resolution using 12-degrees-of-freedom nonlinear transformations with FNIRT (Andersson et al., 2007).
Nuisance Signal Regression
To control for the effects of physiological processes (such as fluctuations related to cardiac and respiratory cycles) and motion, we regressed each subject’s preprocessed 4-D volume on eight predictors that modeled nuisance signals from white matter, cerebrospinal fluid, and six motion parameters. It should be noted that in this study we did not use global signal regression because recent studies have argued against its use in resting state data analysis (Fox et al., 2009; Murphy et al., 2009; Yan et al., 2013). However, given that some previous studies in this area regressed out global signal in this step (Kelly et al., 2009; Kelly et al., 2010; Koyama et al., 2010), we also reported results from global signal regression in supplementary materials (Supplementary Figure 1). Because small amounts of movement from one volume to another could also influence RSFC results, an estimate of motion at each time point was calculated as the framewise displacement (FD), which corresponds to the temporal derivative of the motion parameters (Power et al., 2012; Van Dijk et al., 2012). One subject with mean FD larger than 0.2 was discarded. For the remaining 42 subjects, FDs (mean FD=.09 SD=.037 min=0.02 max=0.18) were used as covariates in the group-level analyses (Hoptman et al., 2012; Yan et al., 2013).
ROI selection, seed generation, and time course extraction
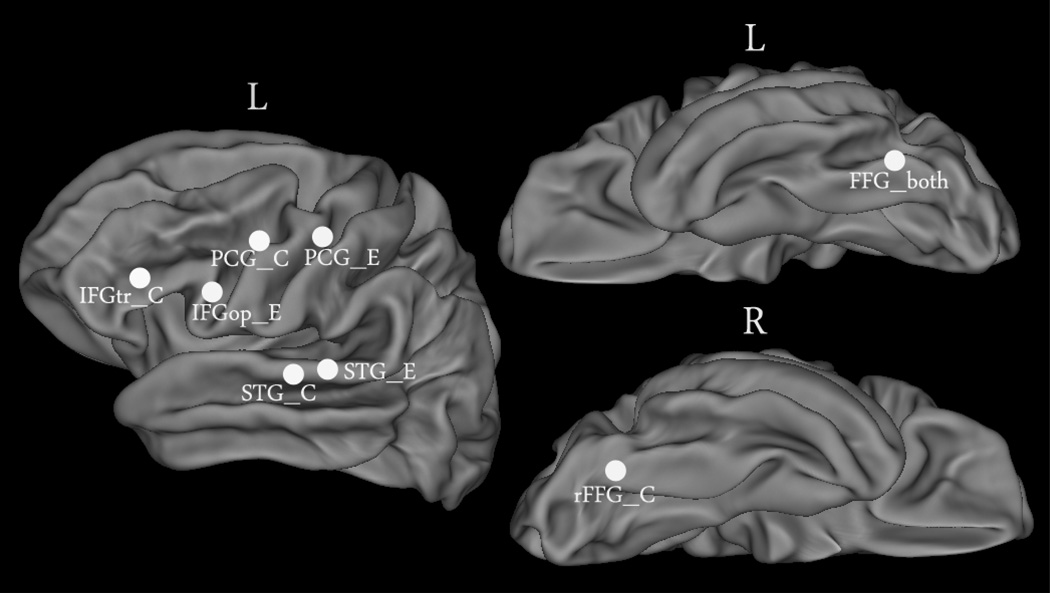
We selected a total of eight ROIs as seed regions on the basis of one previous study on the relationship between RSFC and reading ability (Koyama et al., 2011) and one meta-analysis of the reading network for different writing systems (Bolger et al., 2005). Koyama et al. (2011) selected 11 ROIs to test the RSFC - reading ability relationships in children and adults, six of which are specific for adults (all are in the left hemisphere). In the current study of adult subjects, we focused on four of these six ROIs: IFG, STG, FFG, and PCG. The first three ROIs correspond to Broca's area, Wernicke’s area, and the so-called “visual word form area”, respectively. They are the most frequently reported areas of reading in previous studies (see first paragraph of Introduction). They were suggested to be responsible for visual word analysis (FFG) (Cohen and Dehaene, 2004; Cohen et al., 2002), speech perception (STG) (Scott and Wise, 2004), and speech articulation (IFG) (Wise et al., 1999) in previous studies (Koyama et al., 2010). As for PCG, although it has not garnered as much attention as the other three areas, it is believed to be responsible for phonological motor processing (Vigneau et al., 2006) and Koyama et al (2011) reported the effect of RSFC between this seed and other reading areas on reading ability.
It should be noted that, except FFG, which showed consistent localization across language systems, other three ROIs in Koyama et al., (2011) were selected from a meta-analysis based on alphabetic languages (Bolger et al., 2005). Unlike Koyama et al. (2011), who tested only English reading, the current study included both logographic Chinese reading and alphabetic English reading, so we added four ROIs based on Chinese speakers’ data in Bolger et al.’s meta-analysis, including three ROIs also in IFG, STG and PCG, but with different localizations and one ROI in the right posterior fusiform gyrus (rFFG). In sum, eight ROIs were selected in the current study: one was common to both Chinese and English reading (FFG_both), four were selected from logographic Chinese’s meta-analysis (IFG_C, STG_C, PCG_C, and rFFG_C) and three were selected from alphabetic languages’ meta-analysis (IFG_E, STG_E, and PCG_E). Please see all ROIs and their coordinates in Table 2 and Figure 1. Talairach coordinates used in Bolger et al.,’s study were converted into MNI coordinates in the current study by using a MatLab script from http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach.
Table 2.
MNI coordinates of seed ROIs.
| Seed ROIs | MNI coordinates |
||
|---|---|---|---|
| x | y | z | |
| Regions selected from both logographic Chinese and alphabetic languages’ meta-analyses (xx_both) | |||
| FFG_both: fusiform gyrus (posterior) | −45 | −58 | −18 |
| Regions selected from logographic Chinese’s meta-analysis (xx_C) | |||
| rFFG_C: right fusiform gyrus (posterior) | 33 | −68 | −21 |
| IFGtr_C: inferior frontal gyrus (triangularis) | −45 | 33 | 9 |
| STG_C: superior temporal gyrus (posterior) | −64 | −22 | 1 |
| PCG_C: precentral gyrus (ventral) | −48 | 8 | 33 |
| Regions selected from alphabetic languages’ meta-analysis (xx_E) | |||
| IFGop_E: inferior frontal gyrus (opercularis) | −51 | 10 | 10 |
| STG_E: superior temporal gyrus (posterior) | −53 | −31 | 9 |
| PCG_E: precentral gyrus (dorsal) | −48 | −12 | 45 |
Notes: All ROIs were selected from a meta-analysis of reading (Bolger et al., 2005). All ROIs except rFFG_C are in the left hemisphere.
Figure 1.
Eight seed ROIs. See Table 2 for full names of the seed ROIs. L: left hemisphere; R: right hemisphere.
Although most previous reading literature emphasized left brain regions, many studies reported bilateral brain activity for reading, especially for logographic Chinese reading (Chee et al., 1999; Xue et al., 2006b; Zhang et al., 2013). For readers who may be interested in the relationship between RSFC of seed ROIs in the right hemisphere (besides rFFG) and reading, we used FSL script fslswapdim to convert all ROIs in the left hemisphere (except FFG) into the right hemisphere homologues and examined RSFC of these areas with reading abilities. Please see Online Supplementary Materials (Supplementary Tables and 3) for the results of these ROIs.
For each node in the reading network, we created a spherical ROI centered on the coordinates defined above in the 2-mm3 resolution standard MNI152 template with a 6 mm radius. The time series of all voxels (123 voxels) in each ROI were extracted and averaged to generate a representative time series. These time series served as seeds in subsequent connectivity analysis.
Individual-level RSFC construction
For each subject, a whole-brain analysis was conducted to correlate the time courses of the seed regions with the time courses of all the other voxels in the brain using the general linear model implemented in the FSL program FEAT. Individual subjects’ maps of all voxels that were positively or negatively correlated with the seed time series were created in this step. These maps were converted to Z-value maps using Fisher’s r-to-z transformation for further group-level analysis. All Z-value maps from individual subjects were registered onto the MPRAGE structural image and then to standard MNI152 2 mm3 space again (Jenkinson et al., 2002; Jenkinson and Smith, 2001).
Group-level RSFC construction
Three sets of analyses were conducted. First, we conducted a whole brain calculation on Z-value maps and correlated them with L1 and L2 reading performances separately, using a mixed-effects ordinary least-squares model implemented in FSL. Gender and FD were included as covariates in the current study. (FD was actually not correlated with reading ability in either L1 [r = 0.08, p = .6] or L2 [r = −.08, p = .6]). Corrections for multiple comparisons were conducted at the cluster level for each RSFC map (Z > 2.3, p < 0.05, corrected). The same analysis was also conducted with the scores of Raven’s Advanced Progressive Matrices (RAPM). Results showed no significant relationship between RSFC of the selected regions and the scores of RAPM, and thus these scores were not analyzed further in the whole-brain analysis. Second, to establish common RSFC maps for L1 and L2 reading abilities, we conducted a conjunction analysis. In this analysis, we focused on positive relationships between RSFC and reading abilities. All positive maps from the last step were binarized by using FSL script fslmaths, so that for each ROI, there were two binary maps respectively for L1 and L2, in which voxels of significant correlates of reading ability (L1 or L2) were assigned a value of 1, otherwise 0. Then, the binarized maps of L1 and L2 of each ROI were summed (also using fslmaths) and the common maps for both languages of each ROI were created at the threshold = 2. RSFC in voxels where the value equals 2 were then correlated with reading abilities in both L1 and L2. Finally, to determine divergent RSFC maps in which RSFC-behavior relationship differed between L1 and L2, we conducted direct statistical comparisons (L1 minus L2 [with binarized positively significant RSFC maps for L1 as a pre-threshold mask] and L2 minus L1 [with binarized positively significant RSFC maps for L2 as a pre-threshold mask]) of the RSFC–reading ability relationships. Gender was included as a covariate and a mixed-effects ordinary least-squares model and cluster-wise multiple comparisons correction were used (Z >2.3, p < 0.05, corrected).
ROI-based analysis
In addition to the whole-brain voxel wise analysis, we performed ROI-based analysis in which we calculated RSFC between all possible pairings of the 14 reading areas (including six in the supplementary materials), yielding a total of 91 pairwise correlations representing RSFC strengths for each subject. Then, we correlated the RSFC strengths with L1 and L2 reading abilities as well as RAPM scores across subjects (with gender as a covariate). Finally, to identify ROI pairings that differed in RSFC-reading ability relationship between L1 and L2, we converted the significant RSFC-behavior correlations using Fisher r-to-z transformation and compared the z-transformed correlations between the two languages.
Results
RSFC maps related to L1 and L2 reading abilities
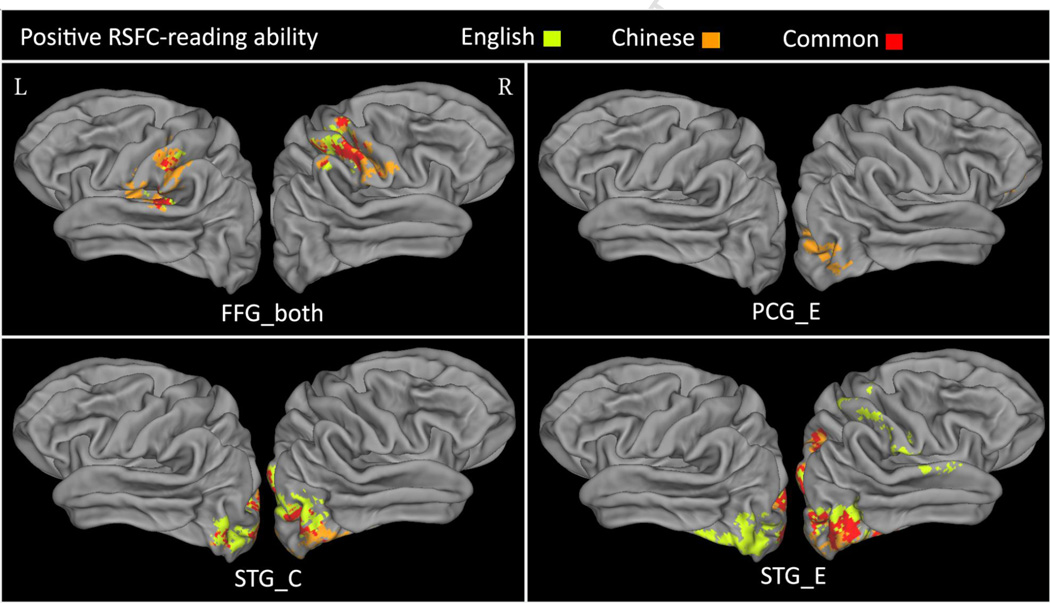
The whole-brain analysis revealed that RSFC between several seeds and other reading areas was positively correlated to reading abilities in both languages. The brain maps of these relationships are shown in Figure 2. Positive correlates of L1 reading ability included RSFC between the FFG_both seed and areas around the bilateral sylvian fissure including the bilateral PCG, postcentral gyrus (PstCG), supramarginal gyrus (SMG) extending to posterior insula, Heschl's gyrus (HG), planum temporale (PT), and posterior STG in the left hemisphere; that between the STG_C seed and the bilateral FFG, occipital pole (OP), lateral occipital cortex (LOC), lingual gyrus(LG); that between the STG_E seed and the bilateral OP, and LOC extending ventrally to the FFG and dorsally to the superior parietal lobule (SPL) in the right hemisphere; and that between the PCG_E seed and the left subcallosal cortex (SCC), right LOC, and right OP. The pattern of results for L2 reading ability was very similar. Peak coordinates and detailed information of these relationships are reported in Table 3. Although most studies focused on positive relationships between RSFC and cognitive performance, our whole-brain analysis also revealed several significant negative RSFC correlates of reading abilities. For readers who may be interested in negative relationships, results are presented in Supplementary Table 1 and Supplementary Figure 2.
Figure 2.
Positive RSFC-reading ability relationships for Chinese (L1, orange), English (L2, yellow), and both of them (red) (Z > 2.3, p < 0.05, cluster-wise corrected). L: left hemisphere; R: right hemisphere.
Table 3.
Positive RSFC-reading ability relationship based on the whole brain analysis.
| ROI seeds |
Cluster size |
Peak (MNI) |
Cluster location(>30 voxels) | Peak Z |
|||
|---|---|---|---|---|---|---|---|
| X | y | z | |||||
| L1 | FFG_both | 2060 | −66 | −30 | 46 | L PstCG/SMG/HG/PT/PCG/insula/STG | 4.42 |
| 1482 | 56 | −4 | 38 | R PCG/PstCG/SMG/ | 4.21 | ||
| STG_C | 3777 | 20 | −104 | 6 | R OP/LOC/FFG/LG | 4.85 | |
| 790 | −50 | −78 | −16 | L OP/LOC/FFG/LG | 4.29 | ||
| STG_E | 3917 | 42 | −72 | −8 | R OP/LOC/FFG/LG/SPL L OP/LOC |
4.35 | |
| PCG_E | 2277 | −4 | 16 | −2 | L SCC | 4.19 | |
| 940 | 34 | −90 | 16 | R LOC/OP | 3.9 | ||
| L2 | FFG_both | 1185 | −36 | −30 | 14 | L PT/PstCG /SMG/HG/STG | 4.1 |
| 1179 | 46 | −18 | 48 | R PstCG/PCG/SMG/ | 3.83 | ||
| 1014 | 4 | −22 | 44 | R PCG/SMC/PstCG | 3.76 | ||
| STG_C | 2785 | 30 | −88 | 32 | R LOC/OP/FFG/ | 4.26 | |
| 1347 | −40 | −86 | 0 | L LOC/OP | 4.01 | ||
| STG_E | 7816 | 12 | −86 | −10 | R LOC/OP/FFG/LG/SPL L LOC/OP/FFG |
4.61 | |
| 692 | 56 | −16 | 46 | R PstCG/PCG/TP | 3.79 | ||
L: left hemisphere; R: right hemisphere; PstCG: postcentral gyrus; SMG: supramarginal gyrus; HG: Heschl's gyrus; PT: planum temporale; PCG: precentral gyrus; STG: superior temporal gyrus; OP: occipital pole; LOC: lateral occipital cortex; FFG:fusiform gyrus; LG: lingual gyrus; SPL: superior parietal lobule; SCC: subcallosal cortex; SMC: supplementary motor cortex; TP: temporal pole.
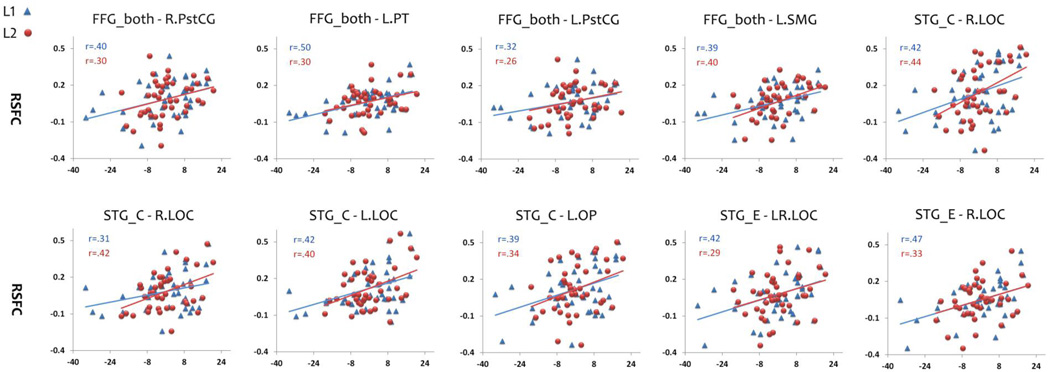
The conjunction analysis identified shared positive RSFC-reading ability relationships for both languages. They included functional connection between the FFG_both seed and bilateral PstCG, the left PT, SMG; between the STG_C seed and the bilateral LOC, the left OP; between the STG_E seed and the bilateral LOC (see Figure 2). In addition, Figure 3 shows the scatter plots of the RSFC-behavior relationship of these clusters in both languages. Because the mean reading scores of the two languages were different, we used the demeaned scores for each language in order to show the results of both languages in the same plots. The contrast analyses (L1 minus L2 and L2 minus L1) revealed no RSFC-reading ability relationship that was specific for either L1 or L2.
Figure 3.
Scatter plots of the common positive RSFC-reading ability relationships for Chinese (L1, blue triangles) and English (L2, red circles). Reading abilities are indexed by demeaned reading scores of L1 and L2. L: left hemisphere; R: right hemisphere.
ROI results
ROI analysis showed positive RSFC–behavior relationships for RSFC between the FFG_both seed and the PCG_E seed in both L1 (p < 0.05) and L2 (p < 0.05), between the FFG_both seed and the STG_E seed in both L1 (p < 0.005) and L2 (p < 0.05), and between the rFFG_C seed and the STG_C seed in L1 (p < 0.005). It revealed negative RSFC–behavior relationships for RSFC between the IFGtr_C seed and the STG_C seed in both L1 (p < .05) and L2 (p < 0.01). Although the correlation coefficients of RSFC strengths and reading abilities were between "medium" and "large" according to Cohen (1988), they did not survive multiple comparisons because of the relatively modest sample size (N = 42) and the large number of multiple comparisons (14 seeds, 91 ROI pairings, correction based on the number of tested ROI pairings: equivalent to p < 0.00055 for 91 tested pairings). The uncorrected results are displayed in Table 4. Consistent with results from the whole-brain analysis, the ROI analysis revealed no significant relationships between RSFC and RAPM scores and no significant differences between L1 and L2 in terms of the correlations between any ROI pairings and reading ability.
Table 4.
ROI-based correlations between RSFC and reading abilities in L1 and L2.
| ROI pairings | RSFC-reading ability in L1 | RSFC-reading ability in L2 |
|---|---|---|
| FFG_both/PCG_E | .30* | .33* |
| FFG_both/STG_E | .45*** | .33* |
| IFGtr_C/STG_C | −.36* | −.42** |
| rFFG_C/STG_C | .44*** | .25 |
p < .05;
p < .01;
p < .005
Discussion
The purpose of the current study was to examine the relationship between RSFC and reading abilities in L1 and L2. Both whole-brain and ROI analyses revealed significant contributions of functional connectivity to L1 and L2 reading abilities and these effects appeared to be specific to reading because they did not exist for general cognitive ability as assessed with a non-verbal reasoning task. Conjunction analysis identified common RSFC maps for both L1 and L2 and contrast analysis revealed no specific RSFC-reading ability relationship for either L1 or L2. In the following sections, we discuss these findings in terms of connectivity-reading ability relationship and theoretical models of L1 and L2 reading.
The contribution of RSFC strength to reading abilities
Brain-behavior analysis is one of the most important approaches to understanding neural basis of reading and investigating neural markers of reading ability. Using this approach, previous functional and structural MRI studies have revealed associations of brain activity and anatomy with reading ability (Brem et al., 2006; Mei et al., 2010; Turkeltaub et al., 2003; Xue et al., 2010; Zhang et al., 2013). In the current study, we used this approach to investigate the relationship between RSFC and reading ability and observed that the RSFC strength within the reading network was correlated to reading abilities in both L1 and L2. In fact, the RSFC-reading ability relationship patterns did not differ significantly between L1 and L2. We discuss each specific RSFC correlate of reading abilities in turn.
First, a positive RSFC-reading ability relationship was observed in functional connection linking the FFG_both seed to areas around the sylvian fissure including motor areas (bilateral PCG and PstCG) and parts of Wernicke’s area (e.g., PT). These areas have been implicated in reading in previous meta-analytical studies (Bolger et al., 2005; Tan et al., 2005; Turkeltaub et al., 2002; Vigneau et al., 2006). The motor areas, together with PT, have been identified as the auditory–motor speech coordination network (Vigneau et al., 2006). This network is involved not only in speech production (Bookheimer et al., 2000; Cohen et al., 1997; Heim et al., 2002), but also in perception (Fadiga et al., 2002; Hickok and Poeppel, 2004), and is hence deemed as the perception–action cycle for reading (Vigneau et al., 2006). The FFG seed region, also known as the VWFA, was suggested for orthographic analysis, an early step in the reading process. Our previous studies revealed that both functional activity (indexed by activity) (Chen et al., 2007; Dong et al., 2008; Mei et al., 2010; Xue et al., 2006a; Xue et al., 2010) and structure (indexed by cortical thickness) (Zhang et al., 2013) of the FFG can predict reading ability and language learning in Chinese adults. The result from the current study suggests that reading ability is not only separately related to visual analysis and phonological processing, but also to the functional cooperation and connection between these two processes.
Second, reading ability was also related to the functional connection linking phonological areas (the PCG and STG seeds) to visual analysis areas other than the FFG, including the clusters extending from bilateral LOC up to the SPL (the STG_E seed). Although the LOC has been studied extensively in object recognition (Beauchamp, 2005; Grill-Spector et al., 2001; Grill-Spector et al., 1999; Kourtzi and Kanwisher, 2001; Lerner et al., 2002), previous meta-analyses have also consistently reported activity in this area in reading tasks (Tan et al., 2005; Vigneau et al., 2006). Studies have also shown that damage to this area can result in pure alexia characterized by letter-by-letter reading (Behrmann et al., 1998; Sakurai et al., 2001). This important role of the LOC would explain our finding that reading ability was related to RSFC between this area and the reading areas. It should be noted, however, that Koyama et al. (2011) also selected one ROI in this region (the inferior part) but did not find any significant RSFC correlates of reading ability with this seed region. It is not clear what contributed to this discrepancy in our results. One possibility is that Chinese subjects may rely more on bilateral visual areas than English-speaking subjects (Bolger et al., 2005; Tan et al., 2005) because the former’s native language (Chinese) is a logographic language with complex strokes that requires greater visual analysis of spatial information.
Although previous studies of reading have not focused on the SPL, significant results about this area have been reported in several studies of logographic language (Chinese) reading (Fu et al., 2002; Tan et al., 2001) and alphabetic language reading (Mechelli et al., 2000; Sakurai et al., 1993; Vigneau et al., 2005). Furthermore, this area has shown decreased activity in children with dyslexia (Corina et al., 2001; Peyrin et al., 2011), perhaps because dyslexia can be caused by disordered visual attention (Peyrin et al., 2011). Previous research has revealed that the functional connectivity (both at rest and during reading) between the FFG and the attention network (e.g., inferior parietal sulcus, the sulcus next to SPL) was correlated with reading level (van der Mark et al., 2011; Vogel et al., 2012). Our finding of the relationship between RSFC of STG_E and SPL and reading ability further confirmed their conclusion.
Third, RSFC between the bilateral temporoparietal junction area (SMG, posterior STG) and FFG_both seed was related to reading ability. The temporoparietal junction area has been linked to the orthography-to-phonology conversion in reading in previous studies (Booth et al., 2002a, b). RSFC between this area and the left FFG was also found to be correlated with adults’ reading ability in Koyama et al. (2011). Finally, our ROI-based analysis showed that RSFC between the rFFG_C seed and STG_C seed was correlated with reading abilities in L1 and L2 (p < .005), which confirmed the bilateral pattern based on the whole brain analysis (Figure 2). Moreover, the similarity between L1 and L2 results seemed to suggest that this particular connectivity may reflect an assimilation effect because the rFFG is believed to a region specific for Chinese processing (Bolger et al., 2005; Nelson et al., 2009).
Our findings of these RSFC-reading ability relationships suggested that the coordination of spontaneous activity within the reading network (e.g., the regions for visual analysis, orthography-to-phonology conversion, and phonological processing) is important for reading skills. We should hasten to add that the precise neural functions as reflected in RSFC are still not well understood. Some researchers suggested that the spontaneous activity serves to maintain the functional integrity of the network by reinforcing the synaptic connections among its neurons (Pinsk and Kastner, 2007; Kelly et al., 2008). Weaker or negative RSFC (as found for poor readers in our study) may indicate less integration or even disintegration among neurons in the network.
Implications for L1 and L2 reading
The conjunction analysis revealed shared RSFC correlates of reading abilities in L1 and L2, especially functional connection between the visual analysis areas (e.g., the bilateral FFG, LOC, and right SPL) and phonological processing areas (e.g., the bilateral PCG, PstCG, left PT and HG). These findings are consistent with previous functional and structural MRI studies that found a shared neural basis of L1, L2, and even a new artificial language (Mei et al., 2008; Mei et al., 2010; Xue et al., 2010; Zhang et al., 2013). As others have argued, there might be “a fixed brain pattern” (or a universal neural basis) for L1 and L2 learning (Hernandez et al., 2005; Perani and Abutalebi, 2005). Alternatively, it may be due to the “assimilation” process, as mentioned earlier, that led the brain to use L1’s neural mechanism to process L2 (Liu et al., 2007a).
In addition to shared neural substrates, studies have found divergent regions for the representation of L1 and L2 in the brain. Indeed, although an “accommodation” process (i.e., relying on dissociated neural mechanisms for L2 processing) also exists, Nelson et al., (2009) found that Chinese subjects were more likely to assimilate than to accommodate. Of course, other factors such as L2 proficiency, ROI selection, and sensitivity of the RSFC in detecting the differences between L1 and L2 should also be considered. In terms of L2 proficiency, for example, our subjects appeared to have a moderate level1, which might have tipped the balance towards assimilation. Future research should include subjects of other languages and of various levels of proficiency as well as other ROI seeds (e.g., some non-reading related regions which may contribute to second language reading [Wang et al., 2009; Wang et al., 2007]).
Conclusions
In this study, we identified RSFC correlates of reading abilities in L1 and L2 and conducted conjunction and contrast analysis to examine shared and divergent correlates for L1 and L2. Results showed that RSFC between the visual analysis areas (e.g., the bilateral FFG, LOC and right SPL) and phonological processing areas (e.g., the bilateral PCG, PstCG, left PT and HG) contributed to reading abilities in both L1 and L2.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no norms for TOWRE for Chinese subjects. Our mean score of 72.6 was about the same as a previous study that used TOWRE with a large sample of Chinese college students (N=226, mean age=21.7 years, Mean score =73) (Zhang et al., 2013). Based on the American norms data (Torgesen et al., 1999), this level of reading speed/efficiency was that of the 5th grader for native speakers.
References
- Andersson J, Jenkinson M, Smith S. Technical Report TR07JA2. Oxford, UK: FMRIB; 2007. Non-linear registration, aka spatial normalisation. [Google Scholar]
- Beauchamp MS. See me, hear me, touch me: multisensory integration in lateral occipital-temporal cortex. Curr Opin Neurobiol. 2005;15:145–153. doi: 10.1016/j.conb.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Nelson J, Sekuler EB. Visual complexity in letter-by-letter reading: "pure" alexia is not pure. Neuropsychologia. 1998;36:1115–1132. doi: 10.1016/s0028-3932(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Hum Brain Mapp. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton TA, Gaillard PW, Theodore WH. Activation of language cortex with automatic speech tasks. Neurology. 2000;55:1151–1157. doi: 10.1212/wnl.55.8.1151. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002a;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Hum Brain Mapp. 2002b;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, Brandeis D. Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage. 2006;29:822–837. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The serendipitous discovery of the brain's default network. Neuroimage. 2012;62:1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chang EF, Rieger JW, Johnson K, Berger MS, Barbaro NM, Knight RT. Categorical speech representation in human superior temporal gyrus. Nat Neurosci. 2010;13:1428–1432. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Tan EW, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci. 1999;19:3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xue G, Dong Q, Jin Z, Li T, Xue F, Zhao L, Guo Y. Sex determines the neurofunctional predictors of visual word learning. Neuropsychologia. 2007;45:741–747. doi: 10.1016/j.neuropsychologia.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Corina DP, Richards TL, Serafini S, Richards AL, Steury K, Abbott RD, Echelard DR, Maravilla KR, Berninger VW. fMRI auditory language differences between dyslexic and able reading children. Neuroreport. 2001;12:1195–1201. doi: 10.1097/00001756-200105080-00029. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Human brain mapping. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cogn Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Dupoux E, Mehler J, Cohen L, Paulesu E, Perani D, van de Moortele PF, Lehericy S, Le Bihan D. Anatomical variability in the cortical representation of first and second language. Neuroreport. 1997;8:3809–3815. doi: 10.1097/00001756-199712010-00030. [DOI] [PubMed] [Google Scholar]
- Dong Q, Mei L, Xue G, Chen C, Li T, Xue F, Huang S. Sex-dependent neurofunctional predictors of long-term maintenance of visual word learning. Neurosci Lett. 2008;430:87–91. doi: 10.1016/j.neulet.2007.09.078. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Buccino G, Rizzolatti G. Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci. 2002;15:399–402. doi: 10.1046/j.0953-816x.2001.01874.x. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Chen Y, Smith S, Iversen S, Matthews PM. Effects of word form on brain processing of written Chinese. Neuroimage. 2002;17:1538–1548. doi: 10.1006/nimg.2002.1155. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Human brain mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. Neuroimage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD. Broca's area in the human brain is involved in the selection of grammatical gender for language production: evidence from event-related functional magnetic resonance imaging. Neurosci Lett. 2002;328:101–104. doi: 10.1016/s0304-3940(02)00494-9. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Li P, MacWhinney B. The emergence of competing modules in bilingualism. Trends Cogn Sci. 2005;9:220–225. doi: 10.1016/j.tics.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, D'Angelo D, Mauro CJ, Butler PD, Milham MP, Javitt DC. Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr Res. 2012;141:1–7. doi: 10.1016/j.schres.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci U S A. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Shehzad Z, Margulies DS, Castellanos FX, Milham MP, Petrides M. Broca's region: linking human brain functional connectivity data and non-human primate tracing anatomy studies. Eur J Neurosci. 2010;32:383–398. doi: 10.1111/j.1460-9568.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Relkin NR, Lee KM, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997;388:171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Castellanos FX, Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. Reading networks at rest. Cerebral cortex. 2010;20:2549–2559. doi: 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Malach R. Object-completion effects in the human lateral occipital complex. Cereb Cortex. 2002;12:163–177. doi: 10.1093/cercor/12.2.163. [DOI] [PubMed] [Google Scholar]
- Ligges C, Ungureanu M, Ligges M, Blanz B, Witte H. Understanding the time variant connectivity of the language network in developmental dyslexia: new insights using Granger causality. J Neural Transm. 2010;117:529–543. doi: 10.1007/s00702-010-0367-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dunlap S, Fiez J, Perfetti C. Evidence for neural accommodation to a writing system following learning. Hum Brain Mapp. 2007a;28:1223–1234. doi: 10.1002/hbm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shu H, Li P. Word naming and psycholinguistic norms: Chinese. Behav Res Methods. 2007b;39:192–198. doi: 10.3758/bf03193147. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Price CJ. The effects of presentation rate during word and pseudoword reading: a comparison of PET and fMRI. Journal of cognitive neuroscience. 2000;12(Suppl 2):145–156. doi: 10.1162/089892900564000. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Penny WD, Price CJ, Gitelman DR, Friston KJ. Effective connectivity and intersubject variability: using a multisubject network to test differences and commonalities. Neuroimage. 2002;17:1459–1469. doi: 10.1006/nimg.2002.1231. [DOI] [PubMed] [Google Scholar]
- Mei L, Chen C, Xue G, He Q, Li T, Xue F, Yang Q, Dong Q. Neural predictors of auditory word learning. Neuroreport. 2008;19:215–219. doi: 10.1097/WNR.0b013e3282f46ea9. [DOI] [PubMed] [Google Scholar]
- Mei L, Xue G, Chen C, Xue F, Zhang M, Dong Q. The "visual word form area" is involved in successful memory encoding of both words and faces. Neuroimage. 2010;52:371–378. doi: 10.1016/j.neuroimage.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada T, Fujii Y, Kwee IL. Brain strategies for reading in the second language are determined by the first language. Neurosci Res. 2001;40:351–358. doi: 10.1016/s0168-0102(01)00247-4. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Hum Brain Mapp. 2009;30:810–820. doi: 10.1002/hbm.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Perani D, Abutalebi J. The neural basis of first and second language processing. Curr Opin Neurobiol. 2005;15:202–206. doi: 10.1016/j.conb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Demonet JF, N'Guyen-Morel MA, Le Bas JF, Valdois S. Superior parietal lobule dysfunction in a homogeneous group of dyslexic children with a visual attention span disorder. Brain Lang. 2011;118:128–138. doi: 10.1016/j.bandl.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Kastner S. Neuroscience: unconscious networking. Nature. 2007;447:46–47. doi: 10.1038/447046a. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Skudlarski P, Marchione KE, Jenner AR, Fletcher JM, Liberman AM, Shankweiler DP, Katz L, Lacadie C, Gore JC. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol Sci. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of cognitive neuroscience. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Ichikawa Y, Mannen T. Pure alexia from a posterior occipital lesion. Neurology. 2001;56:778–781. doi: 10.1212/wnl.56.6.778. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Momose T, Iwata M, Watanabe T, Ishikawa T, Kanazawa I. Semantic process in kana word reading: activation studies with positron emission tomography. Neuroreport. 1993;4:327–330. doi: 10.1097/00001756-199303000-00026. [DOI] [PubMed] [Google Scholar]
- Schinkel S, Zamora-Lopez G, Dimigen O, Sommer W, Kurths J. Functional network analysis reveals differences in the semantic priming task. J Neurosci Methods. 2011;197:333–339. doi: 10.1016/j.jneumeth.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Scott SK, Wise RJ. The functional neuroanatomy of prelexical processing in speech perception. Cognition. 2004;92:13–45. doi: 10.1016/j.cognition.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Reading aloud boosts connectivity through the putamen. Cereb Cortex. 2010;20:570–582. doi: 10.1093/cercor/bhp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Wheless JW, Maggio WW, Fletcher JM, Castillo EM, Papanicolaou AC. Brain mechanisms for reading: the role of the superior temporal gyrus in word and pseudoword naming. Neuroreport. 2000;11:2443–2447. doi: 10.1097/00001756-200008030-00021. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Harris LJ. Handedness, sex, and familial sinistrality effects on spatial tasks. Cortex. 1993;29:115–134. doi: 10.1016/s0010-9452(13)80216-x. [DOI] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta-analysis. Hum Brain Mapp. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13:836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, Fox PT, Gao JH. Neural systems of second language reading are shaped by native language. Hum Brain Mapp. 2003;18:158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Resting functional connectivity of language networks: characterization and reproducibility. Mol Psychiatry. 2012;17:841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. TOWRE: Test of Word Reading Efficiency. Austin, TX: PRO-ED; 1999. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage. 2011;54:2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: what role for the Visual Word Form Area? Neuroimage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, Schlaggar BL. The putative visual word form area is functionally connected to the dorsal attention network. Cereb Cortex. 2012;22:537–549. doi: 10.1093/cercor/bhr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kuhl PK, Chen C, Dong Q. Sustained and transient language control in the bilingual brain. Neuroimage. 2009;47:414–422. doi: 10.1016/j.neuroimage.2008.12.055. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xue G, Chen C, Xue F, Dong Q. Neural bases of asymmetric language switching in second-language learners: an ER-fMRI study. Neuroimage. 2007;35:862–870. doi: 10.1016/j.neuroimage.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353:1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Wu X, Lu J, Chen K, Long Z, Wang X, Shu H, Li K, Liu Y, Yao L. Multiple neural networks supporting a semantic task: an fMRI study using independent component analysis. Neuroimage. 2009;45:1347–1358. doi: 10.1016/j.neuroimage.2008.12.050. [DOI] [PubMed] [Google Scholar]
- Xiang HD, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cereb Cortex. 2010;20:549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Cerebral asymmetry in the fusiform areas predicted the efficiency of learning a new writing system. J Cogn Neurosci. 2006a;18:923–931. doi: 10.1162/jocn.2006.18.6.923. [DOI] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Language experience shapes fusiform activation when processing a logographic artificial language: an fMRI training study. Neuroimage. 2006b;31:1315–1326. doi: 10.1016/j.neuroimage.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Xue G, Mei L, Chen C, Lu ZL, Poldrack RA, Dong Q. Facilitating memory for novel characters by reducing neural repetition suppression in the left fusiform cortex. PLoS ONE. 2010;5:e13204. doi: 10.1371/journal.pone.0013204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Li J, Chen C, Mei L, Xue G, Lu Z, He Q, Wei M, Dong Q. The contribution of the left mid-fusiform cortical thickness to Chinese and English reading in a large Chinese sample. Neuroimage. 2013;65:250–256. doi: 10.1016/j.neuroimage.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Chen C, Loftus EF, Lin C, He Q, Li H, Xue G, Lu Z, Dong Q. Individual differences in false memory from misinformation: cognitive factors. Memory. 2010;18:543–555. doi: 10.1080/09658211.2010.487051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.