Abstract
In clinical cardiac 82Rb PET, globally impaired coronary flow reserve (CFR) is a relevant marker for predicting short-term cardiovascular events. However, there is limited data on the impact of different software and methods for estimation of myocardial blood flow (MBF) and CFR. Our objective was to compare quantitative results obtained from previously validated software tools.
Methods
We retrospectively analyzed cardiac 82Rb PET/CT data from 25 subjects (group 1; 62 ± 11 yrs) with low-to-intermediate probability of coronary artery disease (CAD) and 26 patients (group 2; 57 ± 10 yrs; P = 0.07) with known CAD. Resting and vasodilator-stress MBF and CFR were derived using three software applications: 1) Corridor4DM (4DM), which is based on factor analysis (FA) and kinetic modeling according to Yoshida, 2) with a second option based on region-of-interest (ROI) and kinetic modeling according to Lortie, 3) MunichHeart (MH), which uses a simplified ROI-based-retention model approach and 4) FlowQuant (FQ) based on ROI and compartmental modeling with constant distribution volume (DV).
Results
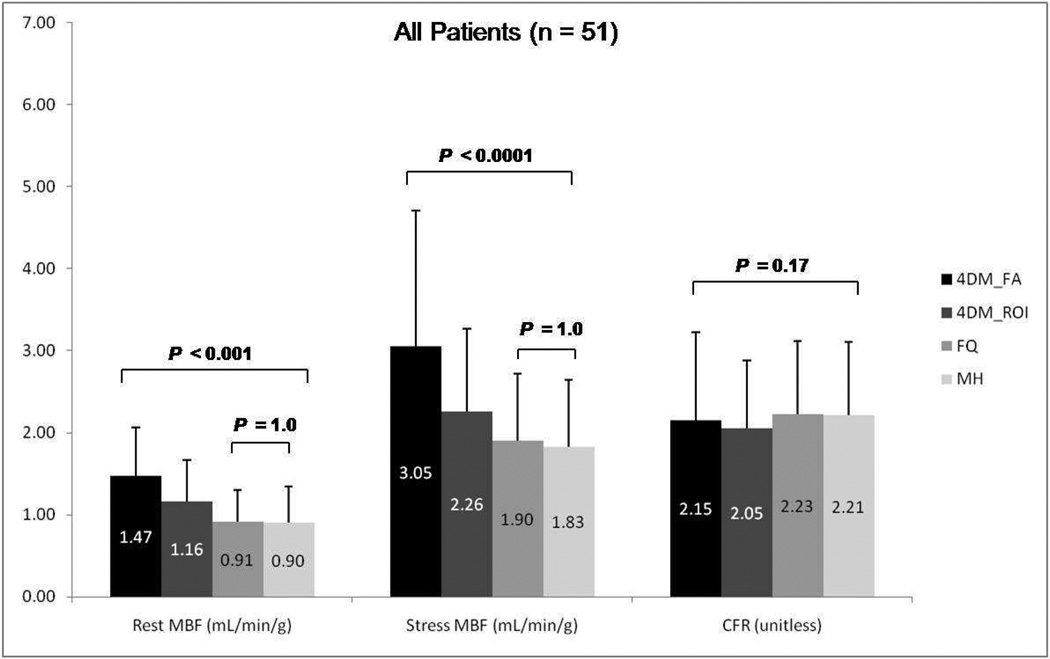
Rest (1.47 ± 0.59, 1.16 ± 0.51, 0.91 ± 0.39, 0.90 ± 0.44; P <0.001), and stress (3.05 ± 1.66, 2.26 ± 1.01, 1.90 ± 0.82, 1.83 ± 0.81; P < 0.001) MBF were significantly different between methods (4DM-FA, 4DM-ROI, FQ, and MH respectively). However there was no statistically significant difference of CFR values (2.15 ± 1.08, 2.05 ± 0.83, 2.23 ± 0.89, and 2.21 ± 0.90; P=0.17) between software tools. Regional MBF and CFR according to vascular territories showed similar results. Linear correlation coefficient for global CFR varied between 0.71 (MH vs. 4DM-ROI) and 0.90 (FQ vs. 4DM-ROI). Using a cutoff of 2.0 for abnormal CFR, the agreement among software programs ranged between 76% (MH vs. FQ) and 90% (FQ vs. 4DM-ROI). Interobserver agreement was in general excellent with all software packages.
Conclusion
Quantitative assessment of resting and stress MBF with 82Rb PET is dependent on the software and methods used, whereas CFR appears to be more comparable. Follow-up and treatment assessment should be done with the same software and method.
Keywords: 82Rb, cardiac PET/CT, myocardial blood flow, coronary flow reserve, kinetic modeling
INTRODUCTION
Myocardial perfusion imaging with 82-rubidium chloride (82Rb) ECG-gated PET/CT has become an important imaging modality for the evaluation of coronary artery disease (CAD) [1–3]. As compared to conventional cardiac SPECT, it has improved diagnostic quality, certainty, and accuracy [4–8]. To date, the interpretation of cardiac PET studies has relied on relative myocardial uptake and traditionally analyzed for the presence of relative regional perfusion defects, similar to cardiac SPECT [9].
However, this does not take advantage of the full potential of quantitative PET. If absolute flow is impaired globally, a comparison of the best and worst perfused regions will underestimate the severity of flow-limiting disease. This lowers the sensitivity of standard relative myocardial perfusion imaging (MPI) for the detection of multi-vessel CAD as compared with single-vessel disease, and of diffuse disease of the microvasculature. Quantification of absolute myocardial blood flow (MBF) and coronary flow reserve (CFR) is desirable [10]. Many researchers have shown the feasibility of MBF and CFR quantification with dynamic 82Rb PET [11–16].
There is cumulative evidence supporting the prognostic value of MBF and CFR in predicting adverse cardiac outcomes [17–22]. Assessment of CFR yields independent and added prognostic information beyond relative MPI and may improve risk stratification in patients investigated for myocardial ischemia.
For the routine clinical quantification and use of MBF and CFR, robust automated quantitative tools are required. Several software packages for absolute quantification of MBF are now in existence. Each package employs different methods of segmenting the left ventricle and sampling the counts in the myocardium and blood pool to obtain time-activity curves. Only one previous study using 13N-ammonia PET has tested the variability of myocardial flow quantification among different software packages and methods [23], however, there is no data on the impact of different software and methods for estimation of MBF and CFR using 82Rb PET.
In this study, we aimed to compare three software packages based on methodologies that have been tested and validated for the quantitative analysis of MBF and CFR with 82Rb myocardial perfusion PET: MunichHeart, Corridor4DM, and FlowQuant.
MATERIALS AND METHODS
Study Group
Cardiac PET/CT image data sets of 51 patients referred for MPI with 82Rb PET at the Johns Hopkins Hospital for the evaluation of ischemic heart disease were included in this study. These were divided into 2 groups: group 1 comprised 25 subjects (8 men and 17 women, Age: 62 ± 11 yrs) with low-to–intermediate probability (and no known history) of CAD, and group 2 comprised 26 patients with known obstructive CAD (14 men and 12 women, Age: 57 ± 10 yrs) as defined as patients with previous history of percutaneous or surgical coronary revascularization.
PET/CT Protocol
All imaging was performed on a 64-slice Discovery Rx VCT PET/CT scanner (GE Medical Systems, Waukesha, WI). Individuals were positioned using a scout scan, and a low-dose CT scan (120 kV, 50–100 mA) for attenuation correction of PET data was acquired during shallow breathing. Using a large intravenous line, 1,480–1,850 MBq [40–50 mCi] of 82Rb (CardioGen-82, Bracco Diagnostics) was infused at 50 mL/min over 30 seconds, and a list-mode 2-D PET scan was acquired for 8 minutes. Vasodilator stress with Dipyridamole (0.56 mg/kg, 4 min) was then started after the rest acquisition, and a second dose of 1,480–1,850 MBq [40–50 mCi] of 82Rb was injected, starting 4 minutes after the end of the dipyridamole infusion, followed by acquisition of an 8-minute list-mode PET scan. Subsequently, rest and stress PET data were aligned with the CT scan, and attenuation correction was performed [24]. The list-mode data were used to reconstruct dynamic images (32 frames for 8 min: 20 × 6 s, 5 × 12 s, 4 × 30 s, and 3 × 60 s). The images were filtered with a Butterworth filter cut off at 20.1 mm. The pixel size was 3.27 mm.
Myocardial Blood Flow Analysis
The three software tools were used to quantify global and regional MBF values in mL/min/g for all 51 PET/CT rest/stress studies. Coronary flow reserve, as the ratio of global stress to rest MBF, was determined for all 51 subjects. Left ventricular (LV) contours and the input function region were obtained automatically with minimal operator intervention in Corridor4DM (Invia, Ann Arbor, Michigan) and FlowQuant (University of Ottawa, Canada) and semi-automatically with MunichHeart (Munich, Germany). Each dataset was analyzed independently by two different expert operators for each software program, who were blind to the results of the other observer.
MunichHeart Analysis
Quantification of the custom software MunichHeart has been previously validated at our institution [15, 17, 25]. Myocardial activity in the last frame of the dynamic dataset is volumetrically sampled using regions of interest (ROI) and polar maps of the LV are generated. Segments are applied to the whole dynamic series to obtain myocardial time-activity curves. Arterial input function is calculated by a small cuboidal region of interest in the center of the LV cavity defined in short-axis planes. MBF is then quantified using a simplified retention approach. Myocardial activity concentration between minutes 4 and 8 is normalized to the area under the arterial input function in the first 120 seconds based on the assumption that myocardial tracer retention is stable during this interval. The resulting index is corrected for partial volume losses, spill-over and nonlinear extraction of 82Rb. Left ventricular (global) MBF (mL/min/g of myocardial tissue) is obtained at peak stress and rest and the ratio of peak stress flow to rest flow is used to obtain global CFR.
Corridor4DM Analysis
The quantitative dynamic analysis software Corridor4DM is based on a methodology that was previously validated [13, 14, 19]. The software uses generalized factor analysis of dynamic sequences (GFADS). The time-activity curves globally and in each vascular and 17-segment polar map sections are modeled as a combination of three contributions: the contribution from myocardial tissue, modeled with compartment analysis, and contributions from right ventricular (RV) and LV blood pools, modeled as fractions of measured LV and RV input functions. LV and RV input functions are obtained automatically as the LV and RV factors estimated for the whole factor image. The user input consisted of choosing the parameters of the kinetic model, here done according to Yoshida [12]. MBF is obtained by fitting the 82Rb time–activity curves to a 2-compartment kinetic model. The main parameters of the model are the kinetic transport constants K1 (mL/min/g) and k2 (1/min), which denote the extraction (forward) and egress (backward) rates of transport between the metabolically trapped space (myocardium) and the freely diffusible space (blood pool), respectively. To estimate MBF from measures of K1, the extraction fraction reported previously for an open-chest procedure on dogs by Yoshida et al. is used [12]. The result is a parametric 17-segment polar map. We also used a second option of determining MBF with the Corridor4DM, based on ROI methodology and the 1-tissue-compartment kinetic modeling according to Lortie [16].
FlowQuant Analysis
FlowQuant (University of Ottawa, Canada) has been previously validated [21, 26] for MBF quantification with 82Rb PET. The rest-stress workflow starts with automatic processing of the rest scan, followed by a nearly identical process for the stress scan, and ending with the stress-rest flow reserve analysis. Uptake images are generated by averaging the last 5 time frames (4 minutes) to maintain high myocardium-to-blood pool contrast and reduce image noise. The uptake images are automatically processed to detect the location, orientation, and size of the LV myocardium. Three blood ROI are placed automatically in the LV cavity, base, and left atrium. In the myocardium, a time activity curve is generated for 576 sample points (36 × 16 rings). The uptake rate of 82Rb, K1 mL/min/g, is quantified using a one-tissue-compartment constant distribution volume (DV) model at both rest and stress states. DV is set to a scan-specific, constant value determined by fitting the unconstrained model to the region of highest uptake in the polar map. K1 is related to flow, MBF in mL/min/g, through an extraction model based on 13N-ammonia MBF measured in humans by Lortie [16].
Quantitative Regional Analysis
Each software package provides default options for regional analysis of the myocardial flow polar map. As such, ROI were applied to each flow polar map to obtain quantitative flow data in the coronary arteries distribution: left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA).
Statistical Analysis
Statistical analysis was performed using SPSS (version 20.0, IBM, Chicago, IL) and MedCalc (version 12.3, MedCalc Software, Mariakerke, Belgium). Continuous variables are presented as mean ± SD. A repeated-measures ANOVA with a Greenhouse-Geisser correction was performed with post -hoc tests using Bonferroni correction. For characterization of inter-observer variability, Pearson correlation coefficients were calculated. The intraclass correlation coefficient (ICC) was also computed. A P value of less than 0.05 was considered statistically significant.
RESULTS
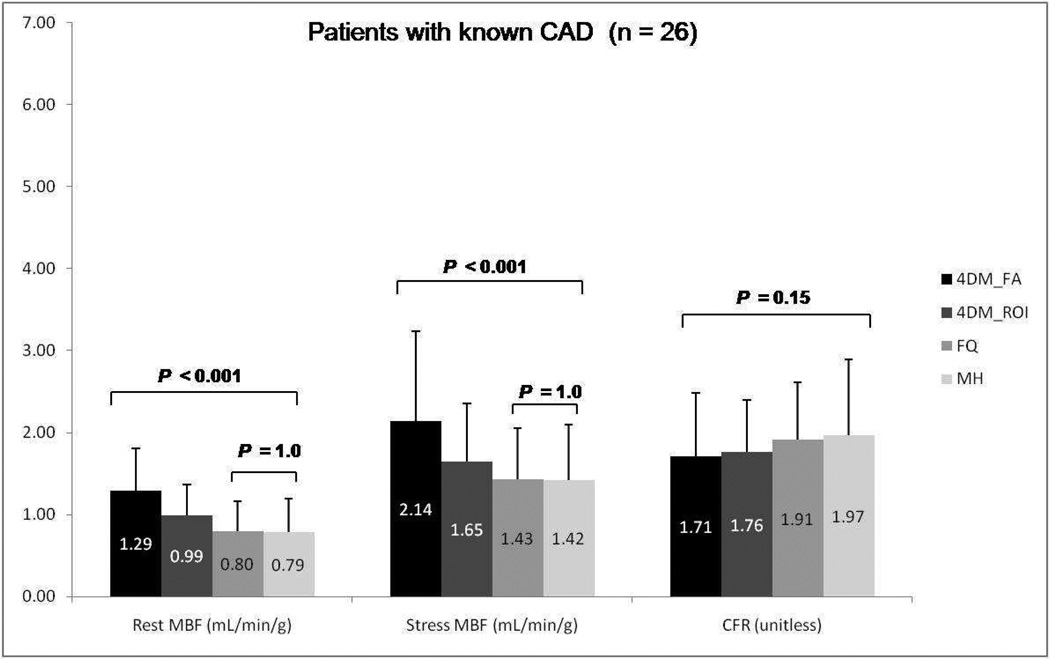
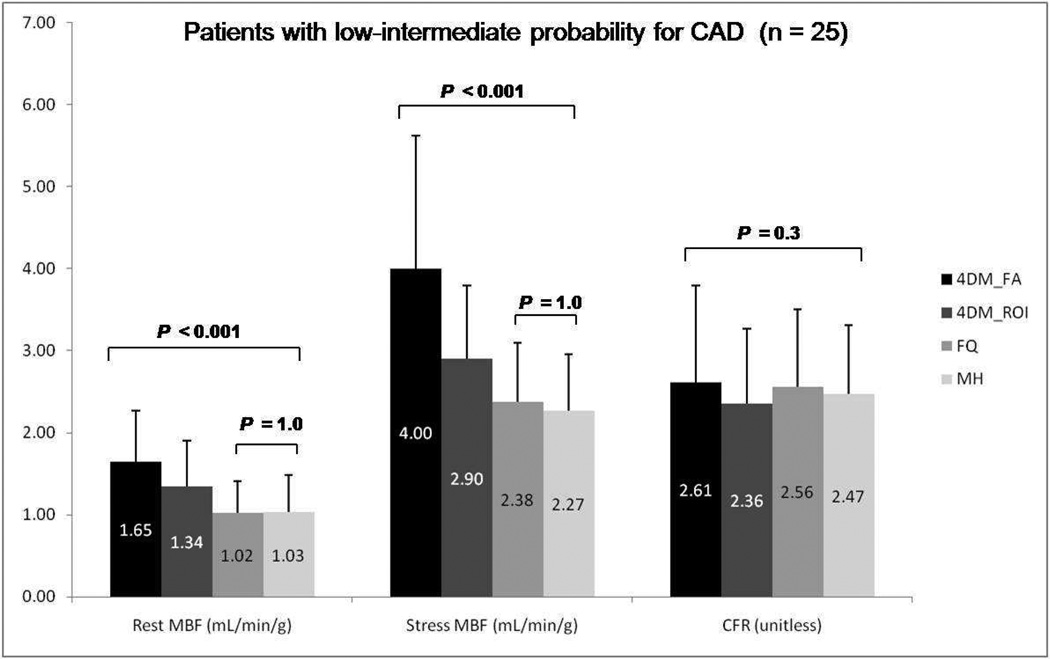
Results for mean rest and stress global MBF along with CFR values using the three different software packages employed in this study are summarized in Figures 1–3. MBF for both stress and rest scans were significantly higher with Corridor4DM as compared to the other two programs (P < 0.001). No significant difference for CFR quantification among all three methods was found (P=0.17). No significant statistical difference was noted between MunichHeart and FlowQuant for MBF quantification at rest or stress images (P=0.1) as summarized in Figure 1. The same statistical trend in differences in MBF and CFR quantification was noted between the three software packages when the analysis was applied separately to the patient subgroups considering prior history or no history of CAD (Figure 2 and 3).
Figure 1.
Group mean rest and stress global myocardial blood flow (MBF) and coronary flow reserve (CFR) among Corridor4DM based on factor analysis, 4DM based on ROI, FlowQuant based on constant DV model, and MunichHeart based on retention model. All 51 patients included. (MH: MunichHeart, 4DM: Corridor4DM, FA: factor analysis, ROI: region of interest, FQ: FlowQuant)
Figure 3.
Group mean rest and stress global myocardial blood flow (MBF) and coronary flow reserve (CFR) among Corridor4DM, FlowQuant, and MunichHeart. Only patients with prior history of CAD included. (MH: MunichHeart, 4DM: Corridor4DM, FA: factor analysis, ROI: region of interest, FQ: FlowQuant)
Figure 2.
Group mean rest and stress global myocardial blood flow (MBF) and coronary flow reserve (CFR) among Corridor4DM, FlowQuant, and MunichHeart. Only patients with low to intermediate probability for CAD included. (MH: MunichHeart, 4DM: Corridor4DM, FA: factor analysis, ROI: region of interest, FQ: FlowQuant)
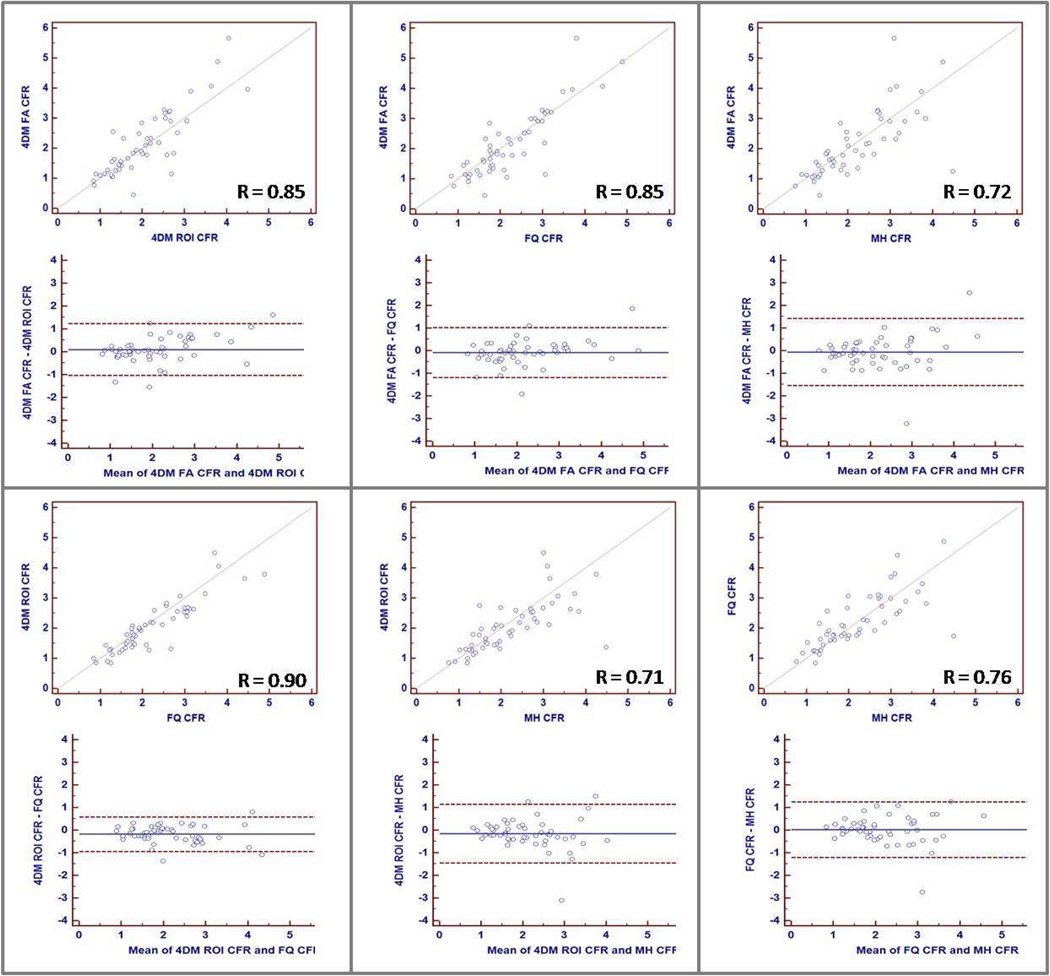
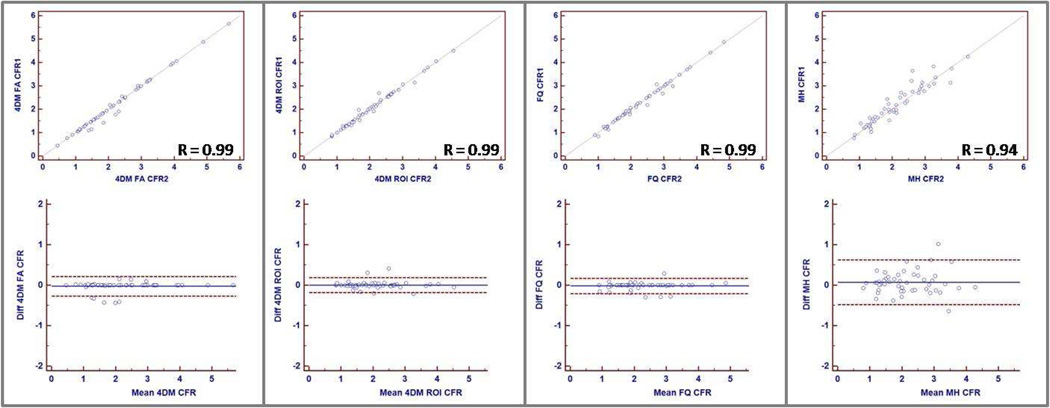
As a means of comparing CFR results from all four methods at the individual patient level, Figure 4 shows linear fit and Bland–Altman plots for CFR results between pairs taken from all four methods (6 pairs). Linear correlation coefficient varied between 0.71 (MunichHeart vs. 4DM ROI Lortie) and 0.90 (FlowQuant vs. 4DM ROI Lortie).
Figure 4.
Variation for CFR as calculated by MunichHeart, Corridor4DM (FA Yoshida and ROI Lortie), and FlowQuant. Upper panel shows graphs depicting linear correlation between observer pairs of methods. Lower panel shows Bland-Altman plots with lines drawn at mean +/− 1.96 SD.
Patient classification agreement between the three software packages was evaluated using a CFR cutoff of 2.0, which is often reported as the value of CFR that separates normal versus abnormal CFR. This has added prognostic value as shown by many groups using 82Rb or 13N-ammonia cardiac PET. Good agreement was reported and varied between 76% and 90% as shown in table 1.
Table 1.
Agreement in patient classification between the 3 software packages considering a cut of 2.0 in CFR, as a threshold between normal and abnormal CFR. (MH: MunichHeart, 4DM: Corridor4DM, FA: factor analysis, ROI: region of interest, FQ: FlowQuant)
| Total = 51 | MH | Total = 51 | 4DM (FA) | ||||
| ≥2.0 | < 2.0 | ≥2.0 | < 2.0 | ||||
| 4DM (ROI) | ≥2.0 | 21 | 3 | MH | ≥2.0 | 20 | 5 |
| <2.0 | 4 | 23 | <2.0 | 3 | 23 | ||
| Agreement 86% | Agreement 84% | ||||||
| Total = 51 | 4DM (FA) | Total = 51 | 4DM (FA) | ||||
| ≥2.0 | <2.0 | ≥2.0 | < 2.0 | ||||
| 4DM (ROI) | ≥2.0 | 19 | 5 | FQ | ≥2.0 | 19 | 6 |
| <2.0 | 4 | 23 | <2.0 | 4 | 22 | ||
| Agreement 82% | Agreement 80% | ||||||
| Total = 51 | FQ | Total = 51 | FQ | ||||
| ≥2.0 | <2.0 | ≥2.0 | < 2.0 | ||||
| 4DM (ROI) | ≥2.0 | 22 | 2 | MH | ≥2.0 | 19 | 6 |
| <2.0 | 3 | 24 | <2.0 | 6 | 20 | ||
| Agreement 90% | Agreement 76% | ||||||
Inter-operator variability of CFR was excellent with all three software packages (Figure 5). MunichHeart, which requires operator input for the manual orientation and short axis sectioning of the LV was found to have slightly higher operator variability as compared to the other 2 software packages (R= 0.94 for MunichHeart, compared to 0.99 for Corridor4DM ROI, Corridor 4DM FA, and FlowQuant), which are nearly fully-automated. Correspondingly, the degree of absolute agreement as measured by ICC was 0.997 for FlowQuant, 4DM FA and ROI. Interobserver ICC was 0.970 for MunichHeart. Figure 5 shows correlation and Bland–Altman plots for the two observers for all four methods.
Figure 5.
Interobserver variation for CFR as calculated by Corridor4DM (FA Yoshida and ROI Lortie), FlowQuant, and MunichHeart. Upper panel shows graphs depicting linear correlation between observer 1 and 2 for each software package. Lower panel shows Bland-Altman plots with lines drawn at mean +/− 1.96 SD.
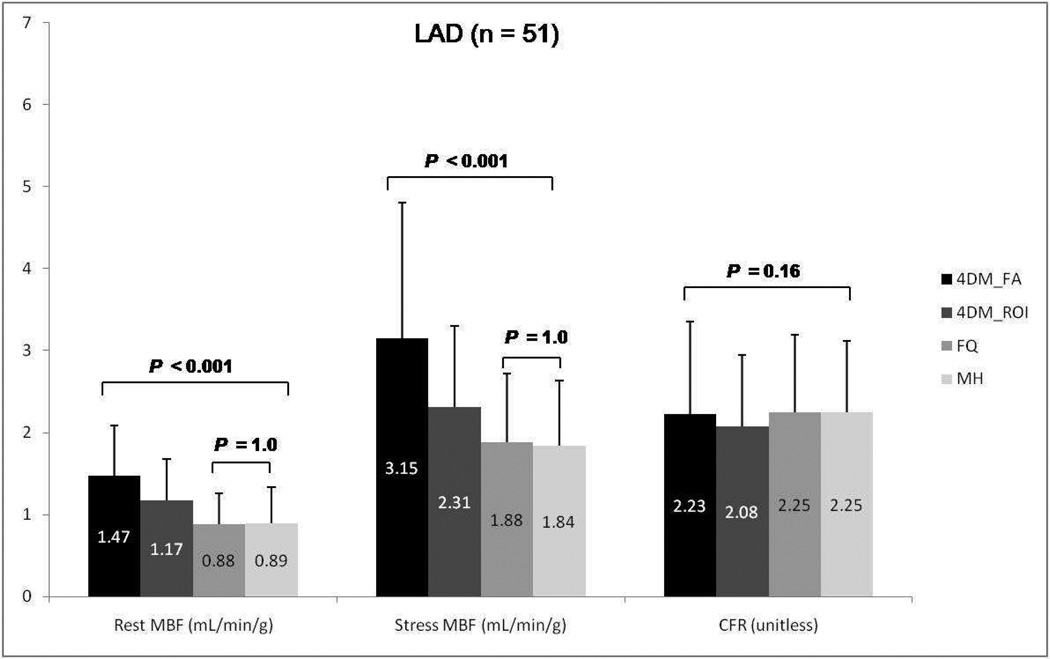
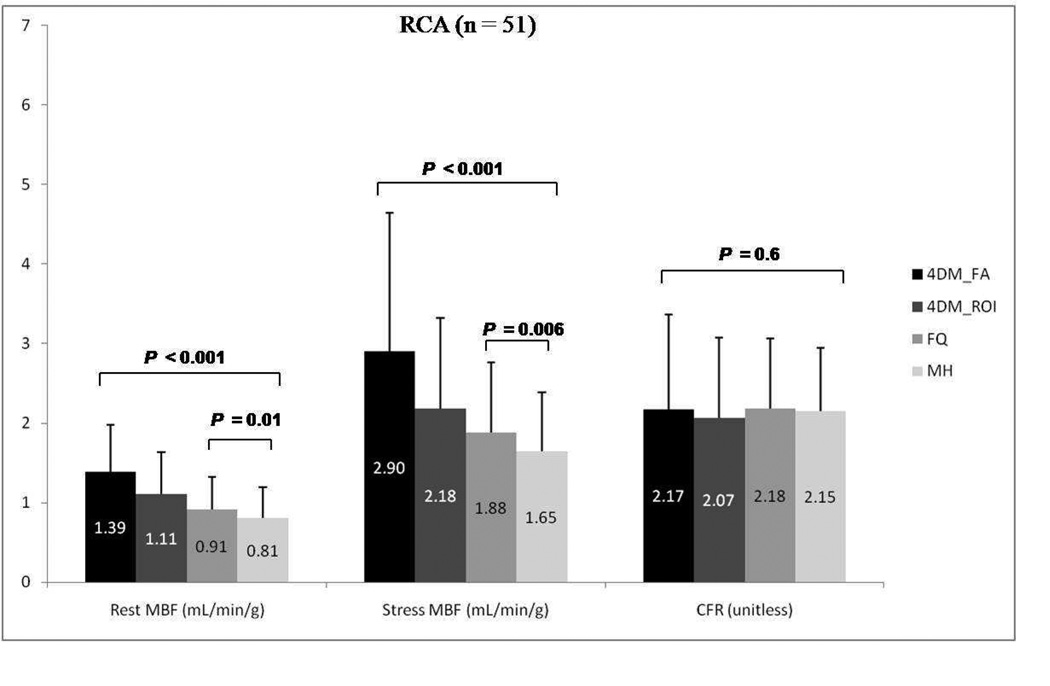
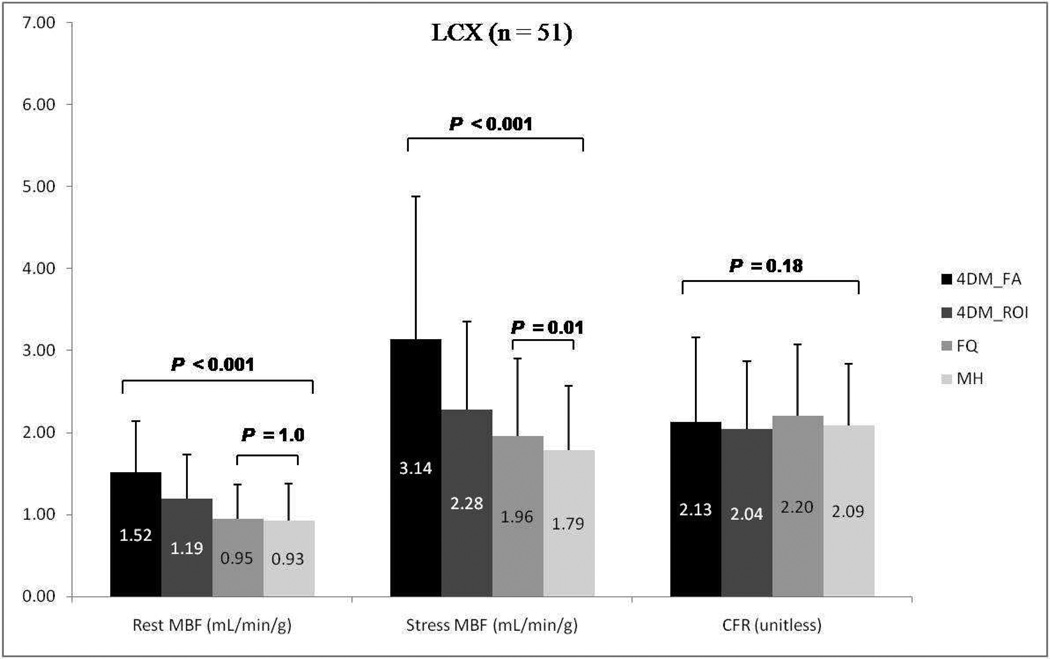
Results for mean rest and stress regional MBF along with CFR according to vascular territories (LDA, LCX, and RCA) using the three different software packages are summarized in Figures 6–8. A similar trend to global blood flow values is seen for each vascular region with statistically significant differences in rest and stress MBF values, but similar CFR results. Intersoftware ICC ranged between 0.93 and 0.95 for regional CFR, was 0.82 for regional stress MBF, and between 0.86 and 0.87 for regional rest MBF.
Figure 6.
Group mean regional rest and stress myocardial blood flow (MBF) and coronary flow reserve (CFR) for the left descending artery (LAD) among Corridor4DM based on factor analysis, 4DM based on ROI, FlowQuant, and MunichHeart. All 51 patients included. (4DM: Corridor4DM, FA: factor analysis, ROI: region of interest, FQ: FlowQuant, MH: MunichHeart)
Figure 8.
Group mean regional rest and stress myocardial blood flow (MBF) and coronary flow reserve (CFR) for the right coronary artery (RCA) among Corridor4DM based on factor analysis, 4DM based on ROI, FlowQuant, and MunichHeart. All 51 patients included. 4DM: Corridor4DM, FA: factor analysis, ROI: region of interest, FQ: FlowQuant, MH: MunichHeart)
DISCUSSION
The main findings of our study are as follows: 1) absolute MBF quantification, both at rest and under pharmacologic stress, yields significantly higher values using Corridor4DM compared to the packages FlowQuant and MunichHeart, particularly when using the GFADS method. In contrast CFR, which is the ratio of stress/rest MBF, appears more similar among the different software packages/methods, 2) CFR values correlated best between FlowQuant and Corridor4DM-ROI and least between MunichHeart and Corridor4DM-FA, 3) the binary agreement between the three software packages when considering a CFR cutoff of 2.0 was highest between FlowQuant and Corridor 4DM-ROI, and lowest between MunichHeart and Corridor4DM-FA and 4) interoperator reproducibility was excellent with FlowQuant and Corridor 4DM and very good with MunichHeart.
To compute absolute flow, several corrections must be made to the data including correcting the myocardial activity for partial volume losses and for spillover from blood-pool activity to the myocardium, correction of blood-pool activity or arterial input for myocardial to blood-pool spillover, and correcting both myocardial and blood-pool activity for random coincidences, physical decay and dead time losses. Reliable tools for the fully automated computation of global and regional blood flow are necessary. Recently, several software packages for absolute MBF quantification have been developed and are being commercialized. This has the potential of making absolute myocardial flow measurements more available in clinical practice and not restricted to academic centers.
In this study, we quantitatively compared and evaluated three such software packages, which were previously tested and validated. Corridor4DM and FlowQuant are fully automated. MunichHeart is semi-automated and relies on operator delineation and orientation of the LV. All three software programs are comprehensive packages and allow the user the choice of different methodologies and kinetic modeling for MBF quantification. We restricted our comparisons to the default methods as recommended by the developers, which were unique to each package. Many researchers have shown the feasibility of MBF and CFR with dynamic 82Rb PET [11–16]. This tracer does not require an on-site cyclotron for its synthesis and thus, it is more available for clinical cardiac PET in the community and has a higher potential for widespread clinical use compared to 13N-ammonia.
Not with standing the different methodologies of the three software applications for finding and segmenting the myocardium and blood pool automatically by Corridor4DM and FlowQuant and semi-automatically by MunichHeart, and different ways of calculating the input and myocardial time-activity curves, no statistically significant differences were found between FlowQuant and MunichHeart in the quantitative determination of global MBF under rest and pharmacological stress conditions. There were however statistically significant differences between MBF values as calculated by Corridor4DM (using either Factor Analysis according to Yoshida, or ROI-based by Lortie, default methods for the application) and FlowQuant and MunichHeart. Interestingly, no statistical difference was found among the three packages in the estimation of global CFR, except for a trend of lower values using ROI-based 4DM.
Previous studies have shown that a CFR threshold of 2.0 separates lower from higher risk individuals for cardiovascular events and therefore warrants prognostic considerations [17–22]. In this regard, agreement between the three software packages when considering a CFR cutoff of 2.0 was good but not excellent. The software agreement varied between 76% and 90%, being highest between FlowQuant and Corridor4DM (ROI). Similarly, global CFR values showed better correlations between FlowQuant and Corridor4DM-ROI and lesser associations between MunichHeart and Corridor4DM.
These discrepancies were likely due to many factors. Different methodologies of segmenting the myocardium and blood pool and different ways of calculating the input and myocardial time-activity curves may play a role. In this regard, both FlowQuant and Corridor4DM-ROI use a similar LV segmentation (ROI-based) and 1-tissue-compartment kinetic modeling according to Lortie, which likely explain the better correlation between these two packages. On the contrary, MunichHeart and Corridor4DM-FA, which employ different LV segmentation (ROI vs. FA) and kinetic modeling (retention vs. compartment) methods, yielded lesser correlations. Corridor4DM uses the concept of Factor Analysis and is unique to this program [13, 14, 27]. The time–activity curve in each 17-segment polar map section is modeled as a combination of myocardial tissue, modeled with compartment analysis, and contributions from RV and LV blood pools, modeled as fractions of measured LV and RV input functions. The spillover is modeled differently in these packages and may lead to differences in quantification of the MBF. Furthermore, it has been recently shown that spatially filtering the data affects the flow values from the 4DM-FA algorithm, especially a lower filter cutoff frequency increases the flow values because the peak activity in the whole image is decreased by smoothing which affects the scaling of the time-activity curves [28]. For the 4DM-FA algorithm, minimal filtering is recommended to improve the MBF estimates [28] and further provide lower standard error than that of ROI methods [29]. Mean CFR values were relatively constant due to similar changes in both stress and rest MBFs.
Not unexpected, the interobserver reproducibility shows better results for the fully automated programs Corridor4DM and FlowQuant over MunichHeart, which is semi-automated, however, automation may not always yield accurate results. In cases of strong subdiaphragmatic radiotracer uptake and scatter, automatic recognition of myocardium may not work and observer intervention may be needed. Fortunately, optional operator intervention is available in FlowQuant and Corridor4DM.
This study had several limitations worth mentioning. A gold standard by which to evaluate the accuracy of MBF measurements is lacking. A large animal study comparing myocardial flow from 82Rb using different kinetic models and microspheres would be ideal, but is also limited by anatomical and pathophysiological differences between animals and humans. An alternative would be to include human studies comparing 82Rb with 15O-water. Our goal, however, was to compare and analyze the results from the clinical implementation of outcome-validated kinetic modeling software tools, using the same scanner and reconstruction protocol within the same patient population. In this work, we studied only 82Rb MBF.
A recent study by Slomka et al. compared three software programs for 13N-ammonia (QPET, syngo MBF, and PMOD) and found excellent correlation between packages [23]. The reason for greater discrepancies in this work may be attributable to fundamentally different tracer kinetic-modeling approaches and the need for extraction correction of K1 values in order to estimate MBF values. 82Rb extraction is MBF dependent and highly non-linear. Model specific extraction functions have previously been demonstrated and can serve to correct for biases in MBF resulting from the image acquisition, reconstruction, and analysis processes [30, 31].
One needs to be aware of these limitations when using 82Rb and that numbers cannot be extrapolated from one software to the other, especially the absolute flow values. Similarly, further discrepancies in reference values between facilities may be possible due to differences in the scanner type, reconstruction, and dynamic protocols applied. Finally, we are aware that the sample size in our study was relatively small, yet, the myocardial flow differences seen among the various software packages were maintained between patients with low-intermediate probability for CAD (higher flow parameters) and those with known CAD (lower flow values), implying that our results are likely reliable across a wide range of myocardial flow values.
CONCLUSION
Quantification of resting and stress MBF is dependent on the software and methods used. CFR, on the other hand, appears to be more comparable. Threshold of abnormal CFR is software-dependant. Normal reference range should be sought and fixed for each application separately. As a consequence, follow-up and treatment assessment should be done with same software and methods.
Figure 7.
Group mean regional rest and stress myocardial blood flow (MBF) and coronary flow reserve (CFR) for the left circumflex artery (LCX) among Corridor4DM based on factor analysis, 4DM based on ROI, FlowQuant, and MunichHeart. All 51 patients included. (4DM: Corridor4DM, FA: factor analysis, ROI: region of interest, FQ: FlowQuant, MH: MunichHeart)
ACKNOWLEDGMENTS
AKT was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number T32EB006351.
COMPETING INTERESTS:
RK and RdeK acknowledge receiving revenue shares from the sale of FlowQuant. EF acknowledges receiving revenue shares from the sale of Corridor4DM. BL acknowledges receiving financial support from INVIA.
REFERENCE
- 1.Machac J. Cardiac positron emission tomography imaging. Elsevier; 2005. pp. 17–36. [DOI] [PubMed] [Google Scholar]
- 2.Bengel FM, Higuchi T, Javadi MS, Lautamäki R. Cardiac positron emission tomography. Journal of the American College of Cardiology. 2009;54:1–15. doi: 10.1016/j.jacc.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 3.Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–1480. doi: 10.1161/CIRCULATIONAHA.106.629808. [DOI] [PubMed] [Google Scholar]
- 4.Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. Journal of nuclear cardiology. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. Journal of the American College of Cardiology. 2007;49:1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Lertsburapa K, Ahlberg AW, Bateman TM, Katten D, Volker L, Cullom SJ, et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. Journal of nuclear cardiology. 2008;15:745–753. doi: 10.1007/BF03007355. [DOI] [PubMed] [Google Scholar]
- 7.Yoshinaga K, Chow BJW, Williams K, Chen L, dekemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? Journal of the American College of Cardiology. 2006;48:1029–1039. doi: 10.1016/j.jacc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Flotats A, Bravo PE, Fukushima K, Chaudhry MA, Merrill J, Bengel FM. (8)(2)Rb PET myocardial perfusion imaging is superior to (9)(9)mTc-labelled agent SPECT in patients with known or suspected coronary artery disease. Eur J Nucl Med Mol Imaging. 2012;39:1233–1239. doi: 10.1007/s00259-012-2140-x. [DOI] [PubMed] [Google Scholar]
- 9.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, et al. Single photon-emission computed tomography. Journal of nuclear cardiology. 2010;17:941–973. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 10.Bengel FM. Leaving Relativity Behind: Quantitative Clinical Perfusion Imaging. Journal of the American College of Cardiology. 2011;58:749. doi: 10.1016/j.jacc.2011.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Herrero P, Markham J, Shelton ME, Bergmann SR. Implementation and evaluation of a two-compartment model for quantification of myocardial perfusion with rubidium-82 and positron emission tomography. Circulation research. 1992;70:496–507. doi: 10.1161/01.res.70.3.496. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida K, Mullani N, Gould KL. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. Journal of Nuclear Medicine. 1996;37:1701–1712. [PubMed] [Google Scholar]
- 13.El Fakhri G, Sitek A, Guérin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. Journal of Nuclear Medicine. 2005;46:1264–1271. [PubMed] [Google Scholar]
- 14.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with 82Rb PET: comparison with 13N-ammonia PET. Journal of Nuclear Medicine. 2009;50:1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lautamäki R, George RT, Kitagawa K, Higuchi T, Merrill J, Voicu C, et al. Rubidium-82 PET-CT for quantitative assessment of myocardial blood flow: validation in a canine model of coronary artery stenosis. European journal of nuclear medicine and molecular imaging. 2009;36:576–586. doi: 10.1007/s00259-008-0972-1. [DOI] [PubMed] [Google Scholar]
- 16.Lortie M, Beanlands RSB, Yoshinaga K, Klein R, DaSilva JN, DeKemp RA. Quantification of myocardial blood flow with 82 Rb dynamic PET imaging. European journal of nuclear medicine and molecular imaging. 2007;34:1765–1774. doi: 10.1007/s00259-007-0478-2. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima K, Javadi MS, Higuchi T, Lautamäki R, Merrill J, Nekolla SG, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. Journal of Nuclear Medicine. 2011;52:726–732. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 18.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-Term Prognostic Value of <sup>13</sup>N-Ammonia Myocardial Perfusion Positron Emission Tomography: Added Value of Coronary Flow Reserve. Journal of the American College of Cardiology. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 19.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved Cardiac Risk Assessment With Noninvasive Measures of Coronary Flow Reserve Clinical Perspective. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tio RA, Dabeshlim A, Siebelink HMJ, De Sutter J, Hillege HL, Zeebregts CJ, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. Journal of Nuclear Medicine. 2009;50:214–219. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 21.Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. Journal of the American College of Cardiology. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 22.Fiechter M, Gebhard C, Ghadri JR, Fuchs TA, Pazhenkottil AP, Nkoulou RN, et al. Myocardial perfusion imaging with <sup>13</sup>N-Ammonia PET is a strong predictor for outcome. International Journal of Cardiology. 2012 doi: 10.1016/j.ijcard.2012.03.076. [DOI] [PubMed] [Google Scholar]
- 23.Slomka PJ, Alexanderson E, Jácome R, Jiménez M, Romero E, Meave A, et al. Comparison of Clinical Tools for Measurements of Regional Stress and Rest Myocardial Blood Flow Assessed with 13N-Ammonia PET/CT. Journal of Nuclear Medicine. 2012;53:171–181. doi: 10.2967/jnumed.111.095398. [DOI] [PubMed] [Google Scholar]
- 24.Rajaram M, Tahari AK, Lee AH, Lodge MA, Tsui B, Nekolla S, et al. Cardiac PET/CT misregistration causes significant changes in estimated myocardial blood flow. J Nucl Med. 2013;54:50–54. doi: 10.2967/jnumed.112.108183. [DOI] [PubMed] [Google Scholar]
- 25.Nekolla SG, Miethaner C, Nguyen N, Ziegler SI, Schwaiger M. Reproducibility of polar map generation and assessment of defect severity and extent assessment in myocardial perfusion imaging using positron emission tomography. European journal of nuclear medicine and molecular imaging. 1998;25:1313–1321. doi: 10.1007/s002590050301. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Renaud JM, Ziadi MC, Thorn SL, Adler A, Beanlands RS, et al. Intra-and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 pet and a highly automated analysis program. Journal of nuclear cardiology. 2010;17:600–616. doi: 10.1007/s12350-010-9225-3. [DOI] [PubMed] [Google Scholar]
- 27.Sitek A, Gullberg GT, Huesman RH. Correction for ambiguous solutions in factor analysis using a penalized least squares objective. Medical Imaging, IEEE Transactions on. 2002;21:216–225. doi: 10.1109/42.996340. [DOI] [PubMed] [Google Scholar]
- 28.Lee B, Moody J, Sitek A, Murthy V, Di Carli M, Corbett J, et al. Effects of filtering on Rb-82 myocardial blood flow estimates. Journal of Nuclear Medicine. 2013;54(Supplement):1659. [Google Scholar]
- 29.Moody JB, Lee BC, Ficaro EF. Error estimation for dynamic PET myocardial blood flow. J Nucl Med. 2012 Sep.v.53(Supplement):323. [Google Scholar]
- 30.Klein R, Beanlands RS, deKemp RA. Quantification of myocardial blood flow and flow reserve: Technical aspects. J Nucl Cardiol. 2010;17:555–570. doi: 10.1007/s12350-010-9256-9. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Beanlands RS, Wassenaar RW, Thorn SL, Lamoureux M, DaSilva JN, et al. Kinetic model-based factor analysis of dynamic sequences for 82-rubidium cardiac positron emission tomography. Med Phys. 2010;37:3995–4010. doi: 10.1118/1.3438474. [DOI] [PubMed] [Google Scholar]