Abstract
Objective
To determine the effect of age on weight loss and weight loss maintenance in participants in the Weight Loss Maintenance trial (WLM).
Design and Methods
We conducted secondary analysis of a randomized controlled trial of overweight/obese adults with CVD risk factors. Participants were 1685 adults with baseline BMI 25–45 kg/m2 with hypertension and/or dyslipidemia. Those who lost at least 4kg in an initial 6-month behavioral weight loss intervention (N=1032) were randomly assigned to a 30-month maintenance phase of self-directed control (SD), monthly personal counseling (PC), or unlimited access to an internet-based intervention (IT). Age groups were defined post-hoc and weight change was compared among age groups.
Results
Participants ≥ 60 years old initially lost more weight than younger individuals, and sustained greater weight loss in IT and PC but not in SD (p-value for trend 0.024, 0.002, and 0.36, respectively).
Conclusions
In WLM, adults age ≥ 60 years had greater initial weight loss and greater sustained weight loss over 3 years, compared to younger adults. Older adults had greater weight loss maintenance with either personal counseling or internet-based intervention. Future research should determine optimal implementation strategies and effects of weight loss on health outcomes in older adults.
Keywords: obesity, weight loss, behavioral intervention, aging, older adults
Introduction
Currently 71% of Americans age ≥ 60 years (“older adults”) are overweight or obese.1 The majority of these individuals have some combination of obesity-related CVD risk factors and/or physical limitation that can be improved with weight loss.2 For example, 66% of older adults have hypertension, 18% have diabetes mellitus, 51% have metabolic syndrome, and 32% report difficulty walking ¼ mile. Given the high risk of CVD and physical decline in the aging US population, effective weight loss strategies have the potential for reducing disability, morbidity and death in older adults. However, the current weight loss guidelines from the National Institutes of Health3 cite inadequate evidence in adults aged 65 and older, but suggest that decisions should be made clinically based on potential benefits and risks as well as patient motivation.
Behavioral weight loss interventions result in clinically significant weight loss4, but the impact of age on responsiveness to behavioral intervention has not been well studied.5 In particular, there is currently great interest in technology-based interventions, but prior studies that have used behavioral weight loss interventions and reported results in older age groups have generally not had technology-based components.6,7 Since older adults are thought to be less familiar with and have lower rates of utilization of the internet and other technologies, particularly when we were enrolling for this study (2003–04)8, we speculated that technical modalities of delivering intervention would not be effective in older adults. Therefore, we evaluated the effectiveness of behavioral weight loss interventions, including an intervention delivered by internet, in different age groups in the Weight Loss Maintenance trial (WLM).9
Methods and procedures
WLM was an NHLBI-sponsored multicenter randomized controlled trial that compared strategies for sustained (3-year) weight loss in a diverse population with CVD risk factors.9 The study design and main results have been published.10 After an initial weight loss phase (Phase I) in which all participants received a 6 month intensive behavioral intervention, participants who lost at least 4 kg (an amount previously associated with prevention of hypertension and diabetes11,12) were randomly assigned to one of three 30-month maintenance conditions: a self-directed control without further intervention (SD), monthly personal counseling (PC), or unlimited access to an internet-based intervention (IT).
WLM was approved by the Institutional Review Board at each of the four participating clinical sites and the Coordinating Center, and by an NHLBI-appointed Protocol Review Committee. All participants provided written informed consent, and a Data and Safety Monitoring Board (DSMB) provided trial oversight.
Post hoc, we defined three subgroups based on age at entry: 50 or less, 51–60, and over 60 years. These age groupings were defined to maximize power for within- and between-group comparisons while also maintaining a meaningful separation between groups, and to allow us to focus on those over 60, the population with the greatest burden of cardiovascular risk factors. In this analysis, we compare weight change among these three age groups.
Participants
WLM participants were overweight or obese adults (body mass index [BMI] 25–45 kg/m2) who were at least 25 years old (no upper age limit), were taking medication for hypertension and/or dyslipidemia, and had telephone and internet access. Participants were excluded if they had a recent cardiovascular event or active malignancy; weight loss of greater than 9 kg in the last 3 months; recent use of weight loss medications; or weight loss surgery. Individuals with diabetes mellitus were also excluded. The primary criterion for randomization into Phase II was weight loss of at least 4 kg during Phase I.
Participant Flow
All eligible participants participated in the 6-month Phase I weight-loss program. Those who completed Phase I and met criteria for Phase II were randomized. Enrollment into Phase I occurred between August 2003 and July 2004; enrollment into Phase II occurred from February to December, 2004. Phase II data collection was completed in June 2007.
Measurements
Data collection visits occurred at the beginning of Phase I (“entry”), at the end of Phase I (i.e., at randomization) and every 6 months after randomization for 30 months (2½ years). Measurements were obtained by trained, certified staff masked to treatment assignment.
Weight was measured with the participant wearing light, indoor clothes without shoes and using a high-quality, calibrated digital scale. Height was measured at entry using a calibrated, wall-mounted stadiometer.
Diet was assessed by the Block Food Frequency Questionnaire (FFQ)13 including a summary of overall diet quality (the Healthy Eating Index (HEI).14
Estimates of total weekly minutes of moderate to vigorous physical activity (MVPA) were obtained from a calibrated, triaxial accelerometer (RT3, Stayhealthy, Inc, Monrovia, California) worn for at least ten hours per day for at least four days, including one weekend day.
Participants completed questionnaires addressing demographics, medication use, perceived current and ideal weight, weight loss history, perceived stress15 and depression (PHQ-8)16.
Initial Weight Loss Intervention (Phase I)
During Phase I, weight loss counselors led 20 weekly group sessions over approximately 6 months. Intervention goals were 180 minutes per week of moderate PA; reduced caloric intake; consumption of the DASH dietary pattern17,18; and weight loss of approximately 1–2 lb/week. Participants were instructed to keep food and PA self-monitoring records, and to calculate caloric intake. The intervention was based on behavior change theory19,20 and incorporated behavior change tools such as self-monitoring, goal-setting, social support, problem solving, relapse prevention,21 and motivational interviewing techniques.22 Participants achieving at least 4 kg of weight loss in Phase I were eligible for randomization into Phase II.
Maintenance Interventions (Phase II)
Randomization was stratified by clinic, self-reported race (Black vs. non-Black), sex, and Phase I weight loss, with equal allocation to a self-directed control group (SD), a group with unlimited access to an investigator-designed intervention website utilizing interactive technology to provide behavioral intervention (IT), or a group receiving monthly personal counseling primarily by phone (PC). Both IT and PC re-enforced the behavior changes made in Phase I, and encouraged continued application of behavior modification techniques. In addition, both incorporated features found to be associated with maintenance of behavior change in observational studies, such as continuing intervention contacts, self-monitoring, and PA. 5,6,23 The behavioral strategies, dietary advice, and PA recommendations were the same in PC and IT. Participants randomized to the SD control condition received printed lifestyle guidelines at randomization and met briefly with a study interventionist after the 12-month data collection visit.10
Outcomes
We evaluated weight change during Phase I (6 months), during Phase II (30 months), and across both Phase I and II (36 months). Continuous weight outcomes are expressed as percent change. In addition, we report the proportion in whom weight at the end of the study was at least 5% below entry weight. This degree of weight loss is associated with clinically significant reduction in blood pressure and incident hypertension and diabetes.7,12,24 We also report changes in diet and physical activity behaviors by age group.
Statistical Methods
All weight changes are expressed as percent change. We compared Phase I weight change across age subgroups without regard for subsequent randomization assignment. However analyses of Phase II or combined Phase I & II weight change do account for treatment as noted below.
Analysis of characteristics at study entry and of changes during Phase I are based on the 1685 individuals who entered Phase I (although one person who died during Phase I was excluded from analyses of Phase I change outcomes). Analyses of post-randomization outcomes, including analyses of the combined Phase I & II weight loss, include all participants randomized into Phase II who were alive at the end of the study (n=1029 of 1032 randomized).
For comparisons of baseline data and both Phase I and Phase II adherence and behavior change across age groups, we used simple one-way ANOVA and Pearson chi-square tests for continuous and categorical data, respectively. For comparisons of weight change outcomes by age group, we used multiple linear regression (continuous data) or logistic regression (binary data). For Phase I weight change only, these models were adjusted for site and entry weight. All other models adjusted for site, race (African American versus not African American), gender, race- gender interaction, entry weight, and treatment group, and in addition included an age-treatment interaction term.
Unless otherwise noted, we used LSMEANS in SAS to calculate model-based mean values for the data in all figures and tables. The models used for these calculations treated age group as a “class” variable in order to allow the means to vary arbitrarily across age groups within each treatment arm. However to calculate p-values for age trends we refit these models treating age group as a continuous variable. We calculated the significance of overall age trends (i.e., ignoring treatment) from a model that did not include an age-treatment interaction.
We used multiple imputation,25 averaging five separate imputation samples, to replace missing end-of-study weights (for 68 individuals), missing interim weights, and selected other measures. Only data missing due to participant death (N = 1 in Phase I and N= 3 in Phase II) were not imputed. All analyses were conducted using SAS, version 9.1 (SAS institute, Cary, NC), and all p-values are two-sided.
Results
The main results of the trial have been reported previously.9 An end of Phase II weight was measured on over 90% of participants in each age group.
Of the 1685 individuals who entered WLM, 536 were age 50 years or younger, 713 were 51–60 years old, and 436 were over 60. Although ages ranged from 25–83 years, on average the three age groups were spaced evenly apart, with mean ages of 45, 55, and 66 years, respectively (Table 1). By design, all participants were overweight or obese, but entry weight tended to be slightly lower in older participants, and a smaller proportion of older participants was obese (73% of those over 60 compared to 81% in those 50 or younger). Eligibility criteria required all participants to have hypertension and/or dyslipidemia, and the majority were being treated for hypertension. Those over 50 were more likely to be taking lipid-lowering medication than were younger participants. However, in general, entry characteristics were comparable across age groups. We observed qualitatively similar results among the subset of 1032 participants who were randomized into Phase II, of whom 272, 471, and 289 were in each age group, respectively.
Table 1.
Participant characteristics at entry into the study (i.e., prior to Phase I), by age group.
| Age, years Characteristic |
≤ 50 | 51– 60 | >60 | p-value for diff across age groups |
|
|---|---|---|---|---|---|
| N | 536 | 713 | 436 | ||
| Age in years, mean (sd) Range 25–83 |
44.6 (4.9) | 55.4 (2.8) | 66.1 (4.4) | <0.001 | |
| BMI in kg/m2, mean (sd) | 34.9 (4.9) | 34.3 (4.8) | 33.1 (4.4) | <0.001 | |
| Weight in kg, mean (sd) | 99.5 (16.8) | 96.4 (16.4) | 92.8 (15.7) | <0.001 | |
| Obese (BMI ≥30 kg/m2) | 81% | 79% | 73% | 0.008 | |
| On BP meds | 88% | 87% | 88% | 0.83 | |
| On lipid-lowering meds | 27% | 41% | 47% | <0.001 | |
| Current smoker | 7% | 5% | 3% | 0.02 | |
| Perceived stress, mean score (sd)1 |
5.0 (2.9) | 4.2 (2.8) | 3.6 (2.8) | <0.001 | |
| Depression, mean sore (sd)2 | 2.5 (1.6) | 2.3 (1.5) | 2.0 (1.5) | <0.001 | |
| Previous weight loss, percent of population |
|||||
| Achieved at least 10lb weight loss | 0.03 | ||||
| Never | 10% | 9% | 13% | ||
| 1–5 times | 68% | 63% | 61% | ||
| 6 or more times | 22% | 28% | 26% | ||
| Maximum amount of weight loss | 0.01 | ||||
| Never tried | 1% | 2% | 4% | ||
| < 20 lbs | 52% | 50% | 55% | ||
| 21–40 lbs | 33% | 31% | 28% | ||
| > 40 lbs | 14% | 17% | 13% | ||
| Difference between current weight and “best weight for me”, mean lbs (sd) |
−24.7 (12.3) | −23.2 (11.4) | −18.9 (10.4) | <0.001 | |
Abbreviations: SD= standard deviation; BMI = body mass index; BP = blood pressure
Range 0–20, with higher score indicating more stress.
Range 0–24 with higher score indicating more depression.
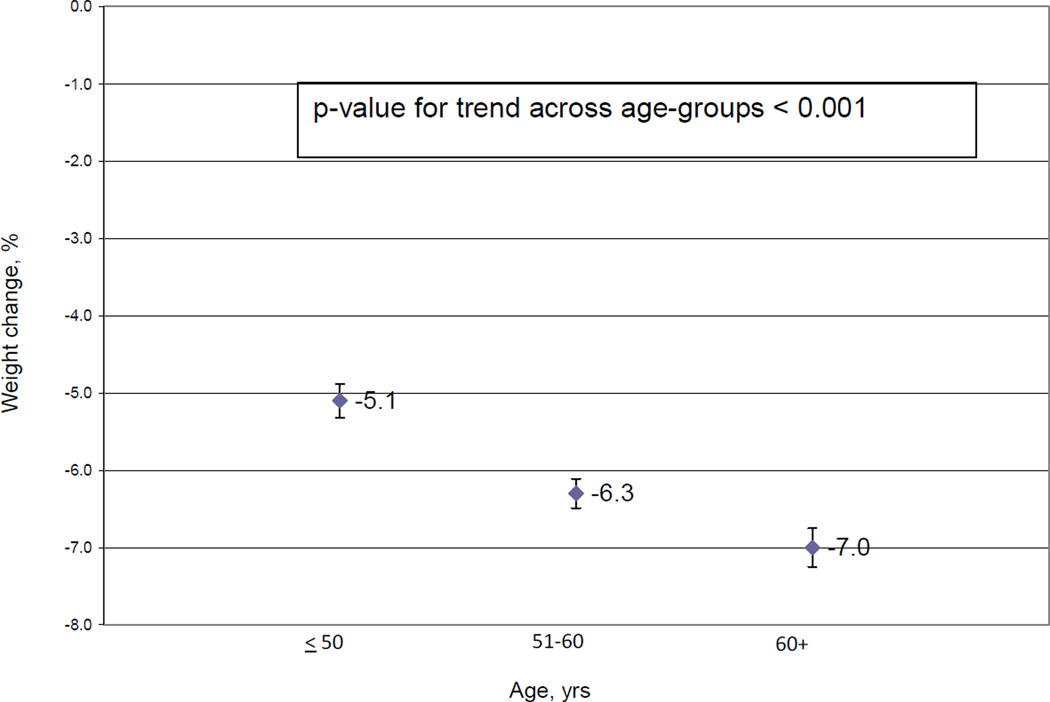
Figure 1 shows Phase I weight loss by age group. Older adults lost significantly more weight during Phase 1 (p for trend <0.001), with 66% of those over 60 achieved at least 4kg of weight loss and were randomized into Phase II versus only 51% of those aged 50 and younger. Among randomized participants, the amount of Phase I weight loss also tended to increase with age, with mean weight loss of 8.54, 8.64, and 9.12 percent in those < 50, 51–60, and > 60 years, respectively. These data are notable because in WLM, Phase I weight loss is the strongest predictor of long-term weight loss.26
Figure 1.
Phase I weight loss by age group N = 1684). Percent change with Standard Error. Adjusted for site and entry weight.
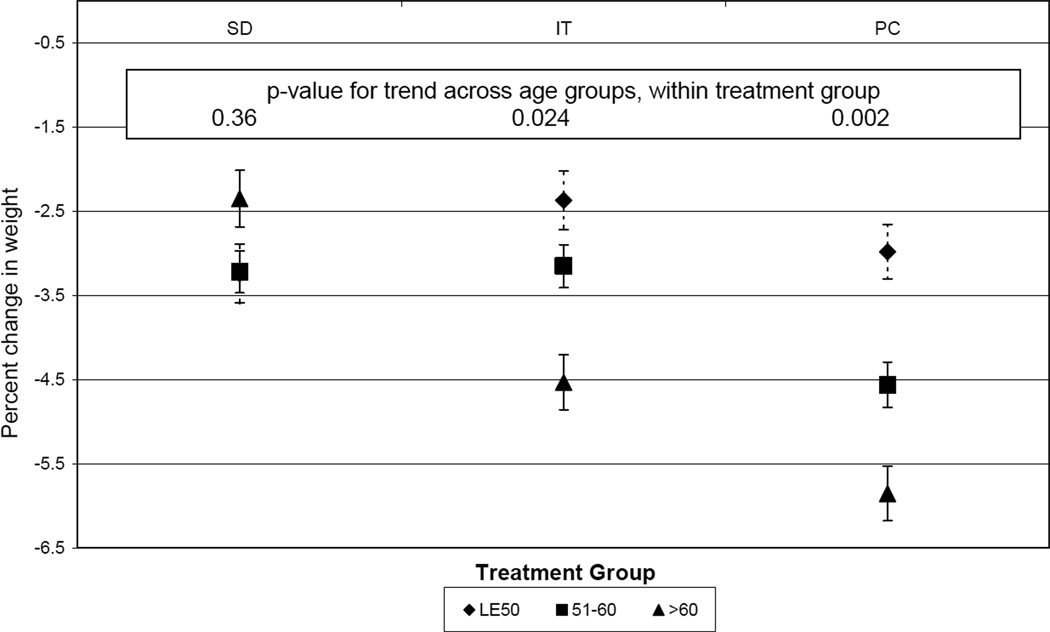
In Phase II, older individuals were more successful at maintaining weight loss. Mean (se) percent weight regain in Phase II was 6.54 (0.42), 5.47 (0.32), and 5.05 (0.41) percent in those ≤ 50, 51–60, and over 60 years old, respectively (p-value for trend = 0.011). Across both Phase I and Phase II (i.e., over 36 months), mean weight change by age group was −2.86 (0.40), −3.64 (0.31), and −4.24 (0.39) percent in those ≤ 50, 51–60, and over 60 years old, respectively (p−value for trend = 0.012). These latter patterns differed significantly by treatment arm (p = 0.005 for interaction), with no significant age trend among SD participants but significant trends of more weight loss maintenance 39 with increasing age among IT and PC participants (Figure 2).
Figure 2.
Effect Of Treatment Group On Percent Weight Change From Entry To End Of Phase II, By Age Group. Adjusted for site, race (African American versus not African American), gender, race- gender interaction, entry weight, treatment group, and age-treatment interaction.
Older individuals also were more likely to sustain at least a 5% weight loss than younger individuals in both PC and IT (49% in those over age 60 compared to approximately 30% in both younger age groups; p = 0.003 and 0.033, respectively).
Table 2 shows intervention adherence and changes in behavior by age group. During Phase I, older age was associated with more attendance at group intervention sessions and more diet self-monitoring. At entry, older participants tended to eat more fruits, vegetables, and dairy products, and had an overall healthier dietary pattern (higher HEI score). During Phase I, older participants tended to show less improvement in these behaviors, potentially because they tended to have better levels at entry. Otherwise, there were no significant associations between age and behavior change during Phase I or Phase II.
Table 2.
Intervention process measures and change (expressed as absolute change) in diet and PA for randomized participants (N = 1029*). Mean (sem). Unadjusted P-values based on Pearson chi-square or 1-way ANOVA.
| Measure | Age, years Time frame |
≤50 | 51–60 | >60 | p-value for differences across age groups |
|
|---|---|---|---|---|---|---|
| Attendance at Phase I groups, sessions attended |
During Phase I | 17.41 (0.13) | 17.75 (0.10) | 18.06 (0.13) | 0.0019 | |
| Food and PA records, number of weeks records were kept |
During Phase I | 13.67 (0.26) | 14.64 (0.20) | 16.37 (0.26) | 0.0000 | |
| Physical activity, MVPA min/wk |
At entry into Phase I | 128.96 (6.99) | 116.74 (5.32) | 106.75 (6.79) | 0.0757 | |
| Change during Phase I | 51.18 (8.78) | 39.21 (6.69) | 52.75 (8.53) | 0.3661 | ||
| Change during Phase II | −40.88 (9.64) | −39.10 (7.34) | −33.49 (9.37) | 0.7359 | ||
| Dietary pattern | ||||||
| Fruits and vegetables, servings/day | At entry into Phase I | 4.61 (0.17) | 5.28 (0.13) | 5.84 (0.17) | 0.0000 | |
| Change during Phase I | 4.17 (0.24) | 3.64 (0.18) | 3.29 (0.23) | 0.0302 | ||
| Change during Phase II | −2.34 (0.24) | −2.07 (0.18) | −1.82 (0.23) | 0.2913 | ||
| Dairy, servings/day | At entry into Phase I | 1.01 (0.06) | 1.09 (0.04) | 1.26 (0.06) | 0.0077 | |
| Change during Phase I | 0.36 (0.06) | 0.41 (0.04) | 0.45 (0.06) | 0.5135 | ||
| Change during Phase II | −0.21 (0.06) | −0.22 (0.04) | −0.29 (0.05) | 0.4674 | ||
| %kcal fat | Entry | 38.70 (0.44) | 38.95 (0.33) | 38.32 (0.42) | 0.4661 | |
| Change during Phase I | −8.58 (0.50) | −8.53 (0.38) | −8.27 (0.48) | 0.8537 | ||
| Change during Phase II | 4.10 (0.47) | 3.74 (0.36) | 3.60 (0.46) | 0.6918 | ||
| Healthy Eating Index (HEI)** |
At entry into Phase I | 59.80 (0.73) | 61.81 (0.56) | 65.74 (0.71) | 0.0000 | |
| Change during Phase I | 15.00 (0.74) | 14.62 (0.57) | 11.87 (0.72) | 0.0045 | ||
| Change during Phase II | −5.74 (0.68) | −6.31 (0.52) | −5.87 (0.66) | 0.6532 | ||
| Frequency of weighing, times per week in the last 30 days |
Based on retrospective questionnaire |
2.72 (0.06) |
2.34 (0.7 |
2.91 (0.08) | <0.0001 | |
Three randomized participants died during Phase II (ages 55, 71, and 59) and are not included in this analysis.
HEI scores range from 0 to 100, with higher scores indicating higher diet quality.9
Abbreviations: HEI: Healthy Eating Index; PA: physical activity; MVPA: moderate-to-vigorous physical activity
Discussion
In WLM, adults age > 60 years had greater initial weight loss, less weight regain, greater sustained weight loss, and a higher proportion with clinically significant weight loss after 3 years compared to younger adults. Older adults had greater weight loss in response to both personal counseling and to an internet-based intervention.
These findings are consistent with the main results of the WLM trial9 in that PC was associated with greater weight loss maintenance than IT. However, it is notable that older individuals had greater weight loss than younger individuals in both interventions. It is clear that this is an intervention effect rather than a reflection that older individuals will sustain more weight loss under any circumstances: without any maintenance intervention (i.e., in the SD group), older and younger participants experienced similar low levels of weight loss maintenance. Despite popular perceptions, an internet-based intervention was effective in older adults, assuming that they had some familiarity with the internet to begin with (an eligibility criterion for WLM).
Clearly, weight loss is achievable and sustainable in older adults. Such a goal seems desirable based on the known benefits of weight loss for prevention and control of CVD risk factors. These benefits appear to occur regardless of age. For example, in the Look AHEAD trial, in which the mean age is 59 years (sd 6.8), weight loss at both 1 and 4 years was associated with improved control of hypertension, diabetes, and dyslipidemia.27,28 Similarly, in the TONE trial, with mean age 66, a behavioral weight loss intervention reduced the need for pharmacologic antihypertensive treatment by 30%.29
There may be additional benefits of weight loss in older adults. For example, although somewhat controversial, obesity in older adults is associated with increased risk of dementia in some studies30, raising the possibility that weight loss can slow cognitive decline. A recent trial in older adults suggests that weight loss coupled with exercise prevents decline in physical functioning and preserves bone mass.31 Observations of increased mortality associated with lower body weight in older adults (the putative “obesity paradox”)32 have been mitigated by a recent cohort study indicating that moderate-to-severe obesity is indeed associated with higher mortality, at least for whites.33 In addition, in a secondary analysis of the ADAPT trial in older adults, randomization to a weight loss intervention was associated with reduced mortality.34 There is additional evidence of potential benefit.35,36
Nonetheless, questions remain concerning the beneficial and potentially harmful effects of weight loss in older adults and whether the degree of overweight/obesity has varying impact by age.37,38 WLM (nor any other trial) did not directly test the effects of weight loss on health outcomes. However, because weight loss in older adults has known beneficial effects on a wide range of risk factors and potential effects on other health outcomes, . the preponderance of evidence suggests that weight loss will lead to health benefits that outweigh potential health risks. However, this hypothesis should be directly tested.
When considering implementation of weight loss intervention in older adults, the success of the IT intervention in older WLM participants is particularly encouraging since this intervention can be efficiently scaled for broad dissemination. Furthermore, , we found that consistent use of the internet intervention was more likely among older than younger participants, and was associated with greater weight loss maintenance.39Although some have expressed concern about the use of technology-based solutions in older adults, our data suggest just the opposite. Currently 46% of US adults age 65 or older report regular use of the internet.40 Therefore, internet-based intervention holds great promise for this age group. However, it is important to bear in mind that all WLM participants received a 6-month intensive behavioral intervention conducted in facilitated group sessions before randomization to SD, IT or PC. Therefore, we cannot speculate about the effects of an internet-based intervention that is implemented without initial weight loss through a more traditional face-to-face intervention.
Potential limitations of our study include the difficulty in understanding the biological or behavioral mechanism(s) of the observations. We did not see differences in key behaviors other than greater attendance and self-monitoring in Phase I (Table 2), but measures of diet and physical activity are imprecise. Alternatively, there may be other behavioral, biological and environmental factors that may contribute to the observed age differences. In addition, we did not measure body composition. If older adults preferentially lose lean rather than fat mass, the health benefits of weight loss may be attenuated. Finally, we chose age categories (age 50 years or younger, 51–60 years, and over 60 years) that allowed us to compare groups of roughly equal size. However, other categories would have facilitated comparisons to other studies and/or been more relevant to health care delivery (e.g. if one category corresponded to Medicare’s age of eligibility) or if we had assessed a category comprising the very old. The latter option was not feasible since there were only two participants who were >80 years old . In sensitivity analyses in which the age categories were defined as <65, 65–74, and >=75 (with N = 868, 149, and 15 randomized participants, respectively), results were similar to our primary analysis.
Nonetheless, our results indicate that older adults can lose weight with behavioral intervention and then sustain weight loss using either personal counseling or internet technology. In the absence of any maintenance intervention, more weight regain occurred, indicating that further intervention is needed after initial weight loss. Ideally, we need trials powered to determine effects of weight loss on clinical outcomes in older adults. But given the range of risk factors favorably affected by weight loss in older adults, future research should determine optimal strategies, including internet-based strategies, for increasing intervention effects and for broad implementation in older adults.
What is already known
-
-
71% of Americans age ≥ 60 years are overweight or obese.
-
-
Overweight/obesity is the leading cause of cardiovascular risk factors.
-
-
Behavioral weight loss interventions result in clinically significant weight loss
What this study adds:
-
-
Our study suggests that older adults respond better than younger adults to weight loss intervention, even when it is provided via internet
Acknowledgments
Sponsored by National Heart, Lung, Blood Institute grants 5-U01 HL68734, 5-U01 HL68676, 5-U01 HL68790, 5-U01 HL68920, and 5-HL68955.
We gratefully acknowledge the contributions of the WLM study participants and the study staff at each participating institution.
Footnotes
Conflict of interest
The authors have no competing interests.
Author contributions: LPS, KF, JFH, JFA, PJB, CML, CMC, WMV and VJS were involved in study design. All authors were involved in study conduct, interpretation of results and preparation and/or editing of the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2001;49:109–116. doi: 10.1046/j.1532-5415.2001.49030.x. [DOI] [PubMed] [Google Scholar]
- 3.Services USDoHaH. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Washington D.C.: 1998. [PubMed] [Google Scholar]
- 4.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.THE PEW INTERNET AND AMERICAN LIFE PROJECT E-GOVERNMENT SURVEY. 2003 Accessed at http://www.pewinternet.org/Shared-Content/Data-Sets/2003/August-2003-eGovernment-Data-Set.aspx.
- 9.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 10.Brantley P, Appel L, Hollis J, et al. Design considerations and rationale of a multi-center trial to sustain weight loss: the Weight Loss Maintenance Trial. Clin Trials. 2008;5:546–556. doi: 10.1177/1740774508096315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 13.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 14.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Appel L, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin P-H, Karanja N. for the DASH Collaborative Research Group. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 18.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–310. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 19.Bandura A. Social foundation of thoughts and actions: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 20.Prochaska JM, Prochaska JO, Levesque DA. A transtheoretical approach to changing organizations. Adm Policy Ment Health. 2001;28:247–261. doi: 10.1023/a:1011155212811. [DOI] [PubMed] [Google Scholar]
- 21.Martin GLPJ. Behavior Modification: What it is and How to Do It. 8th ed. New York: Prentice Hall; 2006. [Google Scholar]
- 22.Miller WR, Rollnick S. Motivational interviewing : preparing people for change. 2nd ed. Guilford Press; 2002. [Google Scholar]
- 23.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 24.Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153:849–858. [PubMed] [Google Scholar]
- 25.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 26.Svetkey LP, Harris EL, Martin E, et al. Modulation of the BP response to diet by genes in the renin-angiotensin system and the adrenergic nervous system. Am J Hypertens. 2011;24:209–217. doi: 10.1038/ajh.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 30.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wee CC, Huskey KW, Ngo LH, et al. Obesity, race, and risk for death or functional decline among Medicare beneficiaries: a cohort study. Ann Intern Med. 2011;154:645–655. doi: 10.7326/0003-4819-154-10-201105170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shea MK, Houston DK, Nicklas BJ, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT study. J Gerontol A Biol Sci Med Sci. 65:519–525. doi: 10.1093/gerona/glp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009;22:93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 36.Eilat-Adar S, Eldar M, Goldbourt U. Association of intentional changes in body weight with coronary heart disease event rates in overweight subjects who have an additional coronary risk factor. Am J Epidemiol. 2005;161:352–358. doi: 10.1093/aje/kwi045. [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008;9:302–312. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Funk KL, Stevens VJ, Appel LJ, et al. Associations of internet website use with weight change in a long-term weight loss maintenance program. J Med Internet Res. 2010;12:e29. doi: 10.2196/jmir.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Internet & American Life Project. [Accessed 2011];Home Broadband. 2010 http://www.pewinternet.org/Static-Pages/Trend-Data/Whos-Online.aspx.