Abstract
Morizane et al. (2013) show that donor-matched differentiated derivatives of induced pluripotent stem cells (iPSC) do not cause an immune response after transplantation, whereas transplantation of HLA-mismatched iPSC derivatives to the same site clearly does. The importance of these results is discussed in this commentary as we assess how best to move forward with iPSC-based cell therapy.
Main Text
One promise of IPSC-based cell therapy is that personalized medicine using cells from the patient may be feasible. Somatic cells could be harvested and reprogrammed with integration-free methods to produce a stock of clinically compliant pluripotent cells ready for use at any time in later life. To ensure that this may really become possible, various groups have been working on the two major hurdles: (1) reduce the cost of development of iPSC and (2) develop clinical grade processes to generate iPSC lines. Other groups have approached the problem by suggesting that, although not fully personalized, one could perhaps develop HLA-matched banks of lines; indeed, the Japanese government and others have proposed building a collection of diverse HLA types that could be then matched between donor and recipient, akin to the current practice using bone marrow and tissue transplants (Taylor et al., 2012, Cyranoski, 2012). Commercial entities have approached this by offering to develop personalized iPSC banks, rather like the private cord blood banks that will store individual samples in anticipation of future need in “for-profit” models (Cave, 2013).
In principle this process seems reasonable; however, several questions need to be addressed. Perhaps the most important is whether the cost of making iPSC lines for a single individual is worth the time and effort or whether cells matched at the major loci of the histocompatibility complex may be adequate. Some experiments have even suggested that mismatched cells may also be an option. The reverse issue also needs to be considered: it is not unreasonable to suppose that autologous iPSC may generate an immune response because of their exposure to foreign antigens during the culture period or because of their expression of embryonic antigens not normally present in the adult. Likewise, viral agents used for reprogramming can activate the innate immunity pathway and thus cause an immune response (Zhao et al., 2011, Guha et al., 2013). These issues are critical, and adequate experimental models to assess these issues directly need to be developed. Unambiguous answers will dictate where resources and time should be allocated to develop iPS cell based therapy.
In this issue of Stem Cell Reports, Morizane and colleagues (Morizane et al., 2013) have developed an elegant system to address these issues. They have taken advantage of a primate colony to generate matched, related, and unmatched iPSC, which they have then differentiated into dopaminergic neurons. These neurons were then transplanted without immune suppression into the monkey striatum as autografts or HLA-mismatched allografts to evaluate immune responses (Figure 1). The animals were followed over a sufficiently long time period to allow examination of acute, hyperacute, and chronic immune responses. The authors were able to make two important observations: (1) mismatched cells generated an immune response—not the acute or hyperacute type of rejection response seen in mismatched organs but a slow chronic rejection phenotype mediated by macrophages and T cells; and (2) no rejection was seen in the matched transplants, suggesting a clear, albeit small, short-term and larger long-term benefit of developing matched iPSC lines. Of importance was the observation that such rejection can occur even in the brain, which is widely considered as somewhat immune privileged.
Figure 1.
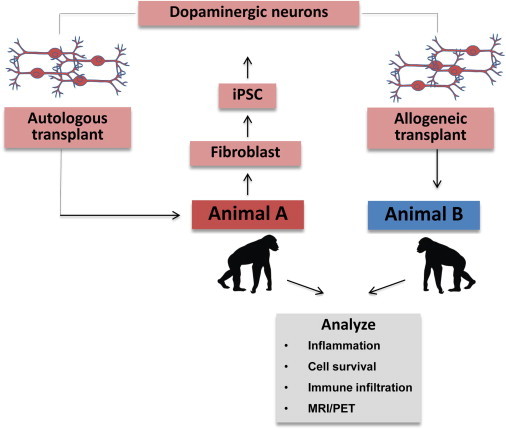
A Schematic Outline of the Experiment
iPSC were made using viral vectors from animals that were maintained in a colony. Differentiation was performed using standard published protocols and transplants were placed within the brain.
The authors have established a system in which they can begin to dissect out even more subtle issues related to immune response in a species that is closely related to humans and has a life span that will allow longer term studies to be performed. These experiments compliment the elegant work of Araki et al. (2013), which showed that autologous or matched ESC and iPSC are equivalent in that they lack of an immunogenic response when differentiated cell products are transplanted and provide an opportunity to perform similar experiments in a primate model. The same models will also allow us to evaluate which immune suppressive regime is best in the long run and when and if any such regime can be discontinued.
Two other advantages of this model system are that we now have a primate model and corresponding pluripotent cells from the same species that can be differentiated into many of the cell types that are being investigated for cell based treatment. Thus, both human and primate iPSC derivatives can be compared in the same animal species. This should make it possible to determine whether the experimental paradigm of the xenograft (human into primate, human into mouse, human into porcine, etc.) and its associated immune suppressive regime has any value in predicting what we really want to know: will human pluripotent stem cell derivatives have therapeutic value in humans and what will be the requirements for immune matching or (long-term) immune suppression. Embryonic stem cell (ESC) lines can be derived from primates, and were, in fact, derived before human ESC lines (Thomson et al., 1995), and iPSC could be made from differentiated derivatives of primate ESC, as has been shown for rodent and human lines (Teichroeb et al., 2011). One could therefore extend these experiments to study subtle differences between ESC and iPSC cells over a long time period in a model that is as close to human as is possible.
Acknowledgments
This work was supported by the NIH Center of Regenerative Medicine, a NIH common fund initiative. The opinions expressed in this article represent the views of the author and in no way can be construed to reflect the opinion or policy of the NIH.
References
- Araki R., Uda M., Hoki Y., Sunayama M., Nakamura M., Ando S., Sugiura M., Ideno H., Shimada A., Nifuji A., Abe M. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- Cave, A. (2013). Stem-cell banks enable wealthy to free “back up version” of their adult selves. The Telegraph, September 9, 2013. http://www.telegraph.co.uk/science/science-news/10295085/Stem-cell-banks-enable-wealthy-to-free-backup-version-of-their-adult-selves.html.
- Cyranoski D. Nature. 2012;488:139. doi: 10.1038/488139a. [DOI] [PubMed] [Google Scholar]
- Guha P., Morgan J.W., Mostoslavsky G., Rodrigues N.P., Boyd A.S. Cell Stem Cell. 2013;12:407–412. doi: 10.1016/j.stem.2013.01.006. Published online Jan 24, 2013. [DOI] [PubMed] [Google Scholar]
- Morizane A., Doi D., Kikuchi T., Okita K., Hotta A., Kawasaki T., Hayashi T., Onoe H., Shinna T., Yamanaka S., Takahashi J. Stem Cell Rep. 2013;1:283–292. doi: 10.1016/j.stemcr.2013.08.007. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Teichroeb J.H., Betts D.H., Vaziri H. PLoS ONE. 2011;6:e23436. doi: 10.1371/journal.pone.0023436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A., Kalishman J., Golos T.G., Durning M., Harris C.P., Becker R.A., Hearn J.P. Proc. Natl. Acad. Sci. USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Zhang Z.-N., Rong Z., Xu Y. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]


