Abstract
Recent findings from studies of two families have shown that mutations in the GABAA-receptor γ2 subunit are associated with generalized epilepsies and febrile seizures. Here we describe a family that has generalized epilepsy with febrile seizures plus (GEFS+), including an individual with severe myoclonic epilepsy of infancy, in whom a third GABAA-receptor γ2-subunit mutation was found. This mutation lies in the intracellular loop between the third and fourth transmembrane domains of the GABAA-receptor γ2 subunit and introduces a premature stop codon at Q351 in the mature protein. GABA sensitivity in Xenopus laevis oocytes expressing the mutant γ2Q351X subunit is completely abolished, and fluorescent-microscopy studies have shown that receptors containing GFP-labeled γ2Q351X protein are retained in the lumen of the endoplasmic reticulum. This finding reinforces the involvement of GABAA receptors in epilepsy.
Generalized epilepsy with febrile seizures plus (GEFS+ [MIM 604233]) is now a well-recognized syndrome (Scheffer and Berkovic 1997; Moulard et al. 1999; Singh et al. 1999; Alekov et al. 2001; Baulac et al. 2001; Escayg et al. 2001; Wallace et al. 2001b). The phenotypes in families with GEFS+ include typical febrile seizures (FS) seen in early childhood, as well as febrile seizures plus (FS+) in which either attacks with fever extend beyond age 6 years or afebrile generalized tonic-clonic seizures (GTCS) occur. Less common phenotypes include FS/FS+ associated with absence, myoclonic, atonic, or partial seizures, and even refractory syndromes with developmental delay, such as myoclonic-astatic epilepsy (MAE) and severe myoclonic epilepsy of infancy (SMEI) (Singh et al. 2001).
Mutations in three voltage-gated Na+-channel genes have been associated with GEFS+. We first described a mutation in the Na+ channel β1-subunit gene (SCN1B) on chromosome 19q in a large family with GEFS+ and showed that such phenotypic heterogeneity could be associated with the segregation of a single mutation (Wallace et al. 1998). Subsequently, mutations were described in the α1-subunit gene (SCN1A) on chromosome 2q in other families with GEFS+ (Escayg et al. 2000, 2001; Wallace et al. 2001b), as were more-severe de novo truncation mutations in patients with SMEI (Claes et al. 2001). Sugawara et al. (2001) also recently implicated the Na+ channel α2-subunit gene (SCN2A) in GEFS+. The spectrum of mutations that so far have been found in these three Na+-channel genes clearly demonstrates Na+-channel dysfunction in GEFS+ in ∼10%–20% of probands (Sugawara et al. 2001; Wallace et al. 2001b). Although sporadic examples of severe phenotypes such as SMEI may be due to de novo truncation mutations (Claes et al. 2001), such syndromes within families are probably due to interaction between mutations at two or more loci.
Recently, it has been shown that mutations in GABAA-receptor subunits also play a significant role in contributing to the GEFS+ phenotype (Baulac et al. 2001; Wallace et al. 2001a). GABAA receptors are pentameric ligand-gated Cl− channels primarily responsible for mediating fast inhibitory neurotransmission in the mammalian central nervous system (Barnard et al. 1998; Schwartzkroin 1998). It has been known for some time that disruption of GABA-mediated pathways is associated with epilepsy (Olsen et al. 1999), but specific mutations contributing to this have only recently been identified. Baulac et al. (2001) described a mutation, in the GABAA-receptor γ2 subunit, that segregates in a French family with GEFS+. This mutation, K289M, changes a highly conserved amino acid in the extracellular loop between the second and third transmembrane domains, and expression of this mutation in Xenopus laevis oocytes resulted in a decrease in the amplitude of GABA-activated currents. This discovery was the first genetic evidence that GABAA-receptor dysfunction is associated with GEFS+.
Simultaneously, we described a missense mutation in the GABAA-receptor γ2-subunit gene in a large Australian family with epilepsy, in which the two main phenotypes were childhood absence epilepsy (CAE) and FS (Wallace et al. 2001a). This mutation, R43Q, changes a highly conserved amino acid residue in the first of two extracellular high-affinity benzodiazepine-binding domains. Although X. laevis oocytes expressing this altered protein were still responsive to GABA, the mutation abolished in vitro sensitivity to diazepam. Because of the complex inheritance of CAE, we hypothesized that this mutation may be primarily responsible for the expression of FS but that a second gene or genes are required for the CAE phenotype (authors' unpublished data). In this pedigree there were three individuals with FS+ and two with MAE, confirming that the R43Q mutation in GABRG2 also contributes to the GEFS+ syndrome.
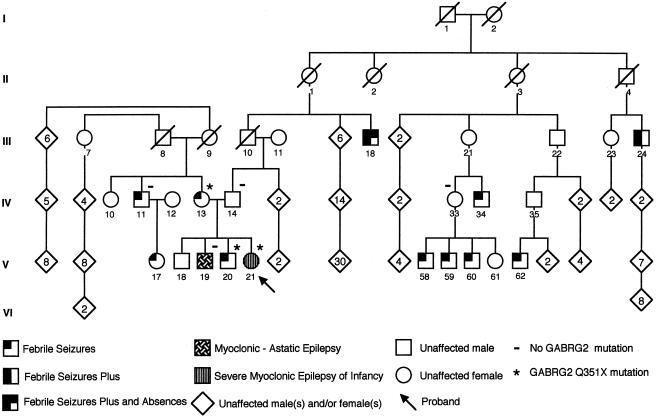
In a screen of >200 unrelated patients with various epilepsy phenotypes (GEFS+, FS, and IGE) by single-strand conformation polymorphism (SSCP) analysis, we found another family with a novel GABRG2 mutation. Hex-labeled primers were designed to amplify all nine exons of the γ2-subunit gene GABRG2 on chromosome 5q (table 1). SSCP and sequencing was performed as described elsewhere (Wallace et al. 2001b). An SSCP variant that was not detected in 96 unaffected control samples was found in exon 9 of GABRG2 in several individuals of a family with GEFS+ (fig. 1). This family was initially described as “family G” by Singh et al. (1999) and has been extended in the present study. The proband (V-21) had a bilineal family history of seizures. Information was obtained on 156 family members. On the paternal side, there were seven individuals with seizures: five with FS, one with FS+, and one with FS and absence seizures. The proband’s mother (IV-13) had multiple FS, and her brother (IV-11) and his daughter (V-17) also had FS.
Table 1.
Sequences of Primers Used for Mutation Analysis of GABRG2
|
Sequence(5′→3′) |
|||
| Exona | Forward | Reverse | Size(bp) |
| 1 | GCATGAGTATACACGAGTGTGC | GCCTCTTGGTTGCAGAAGAATC | 314 |
| 1 | CAGTGAAGGACCTACTAGAGG | GTAAAGCCGCACATCCTAGGAG | 354 |
| 2 | CAGTTAGTCTCCATCTATGCAG | CCTTGCTCTTGAACTACACTG | 414 |
| 3 | TATGCGTGCTTGGTGCATGTGC | GGATCTGGAAGACTATCTTTCAC | 420 |
| 4 | GTGAGACAGTAACCTCCTCAGC | GATAGCATGCCAACCCTGATGC | 520 |
| 5 | CCTGGACTTGGTGGATTTCTTC | TCACCCTAATCGGAGCAAGCTG | 413 |
| 6 | TGCCCTTTGGTCCAAGATCCTC | TCAACTCTGGAAGGGTCACTTG | 323 |
| 7 | GGGATTCAGTTCAGGTTGTG | GGGTTGGTTCCAAGTCTTTGC | 524 |
| 8 | CCACTTATACCTCCTTTCCC | CGTTATGGCCTGGCTAAACTC | 480 |
| 9 | CATCACATTGGTGACATTGTGG | ACATCTCTCCATGAGACTCAGT | 419 |
| 9 | TATTGGGTCTCCTACCTCTACC | CCACTACTGTAAATAGTCAGGGC | 345 |
More than one PCR was required to amplify exons 1 and 9.
Figure 1.
Pedigree of an Australian family with GEFS+. An asterisk (*) indicates members carrying the Q351X mutation in GABRG2, and a minus sign (−) indicates members who were tested and are negative for the Q351X mutation.
The proband (V-21) had a severe phenotype of GEFS+, which was initially regarded as MAE, but further history showed that she had SMEI. She had multiple clonic seizures affecting the right upper limb, associated with fever at age 3 mo. She had many such focal febrile seizures, as well as afebrile GTCS, but she did not have convulsive status epilepticus. Absence, myoclonic, and atonic seizures began at age 3–4 years. Myoclonus involving both upper limbs (right more than left) was prominent on examination and was exacerbated with lamotrigine. The proband had clinical photic sensitivity. From age 17 years, she had continuous irregular myoclonus of her right upper limb; by age 22 years, she had 1–2 GTCS/year and 1–2 absence seizures/d. Electroencephalogram (EEG) readings became more active over time and showed frequent paroxysms of irregular generalized polyspike-wave activity brought out by photic stimulation. Results of a magnetic-resonance imaging brain scan were normal. Early motor developmental milestones were normal, but speech was delayed. Neuropsychological assessment at age 14 years placed her in the moderately intellectually disabled range, without lateralizing features.
The proband had three brothers, two of whom had a history of seizures. The youngest brother (V-20) had six FS at age 2–3 years. The middle brother (V-19) had MAE, with onset of absence and myoclonic seizures at age 2 years. At age 4 years, GTCS developed. Treatment was changed from phenytoin to valproate, at age 4 years 9 mo, and he became seizure free; valproate was ceased at age 8 years. Recurrence of GTCS occurred at age 15 years, and valproate was recommenced. EEG studies at age 4 years showed frequent paroxysms of generalized polyspike-wave activity, and by age 8 years, photic sensitivity was evident. Motor milestones were normal but speech was delayed. He left school after his 10th year there and has subsequently performed factory work.
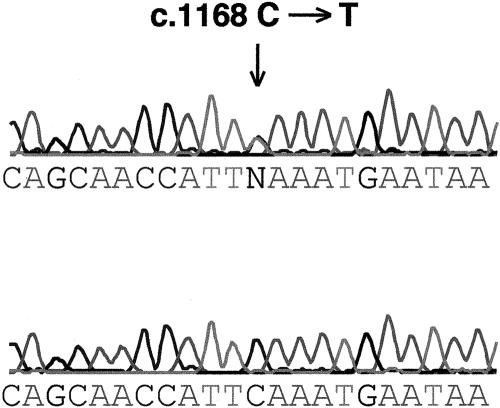
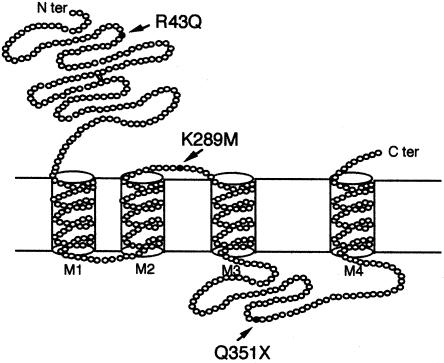
DNA sequencing of the SSCP variant revealed a C→T transition at nucleotide position 1168 of the GABRG2 cDNA sequence (GenBank accession number nm_000816; fig. 2). This single-base substitution introduces a premature stop codon at Q351 in the mature GABRG2 protein in the large cytoplasmic loop between the third and fourth transmembrane domains (fig. 3), resulting in a truncated GABRG2 protein with complete loss of the fourth transmembrane domain. Individual IV-13, who has FS, and her two children, V-20 and V-21, who have FS and SMEI, respectively, all had the Q351X mutation. However, her son (V-19) with MAE and her brother (IV-11) with FS both tested negative for the truncation mutation. This family has bilineal inheritance of seizure disorders, and we speculate that the MAE in individual V-19 is due to inheritance, from his father (IV-14), of a different mutation, which is associated with GEFS+ phenotypes in his extended family. This second mutation may also contribute to the more severe phenotype observed in V-21. The gene or genes responsible for the febrile seizures in IV-11 and V-17 are unknown and could also be contributing to the more severe phenotypes in the proband and in her brother with MAE, where it is likely that a number of genes are involved. Individuals III-8, III-9, IV-12, V-17, and V-18 were not tested, since blood was not available. The generalized epilepsies typically follow a complex inheritance pattern. It is possible that complex inheritance occurs in this multiplex family, since (a) at least three genes are implicated, (b) bilineal inheritance occurs in the proband's nuclear family, and (c) phenotypic heterogeneity is prominent. However, we also acknowledge that it is possible that three separate genes are responsible for the epilepsy seen in this family. Thus, the finding of the Q351X GABRG2 mutation in only three family members forms only one part of the inheritance pattern in this family.
Figure 2.
Sequencing trace of a portion of exon 9 GABRG2, showing the c.1168C→T transition (arrow). The upper chromatogram shows the mutation, and the lower chromatogram shows the control sequence.
Figure 3.
Schematic representation of the GABRG2 protein, with arrows indicating the positions of mutations associated with epilepsy.
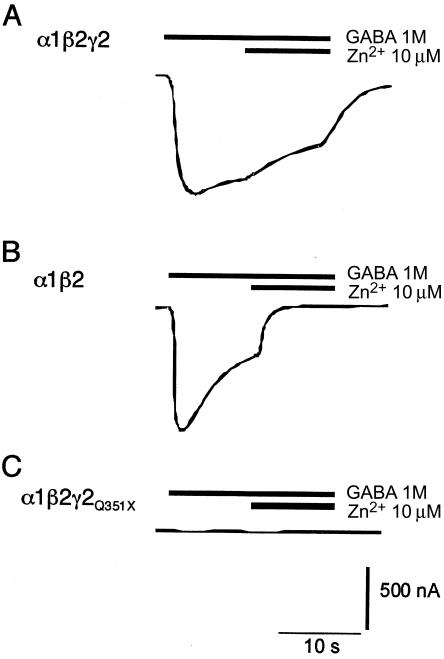
To determine the functional consequences of the Q351X mutation, GABA-mediated currents in X. laevis ooctyes were measured in oocytes expressing the mutant γ2 subunit. A stop codon was introduced at amino acid position 351 in the mature human GABRG2 protein, by PCR-based mutagenesis using the primer pair 5′-GTGAAGACAACTTCCGGAGATTATGTGG-3′ and 5′-GCGAATTCATTAAATGGTTGCTGATCTTGGGCG-3′. Female X. laevis oocytes were co-injected with the cRNAs encoding the α1 (GABRA1) and β2 (GABRB2) subunits of the GABAA receptor, with either the wild-type γ2-subunit cRNA or the mutant truncated γ2Q351X-subunit cRNA, through use of standard procedures (Stuhmer 1998). GABAA receptors occur naturally in a pentameric complex and are thought to consist of two α subunits, two β subunits, and one γ subunit (Klausberger et al. 2000). Oocytes simultaneously expressing all three wild-type subunits produced robust responses to GABA (fig. 4A). In vitro studies have also shown that, in the absence of GABRG2, complexes composed of α and β subunits are functional but have altered pharmacology (Draguhn et al. 1990; Connolly et al. 1996). Coexpression of GABRA1 and GABRB2 results in GABA-activated currents that are extremely sensitive to blockade by Zn2+ (fig. 4B), unlike currents from complexes formed with GABRG2 (fig. 4A). The functional consequences of the Q351X mutation of the GABRG2 subunit was assessed by expression of this mutant with wild-type α and β subunits. In this case, application of GABA failed to activate current responses (fig. 4C), suggesting that the mutant not only fails to assemble correctly with α and β subunits (compare to fig. 4A) but also interferes with the ability of the α and β subunits themselves to assemble into functional complexes (compare to fig. 4B).
Figure 4.
Two-electrode voltage-clamp recordings in oocytes, demonstrating that coexpression of the GABRG2Q351X abolishes the response to GABA. For all recordings, n=6. The current and time scale bars apply to all traces. Holding potential was −80 mV. A, Injection of oocytes with wild-type GABRA1:GABRB2:GABRG2 cRNAs (1:1:10 ratio, to favor assembly of γ subunit–containing complexes), resulting in both a robust inward current response to GABA and a low sensitivity to Zn2+ blockade, characteristic of GABRG2 coexpression. B, Expression of GABRA1:GABRB2 only (1:1 ratio). This also produced robust responses to GABA, but with high sensitivity to blockade by Zn2+. C, Expression of GABRA1:GABRB2:GABRG2Q351X subunits (1:1:10 ratio), abolishing GABA-induced currents.
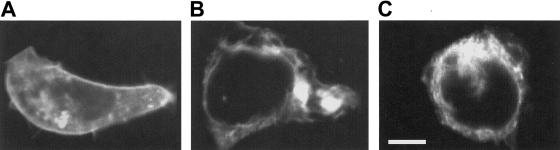
To assess the cellular fate of receptor complexes harboring the GABRG2Q351X mutant subunit, GFP-tagged wild-type and mutant GABRG2 subunits were engineered and then transfected into HEK293 cells, for analysis of spatial distribution with confocal fluorescence microscopy. Previous work has shown that fusion of GFP to the N-terminus of GABRG2 is functionally silent (Kittler et al. 2000). The N-termini of wild-type and mutated GABRG2 were tagged with enhanced green-fluorescent protein (EGFP), by use of Clontech’s pEGFP-C1 vector, to create GFP-GABRG2 and GFP-GABRG2Q351X. Receptor complexes composed of GABRA1:GABRB2:GFP-GABRG2 were found to localize to the cell membrane (fig. 5A) and intracellular compartment. When the wild-type GFP-GABRG2 was replaced by GFP-GABRG2Q351X, plasma-membrane–specific fluorescence was absent (fig. 5B). Instead, a reticular fluorescent pattern was observed, similar to that seen in the intracellular compartment of cells transfected with the wild-type GFP-GABRG2. In no case did we observe membrane localization of the GFP-GABRG2Q351X (n=6 cover slips with >400 cells screened). This fluorescent pattern was also similar to that observed by transfection of HEK-293 cells with either Clontech's endoplasmic reticulum (ER)–targeted pEYFP-ER vector (fig. 5C; BD Biosciences Clontech) or the fluorescent dye, DiOC6. Both markers reveal the localization of ER, suggesting that the GFP-GABRG2 Q351X is retained in the ER. Earlier work on the assembly of truncated nicotinic acetylcholine–receptor subunits that lack transmembrane domains (Verrall and Hall 1992) advanced the idea that, although truncated subunits assemble successfully with full-length subunits, the lack of a transmembrane anchor traps the assembled complex in the lumen of the ER. This proposed mechanism may provide an explanation for the truncated GABRG2 protein's lack of cell-surface expression in the present study. The implication for individuals heterozygous for the Q351X mutation would be that the truncated receptor subunit reduces the density of functional GABAA receptor complexes on the cell surface, leading to a decreased inhibitory response to GABA and, consequently, to increased neuronal excitability and seizures. Therefore, truncation of the GABAA-receptor γ2 subunit most likely contributes to the seizures seen in three affected members of this family (individuals IV-13, V-20, and V-21; fig. 1).
Figure 5.
Imaging of GFP-tagged GABRG2 expressed with GABRA1 and GABRB2 subunits, revealing the fate of GABRG2Q351X-containing receptors in HEK293 cells. Images were obtained by confocal fluorescence microscopy (excitation, 488 nm; emission, >515 nm). The scale bar in panel C corresponds to 5 microns and applies to all three panels. A, EGFP-tagged wild-type GABRG2 subunit, found in both the membrane and the intracellular compartment (n=20 individual cells analyzed). B, EGFP-tagged GABRG2Q351X, found only in the intracellular compartment (n=20 individual cells analyzed). C, ER-targeted EYFP expression, demonstrating subcellular distribution of ER that is remarkably similar to that seen in B.
The Q351X mutation described in the present article appears to cause dysfunction similar to that created by the K289M mutation described by Baulac et al. (2001), in that they both cause complete or partial loss, respectively, of GABA sensitivity and are both associated with families that have GEFS+. In contrast, the R43Q mutation that we previously had described in a family with CAE and FS had no effect on GABA-mediated currents but completely abolished sensitivity to benzodiazepines. It is possible that complete or partial loss of GABA sensitivity is associated with the typical GEFS+ phenotype, whereas disruption of the benzodiazepine receptor, leaving GABA sensitivity intact, results in the CAE/FS phenotype. We hypothesize that the position and/or severity of the mutation in the GABAA-receptor subunit may determine the spectrum of overlapping phenotypes. This has been shown in the Na+-channel–subunit SCN1A gene, where different mutations result in different phenotypes. A number of families with single amino acid substitutions that are associated predominantly with mild GEFS+ phenotypes have been described, whereas severe mutations occur in sporadic SMEI (Escayg et al. 2000, 2001; Claes et al. 2001; Wallace et al. 2001b).
The identification of different GABRG2 mutations in different families reinforces the importance of this subunit in human epilepsies. Like the Na+ channelopathies, where GEFS+-associated mutations have now been identified in a number of different Na+-channel subunits, it is likely that other GABAA-receptor subunits contribute to GEFS+ phenotypes.
Acknowledgments
We thank the family, for their participation, and R. Schultz, for technical assistance. The present study was supported by Bionomics Ltd., the National Health and Medical Research Council, the Women's and Children's Hospital Research Foundation, and the Epilepsy Foundation of Victoria.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human GABRG2 cDNA reference sequence [accession number nm_000816])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for GEFS+ [MIM 604233])
References
- Alekov AK, Rahman MM, Mitrovic N, Lehmann-Horn F, Lerche H (2001) Enhanced inactivation and acceleration of activation of the sodium channel associated with epilepsy in man. Eur J Neurosci 13:2171–2176 [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ (1998) International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50:291–313 [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme J-F, Baulac M, Brice A, Bruzzone R, LeGuern E (2001) First evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat Genet 28:46–48 [DOI] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P (2001) De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet 68:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ (1996) Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem 271:89–96 [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B (1990) Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron 5:781–788 [DOI] [PubMed] [Google Scholar]
- Escayg A, Heils A, MacDonald BT, Haug K, Sander T, Meisler MH (2001) A novel SCN1A mutation associated with generalized epilepsy with febrile seizures plus—and prevalence of variants in patients with epilepsy. Am J Hum Genet 68:866–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulc S, Huberfeld G, an-Gourfinkel I, Brice A, Le Guern E, Moulard B, Chaigne D, Buresi C, Malafosse A (2000) Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet 24:343–345 [DOI] [PubMed] [Google Scholar]
- Kittler JT, Wang J, Connolly CN, Vicini S, Smart TG, Moss SJ (2000) Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using gamma 2 subunit green fluorescent protein chimeras. Mol Cell Neurosci 16:440–452 [DOI] [PubMed] [Google Scholar]
- Klausberger T, Fuchs K, Mayer B, Ehya N, Sieghart W (2000) GABA(A) receptor assembly: identification and structure of gamma(2) sequences forming the intersubunit contacts with alpha(1) and beta(3) subunits. J Biol Chem 275:8921–8928 [DOI] [PubMed] [Google Scholar]
- Moulard B, Guipponi M, Chaigne D, Mouthon D, Buresi C, Malafosse A (1999) Identification of a new locus for generalized epilepsy with febrile seizures plus (GEFS+) on chromosome 2q24-q33. Am J Hum Genet 65:1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, DeLorey TM, Gordey M, Kang MH (1999) GABA receptor function and epilepsy. Adv Neurol 79:499–510 [PubMed] [Google Scholar]
- Scheffer IE, Berkovic SF (1997) Generalized epilepsy with febrile seizures plus—a genetic disorder with heterogeneous clinical phenotypes. Brain 120:479–490 [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA (1998) GABA synapses enter the molecular big time. Nat Med 4:1115-1116 [DOI] [PubMed] [Google Scholar]
- Singh R, Andermann E, Whitehouse WPA, Harvey AS, Keene DL, Seni M-H, Crossland KM, Andermann F, Berkovic SF, Scheffer IE (2001) Severe myoclonic epilepsy of infancy: extended spectrum of GEFS+. Epilepsia 42:837–844 [DOI] [PubMed] [Google Scholar]
- Singh R, Scheffer IE, Crossland K, Berkovic SF (1999) Generalized epilepsy with febrile seizures plus: a common childhood-onset genetic epilepsy syndrome. Ann Neurol 45:75–81 [DOI] [PubMed] [Google Scholar]
- Stuhmer W (1998) Electrophysiologic recordings from Xenopus oocytes. Methods Enzymol 293:280–300 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Tsurubuchi Y, Agarwala KL, Ito M, Fukuma G, Mazaki-Miyazaki E, Nagafuji H, Noda M, Imoto K, Wada K, Mitsudome A, Kaneko S, Montal M, Nagata K, Hirose S, Yamakawa K (2001) A missense mutation of the Na+ channel αII subunit gene Nav1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci USA 98:6384–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrall S, Hall ZW (1992) The N-terminal domains of acetylcholine receptor subunits contain recognition signals for the initial steps of receptor assembly. Cell 68:23–31 [DOI] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser BN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF (2001a) Mutant GABAA receptor γ2 subunit in childhood absence epilepsy and febrile seizures. Nat Genet 28:49–52 [DOI] [PubMed] [Google Scholar]
- Wallace RH, Scheffer IE, Barnett S, Richards M, Dibbens L, Desai RR, Lerman-Sagie T, Lev D, Mazarib A, Brand N, Ben-Zeev B, Goikhman I, Singh R, Kremmidiotis G, Gardner A, Sutherland GR, George AL Jr, Mulley JC, Berkovic SF (2001b) Neuronal sodium-channel α1-subunit mutations in generalized epilepsy with febrile seizures plus. Am J Hum Genet 68:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George AL Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC (1998) Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel β1 subunit gene SCN1B. Nat Genet 19:366–370 [DOI] [PubMed] [Google Scholar]