Abstract
Ranolazine is a novel anti-anginal agent acting through pharmacologic mechanism of inhibition of the late phase of the inward sodium current. In addition, it is a potent inhibitor of rapid delayed rectifier potassium currents, leading to prolongation of the QT interval. However, ranolazine has not yet described to be associated with Torsade de Pointes despite its QT-prolonging effect. In this case report, we describe a patient on ranolazine who developed Torsade de Pointes and discuss about the potential contribution of ranolazine to the development of Torsade de Pointes.
Keywords: Ranolazine, Prolonged QT interval, Torsade de Pointes
1. Introduction
Torsade de Pointes (TdP), is a unique form of ventricular tachyarrhythmia associated with a prolonged QT interval on surface EKG. This arrhythmia may be caused by electrolyte abnormalities, medications, and congenital abnormalities. It is important to diagnose and treat TdP appropriately, since its treatment is different from other common arrhythmias. An inappropriate treatment may be harmful and can result in death.
Ranolazine, a piperazine derivative, is a newly approved anti-anginal agent functioning through the inhibition of the late phase of the inward sodium current (late INa).1 Compared with other conventional anti-anginal agents, ranolazine does not compromise hemodynamic stability. However, ranolazine increases the corrected QT interval by approximately 6 ms, with 5% of patients having prolongation of 15 ms or longer.2 Interestingly, in the MERLIN study, a lower incidence of arrhythmias was noted in the active treatment arm, indicating an antiarrhythmic effect of ranolazine.3 TdP was not seen in the more than 3500 patients included in CARISA and MERLIN trials who received up to 2000 mg ranolazine daily. Indeed, ranolazine has not yet described to be associated with TdP despite its QT-prolonging effect. In this case report, we describe a patient on ranolazine who developed TdP and discuss about the potential contribution of ranolazine to the development of TdP.
2. Case history
A 69 year old women presented to the emergency department (ED) with chest pain and shortness of breath for 1 h. Her past medical history was significant for hypertension, coronary artery disease, s/p coronary artery bypass graft 10 years ago and stenting due to severe angina pectoris. In addition, the patient had dyslipidemia, depression and asthma.
The patient had been feeling sick in the week prior to admission with fever and malaise. On the day of admission, she woke up with sharp and non-radiating persistent substernal chest pain lasting for 1 h. The chest pain was associated with shortness of breath and vomiting.
Her past medical treatment included atorvastatin 80 mg qhs, fluoxetine 20 mg daily, KCl 20 meq daily, losartan 100 mg daily, amlodipine 5 mg daily, alprazolam 0.25 mg TID, atenolol 100 mg daily, isosorbide 60 mg daily, cimetidine 300 mg daily, clopidogrel 75 mg daily, bumetanide 1 mg daily, albuterol and atrovent inhaler PRN, meloxicam 15 mg daily, pantoprazole 40 mg daily, NTG 0.4 mg PRN, and ranolazine (Ranexa®) 500 mg daily.
She had also a history of hyperlipidemia, and a positive family history for coronary artery disease. She denied smoking, ethanol and drug abuse. She did not use over-the-counter medications.
Physical examination on admission: obese white female, lethargic but arousable and responds to questions appropriately. Vital signs: BP-203/105, P-87, RR-18, Temp-103.7, Sat-89% on room air.
Skin: no rash noted. There was no lymphadenopathy. Her head was normocephalic and atraumatic, pupils – normal size, equal and reactive to light and accommodation. Neck was supple, no JVD or bruits were noted. Lungs were clear to auscultation. Heart: S1 and S2 were physiologic, a 2/6 systolic murmur was heard on the left lower sternal border. Her abdomen was soft without tenderness and bowel sounds were normal. Examination of the lower extremities revealed pitting edema (+2) and palpable pulses. The patient was alert and oriented, with no focal neurological deficits.
EKG on admission showed normal sinus rhythm (85 BPM), PR internal, QRS and QT internal within normal range, Q in L3, slight ST elevation (<1 mm) and slight ST depression with inverted T waves in the lateral wall leads (L1 and AVL) (Fig. 1).
Fig. 1.

EKG on presentation.
Chest X-ray revealed and enlarged cardiac shadow with pulmonary congestion. No consolidation or pleural effusions were noted.
Laboratory results: WBC-9200, Hgb-13.5, Hct-40.9, platelets-155,000. Sodium-142, potassium 3.6, chloride-102, CO2-25, BUN-9 and creatinine-0.8. Glucose-180, calcium 9.1, magnesium 1.4, BNP-362. First set of cardiac enzymes was negative. Urine analysis was clear, showing many bacteria with trace leukocyte esterase, positive nitrite and 4 WBC/hpf. Protein, glucose and ketones were negative.
Ceftriaxone 1 g IV was started due to a presumed urinary tract infection. The patient received NTG 0.4 mg twice, aspirin 325 mg, amlodipina 5 mg and atenolol 100 mg in addition to her regular medications to treat her hypertension and suspected unstable angina. Her blood pressure promptly decreased to 134/86. After several hours in the ED, the patient became suddenly unresponsive, in cardio-respiratory arrest. Her monitor showed polymorphic VT (Fig. 2). CPR was started and defibrillation was preformed (200 J) with prompt conversion to normal sinus rhythm. The patient was intubated. Amiodarone, 150 mg IV was given, followed by IV drip (1 mg/min). IV furosemide 80 mg was also given and the patient was admitted to the ICU. After regaining consciousness, the patient self-extubated. She developed further episodes of polymorphic VT, converting spontaneously to NSR (Fig. 3, Fig. 4). Each episode continued for a maximum of 50 s. The patient remained alert with relative bradycardia (55 BPM), and otherwise normal vital signs. She was placed on 100% non-rebreather mask.
Fig. 2.

Monitor recording during cardio-respiratory arrest and further episodes of polymorphic VT (a).
Fig. 3.

A second episode of polymorphic VT in the form of torsade des pointes. Note the onset of the episode with a prolonged QT (>600 ms), associated with an R on T phenomenon and the short/long/short cycles prior to the onset of the VT, which are characteristic of torsades. Note the prolonged QT in the lower strip after spontaneous conversion to sinus.
Fig. 4.
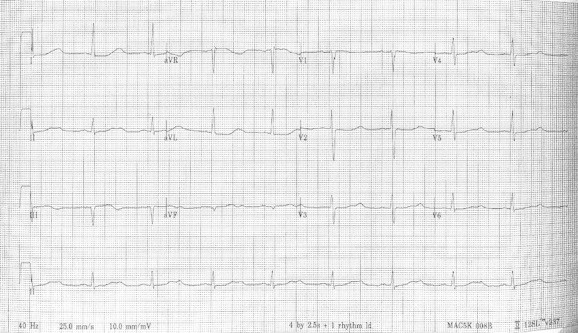
12 lead ECG showing sinus bradycardia (55 BPM) and prolonged QT (640 ms), note the notched appearance of the T wave in L2, AVF, and in V4–V6.
TdP was diagnosed, consistent with the onset of the arrhythmia (short/long/short cycles) and its association with prolonged QT (see Fig. 3). The patient received magnesium 2 g IV and KCl 40 meq IV. Atenolol and amiodarone were discontinued. A transcutaneous pacemaker has been placed, with a defibrillator at bedside. During her ICU stay, the patient was asymptomatic, with no additional episodes of arrhythmia.
3. Discussion
In this case, we describe a 69 year old woman, with a past medical history of coronary artery disease manifesting as severe angina pectoris, status post revascularization (surgical and per-cutaneous), who was admitted because of shortness of breath and chest pain. The patient received multiple medications including fluoxetine, diuretics, beta-blockers and ranolazine (Ranexa). This medication has been started one week prior to her admission due to refractory angina. During her stay in the ED, the patient developed cardiac arrest, with pulseless polymorphic ventricular tachycardia which was promptly defibrillated, and additional episodes of ventricular arrhythmias in the form of Torsade de Pointes.
Torsade de pontes is a unique form of polymorphic ventricular tachycardia that occurs in the setting of acquired or congenital prolongation of the QT interval.4,5 Both congenital and acquired long QT syndrome are caused by abnormalities of the ionic currents underlying ventricular repolarization. In specific settings, such as bradycardia, these conditions may cause polymorphic ventricular tachycardia. This form of VT is characterized by a cyclic alteration of the QRS axis, and a ‘twisting’ of the QRS complexes around the isoelectric line, hence its name ‘Torsade de Pointes’ (twisting of points). This arrhythmia is usually short lived and terminates spontaneously. However, it may degenerate to ventricular fibrillation if untreated.4,5
Numerous conditions can cause prolonged QT and induce TdP. These conditions include inherited mutations of ion channels,6 idiopathic, metabolic abnormalities (hypokalemia, hypocalcemia and hypomagnesemia), and bradyarrhythmias. Medications are an important cause of prolonged QT and TdP. These medications include antiarrhythmic drugs, antibiotics, anti-histamine and psychotropic medications, and their combinations.7 Antiarrhythmic drugs (especially classes Ia and III) are especially prone to cause TdP due to their specific property called reverse use dependence, defined as an inverse correlation between their potency (and their effect on repolarization) and heart rate.8 This phenomenon explains the tendency of antiarrhythmic drugs to cause QT prolongation and TdP during bradycardia.
The patient described in the case report had several predisposing factors for TdP including gender, age, hypokalemia, hypomagnezemia and ischemic heart disease. She was treated by several medications known to increase QT interval (fluoxetine, and ranolazine). In addition, she received cimetidine which is known to interact with cytochrome P450.9 Of the patient's medications, ranolazine was the only new medication, which was started one week prior to this admission.
Ranolazine, a piperazine derivative, is a newly approved anti-anginal medication. In CARISA trial, ranolazine has been shown to provide anginal relief and increased exercise duration in patients with severe chronic angina receiving standard doses of beta-blockers or calcium channel blockers.10 Ranolazine has been found to be a safe medication in a large multi-center randomized study (MERLIN-TIMI-36 study). This medication has a unique mechanism of action. It inhibits the late phase of sodium current (late INa), thus preventing subsequent calcium overload.3,11 In addition it is a potent inhibitor of IKr.12 Through its effect on the delayed rectifier (IKr) ranolazine was found to increase QT interval with a resultant decrease of dose or withdrawing medication in 1% of patients.11 Interestingly, in MERLIN study, a lower incidence of arrhythmias was noted in the active treatment arm, indicating an antiarrhythmic effect.3 Torsades was not seen in more than 3500 patients included in CARISA and MERLIN trials and received up to 2000 mg ranolazine daily. Indeed, ranolazine has not yet described to be associated with Torsade de Pointes despite its QT-prolonging effect.
Multiple causes probably contributed to the development of TdP in this patient. The patient received also fluoxetine, and diuretics causing hypomagnezemia and hypokalemia. In addition, she was treated with cimetidine that could potentiate the effect of other medications through its effect on cyt P450. Thus, the importance of ranolazine as a contributing factor to the development of TdP cannot be ascertained. Yet, ranolazine causes prolongation of the QT interval. In addition, this was the only medication that the patient began receiving recently. Therefore, it reasonable to conclude that indeed, ranolazine contributed to the development of TdP in this patient, among other causes.
The management of TdP secondary to acquired long QT is different from congenital long QT syndrome. The acute treatment in patients with hemodynamically unstable TdP is non-synchronized electric defibrillation.5 Magnesium sulfate is a first line therapy and it is highly effective in treating and preventing recurrence of TdP. Standard therapy includes 2 g. IV followed by a second bolus if needed.5,13–15 Temporary pacing is reserved to treat patients who do not respond to magnesium. Pacing at 100 BPM reduce subsequent recurrences of TdP.5,14 Isoproterenol, titrated to achieve a heart rate of 100 BPM is also useful, but is generally used as a temporizing measure before pacing.5,14 Subsequent management includes discontinuing all medications prone to develop TdP, and treating electrolyte abnormalities (including hypokalemia and hypomagnezemia). Patients at risk of developing bradycardia, or pause related TdP may require a permanent pacemaker.
In summary, Torsade de Pointes is a unique form of polymorphic VT associated with prolonged QT on EKG. TdP can have multiple causes including congenital anomalies, medication and electrolyte abnormalities. Treatment includes correction of electrolyte abnormalities, removal of offending medications, IV magnesium and pacing. In this case, we first demonstrate that ranolazine may contribute to the development of TdP by its QT-prolonging effect.
Conflicts of interest
All authors have none to declare.
Acknowledgment
I would like to thank Dr. Bupesh Panwar and Dr. Deepti Bahl who helped me write this manuscript.
Contributor Information
Zhigang Liu, Email: zgliu0805@gmail.com.
Boaz D. Rosen, Email: rosen.boaz@gmail.com.
References
- 1.Keating G.M. Ranolazine: a review of its use as add-on therapy in patients with chronic stable angina pectoris. Drugs. 2013;73:55–73. doi: 10.1007/s40265-012-0005-z. [DOI] [PubMed] [Google Scholar]
- 2.Hawwa N., Menon V. Ranolazine: clinical applications and therapeutic basis. Am J Cardiovasc Drugs. 2013;13:5–16. doi: 10.1007/s40256-012-0003-2. [DOI] [PubMed] [Google Scholar]
- 3.Scirica B.M., Morrow D.A., Hod H. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 4.El-Sherif N., Turitto G. Torsade de pointes. Curr Opin Cardiol. 2003;18:6–13. doi: 10.1097/00001573-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Passman R., Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85:321–341. doi: 10.1016/s0025-7125(05)70318-7. [DOI] [PubMed] [Google Scholar]
- 6.Roden D.M. Clinical practice. Long-QT syndrome. N Engl J Med. 2008;358:169–176. doi: 10.1056/NEJMcp0706513. [DOI] [PubMed] [Google Scholar]
- 7.Roden D.M. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 8.Hondeghem L.M., Snyders D.J. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- 9.Liu B.A., Juurlink D.N. Drugs and the QT interval – caveat doctor. N Engl J Med. 2004;351:1053–1056. doi: 10.1056/NEJMp048192. [DOI] [PubMed] [Google Scholar]
- 10.Chaitman B.R., Pepine C.J., Parker J.O. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 11.Morrow D.A., Scirica B.M., Karwatowska-Prokopczuk E. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 12.Chaitman B.R. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 13.Tzivoni D., Banai S., Schuger C. Treatment of torsade de pointes with magnesium sulfate. Circulation. 1988;77:392–397. doi: 10.1161/01.cir.77.2.392. [DOI] [PubMed] [Google Scholar]
- 14.Khan I.A. Long QT syndrome: diagnosis and management. Am Heart J. 2002;143:7–14. doi: 10.1067/mhj.2002.120295. [DOI] [PubMed] [Google Scholar]
- 15.Zipes D.P., Camm A.J., Borggrefe M. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]


