Abstract
Introduction
Right ventricular infarction, previously thought to be rare and recently thought to be common, is commonly associated with inferior wall myocardial infarction. We will hereby study the clinical profile of right ventricular infarction (as diagnosed by right precordial electrocardiography) in patients with acute inferior wall myocardial infarction and the clinical course of RV infarction in inferior wall myocardial infarction in terms of complications.
Aims and objectives
1. To study the incidence of right ventricular infarction in patients of acute inferior myocardial infarction using right precordial electrocardiography. 2. To study the complications of right ventricular infarction. 3. To study the response of specific therapy in patients with right ventricular infarction.
Materials & methods
50 patients with a history of chest pain less than 24 h were included in the study, standard 12 leads electrocardiography along with right side chest leads were taken on admission and on daily morning at 7 AM routinely for the first three days. A detailed clinical examination was done to find out the presence of right ventricular failure, left ventricular failure, hypotension and cardiogenic shock at the time of admission. Each patient was subjected to investigations viz. cardiac enzymes. Patients were grouped into two groups group A and group B according to the presence or absence of right ventricular infarction respectively.
Results
Of the total studied 50 patients, 16 patients had right ventricular infarction in association with inferior wall infarction of left ventricle. Complicated course was present in 75% of patients in group A as compared to 29.42% of patients in group B.
Conclusion
Complications and in-hospital mortality rates were more common in patients with right ventricular infarction than in patients without it.
Keywords: Right ventricular infarction, Acute inferior wall myocardial infarction, Electrocardiography
1. Introduction
Myocardial infarction was previously thought to be a disease of mainly the left ventricle. Right ventricular infarction was just a pathological entity. Several authors had recognized the existence of the right ventricular dysfunction in context of acute myocardial infarction but little attention was paid to its clinical aspects. In 1974, Cohn1 for the first time described potentially serious and unique haemodynamic consequences of right ventricular infarction. The advent of more sophisticated diagnostic techniques and more precise haemodynamic measurement has demonstrated that right ventricular infarction is well defined clinical entity and value of recognizing patients with predominant right ventricular dysfunction is related not only to instituting appropriate therapy for severe pump failure but also to avoid inappropriate therapy. Although isolated right ventricular infarction had been described in autopsy reports as less than 3% of all acute myocardial infarction,2 the incidence of right ventricular infarction associated with inferior wall myocardial infarction has been shown to be as high as 30%–50%.3 It has also been shown that right ventricular infarction occurs exclusively in association with inferior myocardial infarction or inferoposterior myocardial infarction4 (Fig. 4). Prompt fluid therapy may abort the vicious cycle set to motion by right ventricular infarction, which if treated in conventional way or neglected tends to lead to true cardiogenic shock.5 It is known that ST segment elevation of 0.1 mV or greater in one or more of right precordial leads V4R to V6R is highly sensitive (90%) and specific (91%) in identifying acute right ventricular infarction. Thus the present study deals with clinical profile of right ventricular infarction as diagnosed by right precordial electrocardiography in patients with acute inferior myocardial infarction. Main purpose of selecting this study was to become aware of right ventricular infarction and its various complications which not only requires appropriate therapy but also avoidance of inappropriate therapy that might reduce right ventricular filling pressure and cardiac output, which, in turn, may, at times, prove disastrous.
Fig. 4.

Electrocardiogram showing inferior wall myocardial infarction.
2. Materials & methods
Fifty patients of acute inferior wall myocardial infarction admitted to a tertiary care centre in Karad between December 2010 and December 2012 were included in the present study.
Exclusion criteria:
-
1.
History of chest pain of more than 24 h duration.
-
2.
Patients whose initial ECG's showed an anteroseptal or anterior wall myocardial infarction were also excluded because these infarctions, may produce an anteriorly oriented ST vector which may also cause ST segment elevation in the right precordial, leads. For the same reason patients with pericarditis were also excluded. Patients with left bundle branch block were also excluded for the same reason.
-
3.
Patients with chronic lung disease for pulmonale were also excluded because they may be associated with a right ventricular dysfunction.
-
4.
Patients with previous history of a myocardial infarction were also excluded to avoid a false positive result for right precordial electrocardiography.
At the time of admission a seventeen lead EKG consisting of twelve conventional leads; and additional right precordial leads V3R, V4R, V5R, V6R were taken and subsequent recordings were made for conventional and right precordial leads. For recordings, a single channel EKG machine was used. The points on chest wall used for recordings chest leads were marked with a skin pencil so that same points were used serially in a given patient. The diagnosis of acute inferior wall myocardial infarction was made as typical history of chest pain, ST segment elevation in leads II, III and avF and by development of pathological q waves in the above mentioned leads. Similar changes in leads V5 & V6 (lateral extension) and lead V2 diagnoses true posterior wall infarction. Tall ‘R’ waves in V1 & V2 and increased serum cardiac enzymes (CK-MB and Troponin-T[quantitative]). The diagnosis of right ventricular was made as by the criteria of – ST segment elevation at 0.1 mV or more in one or more of the right precordial leads (V3R, V4R, V5R & V6R) in those patients who satisfied the criteria for an inferior wall myocardial infarction. After patients were settled haemodynamically and shifted from MICU to recovery room, a ‘M’ mode echocardiography along with colour Doppler study were performed in these selected cases to confirm the diagnosis of right ventricular dilatation. Ventricular diastolic and systolic diameters were measured and considered acceptable only if delineation of septal right and left endocardial surfaces were technically adequate and below the level of mitral valve. Right ventricular dilatation was considered to be present if right ventricular diameter exceeds 25 mm.
2.1. Patients were grouped into two groups
Group A: Inferior wall infarction with right ventricular infarction.
Group B: Inferior wall infarction without right ventricular infarction.
Their clinical course was studied and compared during entire hospital stay. Serial ECG's were taken on admission, at the end of 6 h, end of 12 h and daily till ST segment became isoelectric in right precordial leads. ECG's were also repeated whenever patient complained of chest pain. Patients were continuously monitored. The clinical course, ECG analysis and M mode echocardiography along with colour Doppler study were compared in both the groups. Complications in both the groups were analysed.
2.2. Following clinical findings were considered for comparison viz
-
1.
Hypotension was defined as systolic arterial blood pressure <80 mm of Hg or which has declined by at least 30 mm of Hg below previous level.
-
2.
Right heart failure was considered when the hepatojugular reflux or abdominal compression test was positive. JVP more than 4 cm in 45° position with pedal oedema and systemic venous congestion.
-
3.
Left heart failure was considered present when bilateral pulmonary rales on inspiration; Dullness on percussion over lung bases and on auscultation S3 and S4 was present. Peripheral pulse shows pulsus alternans. Chest X-ray shows Kerley B lines. Present study deals with clinical profile of right ventricular infarction as diagnosed by right precordial electrocardiography in patients of acute inferior wall myocardial infarction. The results obtained were analysed for their statistical significance using fishers exact test and unpaired ‘t’ test.
3. Results
Out of a total of fifty patients of acute inferior wall myocardial infarction, right ventricular infarction was associated in sixteen patients (32%) and thirty four (68%) had inferior wall infarction without right ventricular infarction. Right ventricular infarction in 32% cases positive for right ventricular infarction were more common in inferior wall infarction while it was less common in inferolateral wall infarction. This finding of ours is almost similar to the study done by Overgaard et al.6 There were 32 cases of inferior wall myocardial infarction out of which 12 were positive for right ventricular infarction (RVI). Only 1 case was positive for RVI out of 8 cases of inferolateral infarction. Out of 10 cases of inferior and true posterior infarction, 3 cases were positive for RVI (Table 2). Comparison of the two groups showed no significant differences in any of the observed characteristics.
Table 2.
Distribution of cases according to the site of infarction.
| Type of infarction | Positive for RVI | Negative for RVI | Total |
|---|---|---|---|
| Inferior | 12 | 20 | 32 |
| Inferolateral | 1 | 7 | 8 |
| Inferior and true posterior | 3 | 7 | 10 |
| Total | 16 | 34 | 50 |
There was no significant difference between the two groups regarding mean pulse rate, basal creps, cardiac murmurs or hepatomegaly (Table 3). Raised jugular venous pressure (JVP) central venous pressure >10 cm (CVP) of water, hypotension (systolic Bp <80 mm Hg) and right sided third heart sound were found to be more common in group A than group B and the difference was found to be statistically significant (P < 0.01). Kussmaul's sign was also found to be highly significant physical sign found exclusively in group A patients (P < 0.01). ST segment elevation of ≥0.1 mV (l mm) was seen most commonly in V4R (100%) as compared to V3R (93.75%), V5R (87.5%) and V6R (68.75%). Although the diagnosis of right ventricular infarction was made on ST segment elevation in any of the above mentioned leads, the single most important lead in the diagnosis of right ventricular infarction in the present study was lead V4R.
Table 3.
Clinical profile of the patients.
| Group A |
Group B |
||||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
 |
SEX (FEMALES) | 10 | 62.5 | 14 | 41.2 |
| CIGARETTE SMOKING | 7 | 43.7 | 15 | 44.1 | |
| TOBACCO CHEWING/ALCOHOL INTAKE | 5 | 31.24 | 13 | 38.2 | |
| HYPERTENSION | 6 | 37.5 | 12 | 35.2 | |
| DIABETES MELLITUS | 5 | 31.2 | 11 | 32.3 | |
| CHEST PAIN | 16 | 100 | 34 | 100 | |
| DIAPHORESIS | 8 | 50 | 18 | 52.4 | |
| VOMITING | 6 | 37.5 | 12 | 35.29 | |
| SYNCOPE | 9 | 56.25 | 20 | 58.82 | |
 |
PULSE (mean) | 86/min | 86/min | ||
| BP <80 mm Hg | 9 | 56.25 | 3 | 8.82 | |
| JVP | 7 | 43.75 | 2 | 5.88 | |
| CVP >10 | 8 | 66.60 | 1 | 7.69 | |
| RIGHT SIDED S3 | 8 | 50.0 | 0 | 0 | |
| BASAL CREPTS | 1 | 6.25 | 4 | 11.75 | |
| HEPATOMEGALY | 5 | 31.25 | 3 | 8.82 | |
| KUSSMAUL'S SIGN | 8 | 50.0 | 0 | 0 | |
 |
RVF | 7 | 43.75 | 0 | 0 |
| LVF | 3 | 16.75 | 6 | 11.86 | |
| HYPOTENSION | 9 | 56.25 | 4 | 17.67 | |
| AV BLOCK | 12 | 76.0 | 0 | 0 | |
| BUNDLE BRANCH BLOCK | 2 | 12.5 | 4 | 11.86 | |
| VPBs | 7 | 43.75 | 4 | 11.36 | |
| V.TACH. | 1 | 6.25 | 0 | 0 | |
| V.FIBRILLATION | 2 | 13.50 | 0 | 0 | |
| AV DISSOCIATION | 1 | 6.25 | 0 | 0 | |
| ATRIAL ARRHYTHMIAS | 2 | 12.50 | 0 | 0 | |
| DEATH | 1 | 6.25 | 0 | 0 | |
The incidence of bundle branch blocks was similar between two groups. In group A two out of sixteen patients (12.5%) and in group B four out of thirty four patients (11.76%) had bundle branch blocks. All the above patients had right bundle branch blocks. Ventricular premature beats, requiring treatment were present in seven out of sixteen patients (43.75%) in group A as compared to four out of thirty four patients (11.36%) in group B. One patient in group A developed V. tachycardia followed by V. fibrillation. He also had A.V dissociation for which a temporary pacemaker was inserted. He however expired despite all management. None of the patients' in-present study had pericarditis. The total incidence of all arrhythmias was higher in group A (75%) as compared to group B (35.29%). There was however no statistically significant correlation between high incidence of arrhythmia in group A with the amplitude of ST segment elevation in lead V4R and M mode echocardiography in present study. However this could be due to small sample size. On comparing the clinical course in patients of both the groups, in group A – three out of sixteen patients (18.75%) had no complications, twelve out of sixteen patients (75%) had a complicated course and one patient (6.25%) expired. In group B – twenty four out of thirty four patients (70.58%) had no complications. Ten out of thirty four patients (29.42%) had a complicated course. There was a death in group B.
The ST segment elevation of 1 mm or more was most consistently seen in the maximum number of patients in leads V3R, V4R, and V6R of these maximum elevation (>1.5 mm) was seen most commonly in lead V4R. This lead was found to be highly positive.
In most patients the ST segment elevation in right precordial leads became isoelectric within 24 h (62.5%). In the remaining patients it was within 72 h (31%) only in one patient was ST segment still elevated at end of 72 h. The jugular venous pressure was raised in only two patients in group B and in one of them central venous pressure (CVP) was also raised (>10 cm of water). The same patient developed signs of left ventricular failure. Complications were seen more commonly in group A as compared to group B. On comparing the complications in both groups, right heart failure, hypotension and arrhythmias were more common in group A as compared to group B (P < 0.01).
4. Discussion
In 1974, the clinical significance of right ventricular infarction has stimulated many noninvasive modalities for its diagnosis and management. In 1982, simple ECG criteria for the diagnosis of this condition were defined which have proved to be both highly specific and sensitive. Overgaard et al6 in 2002 reported the incidence of right ventricular infarction is 23.7% of patients (384 patients, out of 1619 patients) in patients with inferior wall Ml with the mortality rate of 8.1% in patients with associated right ventricular infarction. The incidence of right ventricular infarction associated with inferior wall myocardial infarction in the present study was 32%. This is comparable with the incidence described in other studies. Six large Indian studies have been published so far. These results are as follows: (Fig. 3)
Fig. 3.

Diagram showing the comparison of results of major Indian studies with our present study for the right ventricular infarction in context to the inferior wall myocardial infarction.
The incidence in present study corroborates well with the other studies (Fig. 3). Criteria of exclusion used were similar to those employed by Saran et al.7
4.1. Clinical features
Only patients who arrived within 24 h of onset of chest pain were included in this study. The incidence in the present study is lower than 52.73% which is reported by Klein et al.8 This could be due to the fact that the patients in the series by Klein et al8 were reported within 10 h of the onset of symptoms, whereas in this study it is up to 24 h. Since Klein et al found that ST deviation in V4R is a very transient finding and sometimes it disappeared within 2 h after the onset of chest pain. In our series ST elevation of 1 mm or more in leads V3R and V4R suggests infarction (Fig. 1), while Klein et al considered ST elevation of 0.5 mm or more as an evidence of right ventricular infarction. Several other factors also influence the degree of ST elevation in V3R and V4R. 1) It will be expectedly less prominent in right precordial leads, if it is not prominent in leads II, III and avF. 2) ST segment in V3R and V4R is rightward as well as interiorly oriented vector. Thus ST deviation seen in leads V5 & V6 produced by lateral wall extension of myocardial infarction could cancel out ST elevation in V3R & V4R. 3) ST elevation indicates transmural ischaemia rather than infarction. There was no difference between the two groups in relation to cigarette smoking, tobacco chewing, alcohol intake, hypertension, diabetes mellitus or ischaemic heart disease. All patients with previous history of myocardial infarction were excluded as per the criteria of exclusion applied in this study. Similar findings have been observed earlier in other studies.
Fig. 1.

ST segment elevation >1 mm in right precordial leads in group A patients.
Jugular venous pressure was raised in seven out of sixteen patients (43.75%) in group A. Two out of thirty tour patients (5.88%) in group B had a raised JVP jugular venous pulse pressure. This difference was highly significant for the two groups (P < 0.01). Croft et al found a raised jugular venous pressure in 45% of patients with right ventricular infarction in their study. In none of these patients, there were signs of left ventricular failure, pulmonary disease or pericardial effusion. Ashutosh Mohan et al found a raised jugular venous pressure in 77.7% of their patients. Experimental necrosis of the right ventricle has been shown to decrease the ability of the right ventricle to handle a volume load. Any maneuver that stress the right ventricle would help uncover subclinical dysfunction. This is best done at bed side by observing for a Kussmaul sign. This physiological response is altered in right ventricular infarction and may be the earliest haemodynamic change in right ventricular infarction which produces a Kussmaul's sign. None of the patients in present study had tricuspid regurgitation which may complicate right ventricular infarction.
Morphine has been shown to increase infarct size10 and lessen blood flow through coronary arteries by as much as 13%.11 Fentanyl may be given instead of morphine, since morphine also causes vasodilation. Some have questioned the efficacy of morphine in MI, but the American Heart Association still grants it a Class IC classification with the stated precaution: “Use with caution in right ventricular infarction.”12
4.2. Electrocardiography
ST segment elevation in lead V4R correlates closely with occlusion of the proximal right coronary artery, and has 88% sensitivity and 78% specificity for concurrent RVMI in patients with IWMI.13 Erhardt et a114 used a more complicated single lead CR4R and found it to be positive in twenty five out of ninety two cases, all of whom had right ventricular infarction on autopsy. Erhardt et a1 found out & pointed out that the pathogenesis of changes in right precordial leads may be a result of either transmural right ventricular damage or infarction of the posterior interventricular septum, reflected through the necrosis and electrically silent right ventricular myocardium. ST segment elevation in right precordial leads in right ventricular infarction is found to be transient. Fifteen out of sixteen patients in group A survived and in them ST segment position was studied till it became isoelectric in the right precordial leads. Time taken was less than 24 h in ten patients (62.5%); 24–48 h in two patients (15.5%); 48–72 h in two patients (15.5%); >72 h in one patient (6.25%). In the last patient it was isoelectric by almost fifth day. Garg et al15 in 1984 reported ST segment normalization in 30.3% within 24 h in 36.4% within 24–48 h, in 24.2% within 48–72 h and in 9.1% in more than 72 h. Klein et al8 reported ST segment normalization within 24 h in 31.03% of patients and in 57.5% of more than 24 h. Our findings are in agreement with these studies reported by Klein et al,8 Garg et al.15 Considering the transient nature of ST segment elevation in right precordial leads, it is suggested that right precordial electrocardiography be done on admission itself to diagnose the possible association of right ventricular infarction in patients with inferior wall myocardial infarction.
4.3. Clinical course
We studied the clinical course of patients in both groups and compared it. In group A three out of sixteen patients (18.75%) had an uncomplicated course and twelve out of sixteen (75%) patients had complications with death in one (6.25%). In group B twenty four out of thirty four patients (70.58) had an uncomplicated course; ten out of thirty four patients (29.42) had complications with no death. Garg et a115 reported a complicated course in 57.65% of patients with right ventricular infarction and 41.8% of patients of isolated inferior wall infarction. Klein et al8 in 1981 reported complicated course in 59% patients with right ventricular infarction as compared to 33% patients with acute isolated inferior wall infarction.
In the present study our findings are also consistent with their studies. There is a high incidence of complications in both the groups but the complication rate is definitely higher in group A as compared to group B.
4.4. Complications
On comparing the complications in both the groups, right heart failure without the presence of left heart failure was seen exclusively in group A patients i.e. seven out of sixteen patients (43–75) (Fig. 2). Ashutosh Mohan et al reported right heart failure in 77.7% of their patients of right ventricular infarction and none in their patients of isolated inferior wall infarction. Garg et a115 found right heart failure in 21.2% of their patients of right ventricular infarction and in 3% of patients of isolated inferior wall myocardial infarction (P < 0.01). The present study is in consistent with the above mentioned studies. Thus right heart failure is due to impaired right ventricular contraction which in turn will lead to an elevated right ventricular end diastolic volume, increased right atrial pressure and features of right ventricular failure. In the present study the clinical severity of right heart failure as studied by CVP >10 cm, right ventricular third heart sound and hypotension (systolic blood pressure <80 mm Hg) correlated well with ST segment elevation (mean 1.5 mm) in lead V4R. Patients with right heart failure also had more incidences of arrhythmias; however the sample size was too small to draw any conclusion. Hypotension (systolic blood pressure <80 mm Hg) was found in nine out of sixteen patients (56.25%) in group A four out of thirty four patients (11.86%) in group B. Garg et al found hypotension in 21.2% of patients with right ventricular infarction and 7.5% of patients with inferior wall infarction alone. Thus there is a significant difference in hypotension seen in patients with and without associated right ventricular infarction. Infarction of right ventricle leads to an impaired contraction, which in turn leads to decreased ejection fraction of the right ventricle, decreased left ventricular filling and hence a low cardiac output state and hypotension. This hypotension can progress to frank cardiogenic shook if left untreated or treated inadequately. Left ventricular failure was present in three out of sixteen patients 18.75% in group A while it was seen in six out of thirty four patients 17.64% in group B. In these circumstances right ventricular infarcts when specially combined with septal infarcts may eventuate in syndrome of predominant right ventricular dysfunction. Left ventricular failing could then be supported by any of these possible mechanism. 1. Increased right ventricular end diastolic volume, which could augment contractile force of the residual functioning fibres in the damaged right ventricle. 2. Increased right atrial contracting force, which might help to propel blood into pulmonary artery in the absence of right ventricular infarction. 3. Passive flow along a right atrial to left atrial pressure gradient through the low resistance pulmonary vascular bed. The recognition of venous engorgement in this setting usually leads the physician to administer a potent diuretic agent. Such therapy is probably contraindicated, if the pulmonary wedge pressure is not greatly increased and left ventricular tilling pressure is largely dependent on passive flow from distended right heart. The goal of therapy is to increase left ventricular output which requires achieving a greater flow into the left ventricle without benefit of a strong right ventricular contraction. Pulmonary vascular resistance must be minimised by maintaining good oxygen. Judicious use of plasma volume expanders or Intravenous infusion of saline may augment left ventricular input by increasing pressure gradient, favouring passive flow through lungs as well as by possibly increasing force of right atrial contraction.
Fig. 2.
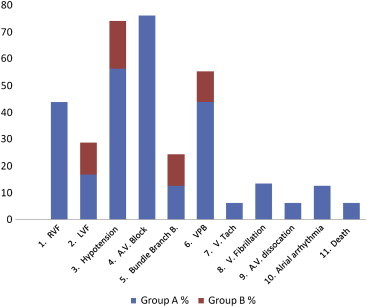
Diagram showing the incidence of complications in both groups.
4.5. Arrhythmias
Our findings are comparable with those reported by Braat et al9 & Garg et al15 several other series have reported a high incidence of II0 and III0 atrioventricular block associated with right ventricular infarction. In higher incidence of heart blocks of grade II or III in association with right ventricular infarction is probably due to the involvement of the region of atrioventricular node which is supplied by right coronary artery. Inferior wall infarction can result either from an occlusion of right coronary artery (RCA) the circumflex branch from left coronary artery or the left anterior descending coronary artery if it comes over apex. Blood supply to AV node is by the way of AV nodal artery. In 90% of cases, the AV nodal artery comes from right coronary artery. In a varying percentage of cases however there is dual blood supply to AV node because not only AV nodal artery but also branches of left anterior descending artery bring blood to AV node. One would expect the incidence of AV nodal conduction disturbances to be close to 90% in patients with right ventricular involvement. The incidence was higher in group A (76%) than group B as expected. An alternative explanation for the AV nodal conduction disturbances would be stimulation of vagal afferents in inferior wall of right and left ventricle. In this case one would expect a higher incidence of sinus bradycardia with a satisfactory response to atropine which was not the case in our group. Complete heart block with right ventricular infarction was more long lasting and required transvenous pacing in the present study. Two out of sixteen patients (12.5%) of group A required the same while none in group B needed it. In group A, nine patients had hypotension with high CVP (range 9–18 cm). These patients were closely monitored for development of signs of right heart failure. Diuretics were not given and vasodilators were withheld. Seven out of these patients went on to develop signs of right ventricular failure and they were treated with I.V fluids in the form of isotonic saline. The amount varied from 1 to 4 lies in 24 h. Four out of seven patients so treated improved whereas three remaining were started on intravenous dopamine hydrochloride in the form of a continuous drip at the rate of 2–10 μg/kg/min after a trial of isotonic saline.
4.6. On-going trials
Certain trials are already going on regarding the ischaemic preconditioning (IPC) where it is believed that IPC reduces RV myocardial infarct size and improves postischemic RV contractile function in the isolated rat heart, possibly through opening of the mitochondrial KATP channel.16 Even the use of levosimendan could be potentially beneficial option.17 Furthermore studies are required to properly manage the right ventricular infarction and prevent its complications.
5. Conclusion
Thus, we, hereby, want to conclude that right ventricular infarction, whenever present in conjunction with inferior wall myocardial infarction, requires aggressive fluid-resuscitation strategies to start with, and once haemodynamically stable, aggressive treatment as per the ACS protocol should be continued and one should be always on their toes for the possibilities of arrhythmias (Table 1).
Table 1.
Stepwise approach in diagnosis and management of right ventricular infarction and its common complications.
| Recognition | ST segment elevation in leads II, III and aVF plus ST segment elevation in leads V3 through V6R. |
| Reperfusion | Thrombolysis |
| Streptokinase | |
| Urokinase | |
| Recombinant tissue plasminogen activator | |
| Tenecteplase | |
| Angioplasty. | |
| Coronary bypass surgery. | |
| Volume loading | Normal saline, 40 ml per minute given IV till to total of 2 L. Keep right atrial pressure of less than 18 mm Hg; haemodynamic monitoring required. |
| Inotropic support | Dobutamine – 2–5 μg per kg per minute given IV, with dose increased every 5–10 min up to 15–20 μg per kg per minute. |
| Rate and rhythm control | Symptomatic bradycardia: atropine, 0.6 mg given IV every 5 min up to total of 2.5 mg. AV block: AV sequential pacing (usually short term) |
| Common complications |
LV ischaemic dysfunction: judicious afterload reduction, volume restriction. Cardiogenic shock: intra-aortic balloon pump. Interventricular septal rupture: emergency surgical repair. RV papillary muscle ruptures and tricuspid regurgitation: emergency surgical repair. |
Conflicts of interest
All authors have none to declare.
References
- 1.Cohn J.N. Right ventricular infarction revisited. Am J Cardiol. 1979;43:666. doi: 10.1016/0002-9149(79)90030-4. [DOI] [PubMed] [Google Scholar]
- 2.Lovell B., Leinbach R.C., Pohost J.M. Right ventricular infarction; clinical diagnosis and differentiation from cardiac tamponade and pericardial constriction. Am J Cardiol. 1979;43:465. doi: 10.1016/0002-9149(79)90001-8. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein J.A. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002;40:841. doi: 10.1016/s0735-1097(02)02048-x. [DOI] [PubMed] [Google Scholar]
- 4.Khan S., Kundi A., Sharieff S. Prevalence of right ventricular myocardial infarction in patients with acute inferior wall myocardial infarction. Int J Clin Pract. 2004;58:354–357. doi: 10.1111/j.1368-5031.2004.00030.x. [DOI] [PubMed] [Google Scholar]
- 5.Dimitrios G., Ketikoglou M.D., Karvounis H.I., Papadopoulos C.E., Theodra A. Echocardiographic evaluation of spontaneous recovery of right ventricular systolic and diastolic function in patients with acute right ventricular infarction associated with posterior wall left ventricular infarction. Am J Cardiol. 2004;93:911–913. doi: 10.1016/j.amjcard.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard C., Fitchett D. Cardiogenic shock from right ventricular infarction. Cardiol Rounds. 2002;VII(8) [Google Scholar]
- 7.Saran R.K., Ahuja R.C. The value of serial right precordial ECGs in acute myocardial infarction. J Assoc Physicians India. 1987;35:263. [PubMed] [Google Scholar]
- 8.Klein H.O., Tordjman, Ninio R. The early recognition of right ventricular infarction diagnostic accuracy of electrocardiographic lead V4R. Circulation. 1983;67:558. doi: 10.1161/01.cir.67.3.558. [DOI] [PubMed] [Google Scholar]
- 9.Braat S.H., Braguda P., Dulk K.D., Ommen V., Wellens H.J.J. Value of lead V4R for recognition of the infarct coronary artery in acute inferior myocardial infarction. Am J Cardiol. 1984;53:1538–1541. doi: 10.1016/0002-9149(84)90575-7. [DOI] [PubMed] [Google Scholar]
- 10.Markiewicz W., Finberg J., Lichtig C. Morphine increases myocardial infarction size in rats. Anesth Analg. 1982;61:843–846. [PubMed] [Google Scholar]
- 11.Hilfiker O., Larsen R., Brockschneider B. Morphine in coronary blood flow and oxygen consumption in patients with coronary artery disease. Anaesthesist. 1982;31:371–376. [PubMed] [Google Scholar]
- 12.American Heart Association Handbook of Emergency Cardiovascular Care, Guidelines CPR-ECC. 2005. p. 58. [Google Scholar]
- 13.Menown I.B., Allen J., Anderson J.M. Early diagnosis of right ventricular or posterior infarction associated with inferior wall left ventricular acute myocardial infarction. Am J Cardiol. 2000;85:934–938. doi: 10.1016/s0002-9149(99)00904-2. [DOI] [PubMed] [Google Scholar]
- 14.Erhardt L.R., Sjogren A. Electrocardiographic changes in right ventricular infarction. A case report. Acta Med Stand. 1978;204:331. doi: 10.1111/j.0954-6820.1978.tb08448.x. [DOI] [PubMed] [Google Scholar]
- 15.Garg K.C., Pathak P.K., Agrawal A. Right ventricular infarction in acute inferior myocardial infarction. The importance of right precordial electrocardiography. Am Heart J. 1984;36:202. [PubMed] [Google Scholar]
- 16.Andersen Asger, Povlsen Jonas Agerlund, Bøtker Hans Erik, Nielsen-Kudsk Jens Erik. Ischemic preconditioning reduces right ventricular infarct size through opening of mitochondrial potassium channels. Cardiology. 2012;123:177–180. doi: 10.1159/000342481. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz Bailén M., Ruiz García M.I., Ferrezuelo Mata A., Quirós Barrera R. Cardiogenic shock: management of right ventricular infarction shock. Minerva Cardioangiol. 2012 April;60(2):167–174. [PubMed] [Google Scholar]


