Abstract
Fingolimod hydrochloride (FTY720) is the first in class of sphingosine 1-phosphate (S1P) receptor modulator approved to treat multiple sclerosis via down-regulation of G protein-coupled S1P receptor 1 by its phosphorylated form (FTY720-P). Many studies have revealed that FTY720 exerts various biological effects, including antitumor activities, angiogenesis inhibition, Ca2+ mobilization and apoptosis, independently of S1P receptors. However, the exact mechanisms underlying their effects or signaling pathways mediated by FTY720 have not been completely established. To gain further insights into molecular mechanisms of FTY720 action, the effect of FTY720 on Ca2+ signaling in fission yeast was analyzed. The addition of Ca2+ enhanced the sensitivity induced by FTY720, and mutants lacking genes required for calcium homeostasis, including calcineurin and its downstream transcription factor, Ppb1-responsive zinc finger protein (Prz1), were hypersensitive to FTY720 and CaCl2. The effect of FTY720 on calcineurin signaling was monitored by utilizing a luciferase reporter construct fused to three tandem repeats of the calcineurin-dependent response element (CDRE), which gives an accurate measure of calcineurin activity. The addition of FTY720 increased calcineurin activity as well as Ca2+ influx in a concentration-dependent manner. Notably, the FTY720-mediated Ca2+ influx and calcineurin activation were reduced markedly by the deletion of yam8 + or cch1 + encoding putative subunits of a Ca2+ channel. Consistently, the deletion of Pmk1 mitogen-activated protein kinase (MAPK), which plays an important role in the activation of the Yam8/Cch1 channel, markedly decreased the intracellular Ca2+ levels upon FTY720 treatment. These results suggest that the FTY720-stimulated Ca2+/calcineurin signaling activation partly involves the Yam8/Cch1 channel in fission yeast.
Introduction
Fingolimod hydrochloride (FTY720) is a novel sphingosine 1-phophate (S1P) receptor modulator that was found by chemical modification of myriocin, a natural product isolated from culture filtrates of the ascomycete Isaria sinclairii [1]. FTY720 inhibits lymphocyte egress from lymph nodes to efferent lymphatics and blood, and the immunomodulating effects of FTY720 are largely elicited following its phosphorylation by sphingosine kinase (SphK)2 and the subsequent modulation of G protein-coupled S1P receptor 1 [2,3]. Although the biological effects of FTY720 have been generally attributed to its actions as an S1P mimetic upon its phosphorylation, considerable evidence suggests that FTY720 may act through more than one target.
Interestingly, in addition to its therapeutic use as an immunomodulating drug, FTY720 was also shown to exert potent antitumor and antimetastatic activities in different tumor types, including breast cancer, bladder cancer, hepatocellular carcinoma, and leukemia [4,5]. Several hypotheses explain the antitumor activity of FTY720. Reports have shown that FTY720 induced the mitochondrial permeability transition and consequent activation of caspases, with the modulation of these processes by the mitochondrial gatekeeper Bcl-2 family proteins [6,7]. FTY720 is also known to downregulate prosurvival mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase/Akt pathways and upregulate stress-activated kinases such as p38 [8,9]. FTY720 also increases the intracellular concentration of calcium ions and induces apoptosis in HL-60 [10].
Accumulating evidence also suggests that FTY720 may exert some of these effects independently of S1P receptors by modulating a range of other recently described proteins targeted by nonphosphorylated FTY720 [11]. For example, FTY720 inhibits cytosolic phospholipase A2 independently of its phosphorylation and S1P receptor functions [12]. However, although diverse physiological and therapeutic effects have been documented for this compound, the multifaceted mechanism of the action of FTY720 remains unclear.
This study uses fission yeast as a model eukaryotic system to dissect the biological activity of FTY720. The fission yeast Schizosaccharomyces pombe and the budding yeast Saccharomyces cerevisiae are among the simplest eukaryotic organisms that are widely used as valuable tools for the study of basic cellular processes and pathways relevant to higher eukaryotes, including mechanisms of cell cycle control, metabolism, and membrane trafficking [13,14]. Both these strains are also excellent organisms for the identification of molecular targets and elucidation of molecular/cellular mechanisms of sensitivity to various drugs because the major signaling pathways and processes involved in the cellular response to cytotoxic agents are conserved between yeasts and mammalian cells [15-18]. In budding yeast, it has been reported that ubiquitin pathway proteins are involved in the mechanism of action of FTY720 [19] and that FTY20 and phytosphingosine influence a similar pathway in yeast cells [20].
To better understand the signaling pathways mediated by FTY720, the effect of FTY720 on Ca2+/calcineurin signaling was analyzed. In fission yeast, a mutation in ppb1 + encoding the Ca2+/calmodulin-dependent protein phosphatase [17,21] as well as a mutation in prz1 + encoding a transcription factor downstream of calcineurin involved in Ca2+ homeostasis [22] affected the sensitivity of cells to FTY720, suggesting that a disturbance in Ca2+ homeostasis results in increased sensitivity to FTY720. We also demonstrated that FTY720 activates calcineurin signaling by stimulating Ca2+ influx mediated by the Yam8/Cch1 Ca2+ channel in fission yeast.
Materials and Methods
Strains, Media, and Genetic/Molecular Biology Methods
The S. pombe strains used in this study are listed in Table 1. The complete and minimal media used were yeast extract-peptone-dextrose (YPD) and Edinburgh minimal medium (EMM) [23], respectively. Standard genetic and recombinant DNA methods [24] were used, except where stated otherwise. Gene knockouts are denoted by lowercase letters representing the disrupted gene, followed by two colons and the wild-type (wt) gene marker used for the disruption (e.g., ppb1::ura4 +). In addition, gene knockouts are abbreviated by the gene, which is preceded by Δ (e.g., Δppb1). Proteins are denoted by Roman letters, and only the first letter is capitalized (e.g., Ppb1). Genome DNA clones were provided by the National Bio Resource Project, Yeast Genetic Resource Center (Graduate School of Science, Osaka City University).
Table 1. Schizosaccharomyces pombe strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h- leu1-32 | Our stock |
| KP119 | h+ leu1-32 ura4-D18 ppb1::ura4+ | Ma et al., (2011) |
| KP1003 | h -leu1-32 ura4-D18 prz1::ura4+ | Hirayama et al., (2003) |
| KP2758 | h- leu1-32 ura4-D18 yam8::ura4+ | Deng et al., (2006) |
| KP2784 | h- leu1-32 ura4-D18 cch1::ura4+ | Deng et al., (2006) |
| SP983 | h - leu1-32 ura4-D18 yam8::KanMX6 cch1::ura4 + | Our stock |
| SP385 | h – leu1-32 ura4-D18 pmk1::KanMX6 | Bimbó et al., (2005) [44] |
| SP1937 | h – leu1-32 ura4-D18 bfr1::ura4 + pmd1::hisG | kind gift from Minoru Yoshida |
Chemicals
FTY720 and FTY720-P (phosphorylated FTY720) were kindly received as gifts from the Mitsubishi Tanabe Pharma Corporation (Yokohama, Japan). Yeast growth media containing each of these chemicals were prepared by mixing stock solutions of FTY720 or FTY720-P with the YPD or YES medium to achieve the desired drug concentration. For agar media, the stock solution of the appropriate drug was added after autoclaving and cooling of the media to approximately 50°C. For FTY720, the stock solution was prepared using water, whereas for FTY720-P, the stock solution was prepared using 80% ethanol containing 10 mM NaOH [25]. When comparing the effects of FTY720 and FTY720-P, FTY720 was dissolved in the same vehicle as FTY720-P to unify the basal conditions. Each vehicle was added to control media at concentrations equivalent to that in the media supplemented with FTY720 or FTY720-P.
Microscopy and Miscellaneous Methods
Light microscopy methods (e.g., fluorescence microscopy) used to observe the localization of GFP-Prz1 was performed as described previously [26].
Image Quantification
All the image quantifications were performed for three individual datasets, which summed up to 300 counted cells.
Statistical Analysis
The mean and the standard deviation (SD) of the relative light units (RLU) values for three different experiments were calculated for each sample. Data were analyzed using a two-way ANOVA, followed by a posthoc test using Williams’ multiple comparison.
Live Cell Monitoring of Calcineurin-mediated Transcriptional Activity
Calcineurin-dependent response element (CDRE)-reporter activity in living cells was monitored by the multicopy 3×CDRE::luc (R2.2) reporter vector pKB5723 as described previously [27]. We also created the mutant version of the 3×CDRE::luc (R2.2) reporter vector by replacing the wt CDRE oligonucleotide (AGCCTC or GAGGCT) in pKB5723 with the mutant CDRE oligonucleotide (sense 884: 5′-GGC TTA GAT CTA TAC AAG ATC TAT ACA CAA GAT CTA TGCAC-3′; antisense 885: 5′-TCG AGT GCA TAG ATC TTG TGT ATA GAT CTT GTA TAG ATC TAA GCC TGCA-3′) that contains three tandem repeats of the mutated CDRE (AGATCT, underlined), thereby yielding the multicopy 3×mtCDRE::luc(R2.2) reporter vector pKD2767.
Measurement of Cytoplasmic Ca2+ Levels
Cytoplasmic Ca2+ levels were determined using a previously described method with minor modifications [28]. In brief, cells containing GFP-19-apoaequorin (AEQ) were grown at 27°C in the EMM medium and harvested (to midlog phase) in the early logarithmic growth phase. The cells were resuspended in fresh EMM containing 10 μM coelenterazine (Promega), and the optical density was adjusted to 0.6 at 660 nm. To convert AEQ to aequorin, the cells were incubated for 3 h at 27°C. The cells were washed twice, resuspended in fresh EMM, and the optical density was adjusted to 0.6 at 660 nm. The cell culture was then incubated at 27°C for 30 min before initiating the experiment. The light emission levels expressed as RLU were measured using a luminometer (Centro XS3 LB960: Berthold) at 30-s intervals.
Results
FTY720 inhibits growth of S. pombe.
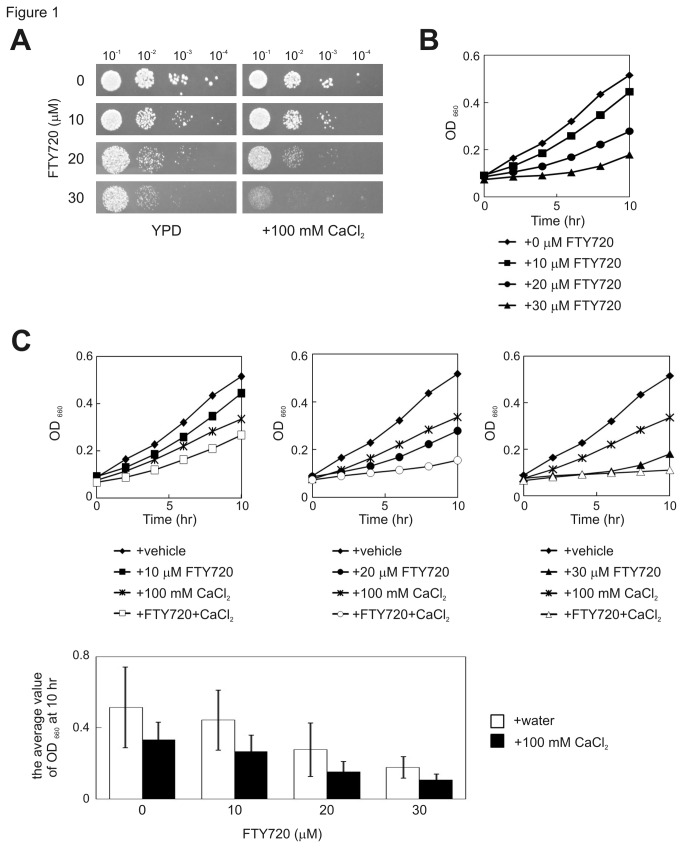
FTY720 was tested at a range of concentrations and found to inhibit growth of wt cells in a dose-dependent manner (Figure 1A). To quantitatively measure the effect of FTY720, the influence of the compound on the growth of wt cells in the liquid medium was assessed, and dose-dependent growth inhibition induced by FTY720 was confirmed (Figure 1B). To analyze the relationship between Ca2+ signaling and FTY720 sensitivity, the effect of Ca2+ on the growth of wt cells in the medium supplemented with FTY720 was first tested. As shown in Figure 1A, the addition of 100 mM CaCl2 alone only marginally affected the growth of wt cells in the solid YPD medium. However, the same concentration of CaCl2 exacerbated the growth of wt cells treated with various concentrations of FTY720 (Figure 1A). The effect of Ca2+ on FTY720-induced cell growth defects was also confirmed by measuring the OD at 660 nm in a microplate reader (Figure 1C).
Figure 1. Fission yeast sensitivity to FTY720.
(A) Fission yeast cells are sensitive to FTY720. A serial dilution assay of the wild-type strain grown in YPD medium or YPD medium containing the indicated concentrations of FTY720 in the absence (Left: YPD) or presence (Right: + 100 mM CaCl2) of 100 mM CaCl2. Cells were incubated for 3 days at 27°C. (B) Quantitative measurements of cell growth in the presence of FTY720. The cells were grown in liquid YES cultures to an OD660 of 0.3 and were treated with the drugs (FTY720) at the concentrations indicated, and the quantitative measurements of cell growth rates were performed using a microplate reader (SunriseTM series, Tecan, Switzerland). A representative for three independent curves is presented. (C) Addition of CaCl2 exacerbated the fission yeast sensitivity to FTY720. Wild-type cells were cultured in YES liquid medium and treated with 100 mM CaCl2 in the absence or presence of indicated concentrations of FTY720, and the growth curve of the cells were shown by measuring OD660 for 10 h. (D) Graph shows the OD660 at 10 h of the cells, as indicated in Figure 1(B) and (C). The data were averaged from three independent experiments. Bars, SD.
Fission yeast mutants with defects in Ca2+/calcineurin signaling are hypersensitive to FTY720.
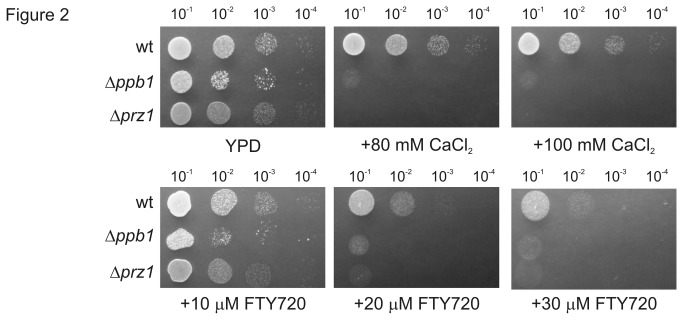
The effect of mutated Ca2+ signaling on FTY720 sensitivity was next examined. The addition of 10 μM FTY720 only slightly inhibited the growth of wt cells (Figure 2, +10μM FTY720, wt). In contrast, knockout of ppb1 +, which encodes the Ca2+/calmodulin activated protein phosphatase [28], resulted in significant sensitivity to the same concentration of FTY720. The Δppb1 cells almost failed to grow in the presence of 30 μM FTY720, whereas the wt cells formed colonies (Figure 2, Δppb1). In fission yeast, calcineurin dephosphorylates and activates Prz1, a zinc finger-type transcription factor involved in Ca2+ homeostasis [26]. Deletion of Prz1 also enhanced the sensitivity to FTY720 because Δprz1 cells failed to grow in media containing more than 20 μM FTY720 (Figure 2, Δprz1). Notably, these mutant cells also displayed enhanced sensitivities to CaCl2 compared with the wt cells because they failed to grow in the medium containing more than 80 mM CaCl2 (Figure 2). These results suggest that the mechanism underlying FTY720 sensitivity may involve impaired Ca2+ signaling.
Figure 2. Defects in calcineurin signaling caused hypersensitivity to FTY720.
A serial dilution assay of the wild-type (wt), Δppb1, and Δprz1 cells grown in rich YPD medium containing the indicated concentrations of FTY720 or CaCl2.
FTY720 stimulates the calcineurin/Prz1 signaling pathway.
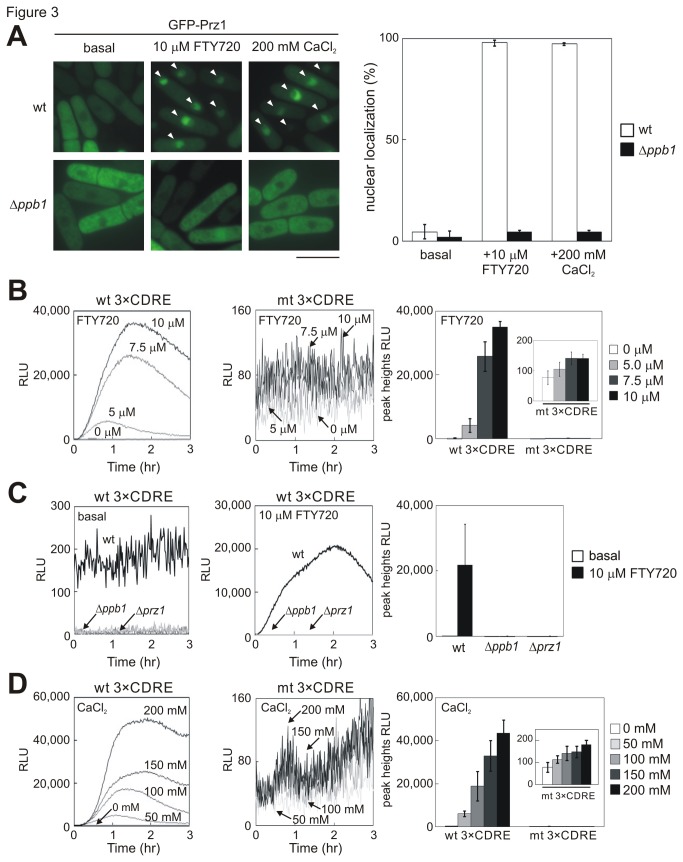
The above findings prompted the examination of the effect of FTY720 on calcineurin/Prz1 signaling. The intracellular localization of GFP-Prz1 was examined because the activation of calcineurin causes the translocation of GFP-Prz1 from the cytoplasm to the nucleus [26]. The addition of 10 μM FTY720 stimulated the nuclear accumulation of GFP-Prz1 because more than 95% of wt cells exhibited nuclear localization of GFP-Prz1 (Figure 3A, wt, +10 μM FTY720, arrowheads). On the other hand, in calcineurin-null cells treated with the same concentration of FTY720, GFP-Prz1 almost remained cytosolic, with less than 5% of the cells localizing to the nucleus (Figure 3A, Δppb1, +10 μM FTY720). The addition of 200 mM CaCl2 exerted similar effects on GFP-Prz1 localization (Figure 3A, +200 mM CaCl2, arrowheads). Thus, FTY720 stimulates nuclear translocation of Prz1 as efficiently as Ca2+.
Figure 3. FTY720 stimulates the calcineurin/Prz1 signaling pathway.
(A) Left: Translocation of GFP-Prz1 to the nucleus is induced by FTY720 addition and requires calcineurin. Wild-type (wt), or calcineurin-null cells (Δppb1) expressing GFP-Prz1 were grown in EMM medium at 27°C and analyzed by fluorescence microscopy to observe GFP-Prz1 localization (GFP-Prz1). The cells were incubated with or without 10 μM FTY720 for 10 min or in the presence of 200 mM CaCl2 for 10 min at 27 °C. Arrowheads indicate cells whose nuclei show intense GFP fluorescence. The bar indicates 10 μm. Right: The percentage of cells in (A) showing intense nuclear fluorescence of GFP-Prz1 was measured. At least 300 cells were counted. (B) Real-time monitoring of calcineurin activity in living cells stimulated by FTY720. Wild-type cells harboring the multicopy plasmid wt 3×CDRE::luc(R2.2) reporter vector pKB5723 (wt 3×CDRE) or the multicopy plasmid carrying the mutant version of the CDRE (mt 3×CDRE::luc(R2.2)) reporter vector pKD2767 (mt 3×CDRE) were incubated with D-luciferin and treated with various concentrations of FTY720, as indicated. Using a luminometer, light emission levels expressed as relative light units (RLU) were measured per minutes for 3 h. The data shown are representative of multiple experiments. Right: Graph shows the peak heights of 3×CDRE::luc(R2.2) (wt 3×CDRE) or the mutant 3×CDRE::luc(R2.2) (mt 3×CDRE) reporter activity. The data were averaged from three independent experiments. Bars, SD. (C) Stimulation of calcineurin signaling induced by FTY720 addition requires calcineurin/Prz1 signaling. Wild-type and ppb1─ and prz1─null cells harboring the multicopy plasmid (wt 3×CDRE::luc(R2.2)) reporter vector were treated with 10 μM FTY720 and monitored as described in Figure 3B. (D) Real-time monitoring of calcineurin activity in living cells stimulated by CaCl2. Cells as indicated in Figure 3 (B) were treated with various concentrations of CaCl2, as indicated, and monitored as described in Figure 3 (B).
The effect of FTY720 on calcineurin/Prz1 signaling was then further tested using the reporter construct 3×CDRE fused to R2.2 destabilized luciferase (3×CDRE::luc(R2.2)), which was established as an accurate measure of the calcineurin activation in living cells [27]. The wt cells harboring the reporter plasmid 3×CDRE::luc(R2.2) were stimulated with various concentrations of FTY720 (5.0–10 μM) or CaCl2 (50–200 mM). FTY720 treatment caused a dose-dependent increase in the 3×CDRE::luc (R2.2) response, exhibiting a peak rise and then approaching a constant level (Figure 3B, wt 3×CDRE). On the other hand, in calcineurin-null (Δppb1) and prz1-null (Δprz1) cells treated with 10 μM FTY720, the reporter response both in the absence (basal) and the presence of FTY720 was reduced markedly (Figure 3C), consistent with the notion that the 3×CDRE reporter gives an accurate measure of the calcineurin/Prz1 system. The reporter response obtained by FTY720 treatment was similar to that obtained by CaCl2 addition (Figure 3D, wt 3×CDRE). Thus, FTY720 as well as Ca2+ stimulates calcineurin/Prz1 signaling.
The mutant version of the 3×CDRE reporter plasmid 3×mt CDRE::luc (R2.2) was next created, in which the consensus motif AGCCTC in the CDRE was mutated to AGATCT (Materials and Methods). The mutation in CDRE dramatically decreased the reporter activity upon FTY720 and CaCl2 treatment (Figure 3B, D, mt 3×CDRE), confirming that the above mentioned reporter activity stimulated by FTY720 and CaCl2 was exerted via Prz1 binding to the CDRE motif.
While this range of concentration is higher than that required for immune modulation (10-100 nM) in cultured mammalian cells, it should be noted that significantly higher concentrations of FTY720 were used (5-25 μM) when examining the inhibition of tumor development, induction of apoptosis and stimulation of the intracellular concentration of calcium ions in mammalian cells [4,9,10,29-32].
FTY720 stimulates Ca2+ influx.
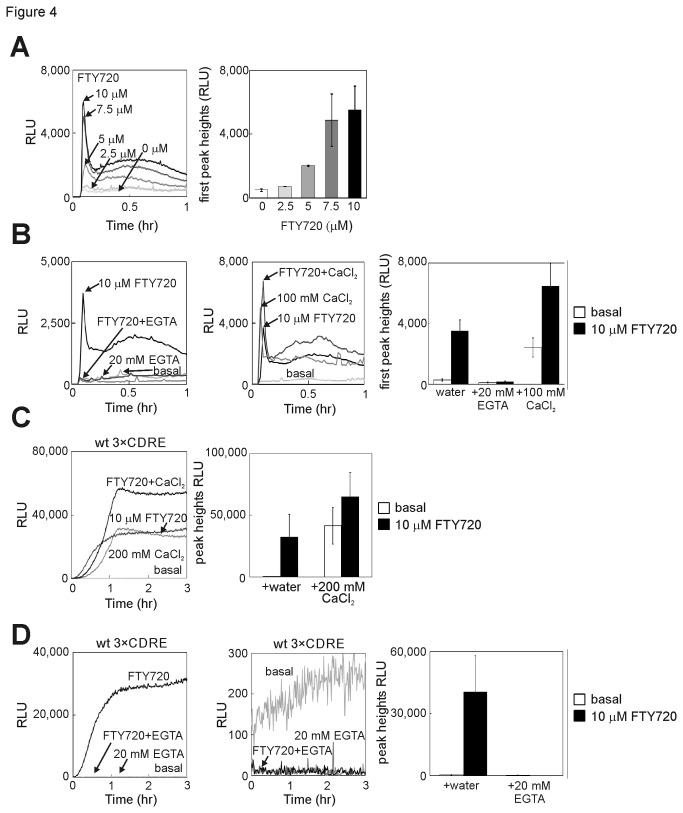
Various agents known to cause Ca2+ influx reportedly induce calcineurin activation [27]. To provide additional information on the effect of FTY720 on calcineurin signaling, intracellular Ca2+ levels upon FTY720 treatment were monitored in fission yeast, using adh1-GFP-19-AEQ, which have been established as a sensitive method to monitor the cytoplasmic Ca2+ levels [28]. FTY720 induced a dose-dependent increase in cytoplasmic Ca2+ levels, which rapidly increased and reached a peak level immediately after the addition of FTY720, rapidly decreased thereafter and exhibited a less pronounced second peak, and then reached a steady state level (Figure 4A). To investigate whether the increase in cytoplasmic Ca2+ levels following the addition of FTY720 was due to the influx from the extracellular medium or due to the release from an internal store, the effect of EGTA (extracellular Ca2+ -chelator) was examined in the same assay. The results demonstrated that the peak responses and increase in cytoplasmic Ca2+ levels elicited by FTY720 were almost inhibited by the addition of EGTA to the culture medium (Figure 4B, FTY720 + EGTA). Thus, it is suggested that the increase in cytoplasmic Ca2+ levels upon FTY720 treatment is dependent on the influx across the Ca2+ machinery involved in Ca2+ entry that exists on the plasma membrane. The effect of CaCl2 on FTY720-induced Ca2+ influx was also examined. As shown in Figure 4B, the addition of 100 mM CaCl2 to the medium containing FTY720 markedly increased the peak response compared with that obtained by the addition of 100 mM CaCl2 alone, indicating the presence of synergy between CaCl2 and FTY720 in stimulating Ca2+ influx in fission yeast.
Figure 4. Intracellular Ca2+ levels on FTY720 treatment.
(A) Left: Monitoring of intracellular Ca2+ levels in wild-type (wt) cells harboring adh1-GFP-19-AEQ (pKB6892) treated with various concentrations of FTY720 at a time point of 5 min and the luminescence was followed for 1 h. An aequorin assay was performed as described in the Materials and Methods. The data shown are the representative of multiple experiments. Right: Graph shows the average of peak heights from three independent experiments shown in the left column of Figure 4 (A). Bars, SD. (B) Effects of EGTA and CaCl2 on the FTY720-induced increase in the cytoplasmic Ca2+ level. The experiments were performed as described in Figure 4 (A), except that prior to the addition of FTY720, 20 mM EGTA (left) or 100 mM CaCl2 (middle) were added to the EMM medium. Right: The histogram was calculated as described in Figure 4A. (C) (D) Effects of EGTA and CaCl2 on the FTY720-induced increase in the calcineurin activity. The experiments were performed as described in Figure 3 (B) with wt 3×CDRE, except that prior to the addition of 10 μM FTY720, 10 mM or 20 mM EGTA or 200 mM CaCl2 were added to the EMM medium. The histogram was calculated as described in Figure 3 (B).
Similarly, effects of EGTA and CaCl2 on FTY720-induced calcineurin activation were examined using the reporter plasmid 3×CDRE::luc(R2.2). The addition of CaCl2 induced the additive response of calcineurin activity upon FTY720 treatment (Figure 4C). In addition, the addition of EGTA to the culture medium reduced calcineurin activation by FTY720 (Figure 4D). Therefore, the FTY720-stimulated response activated calcineurin-signaling pathway by a mechanism requiring influx of extracellular Ca2+. Thus, these results suggest that FTY720 induced calcineurin activation by stimulating Ca2+ influx.
FTY720 increased the cytoplasmic Ca2+ level partly via the Yam8-Cch1 channel complex.
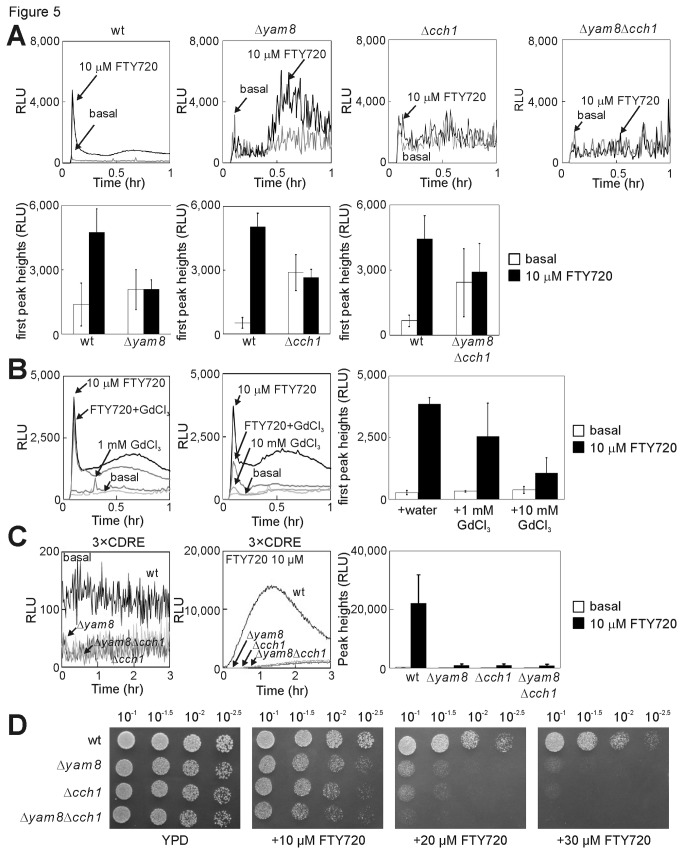
The increase in intracellular Ca2+ levels induced by FTY720 was next examined in cells lacking yam8 +- or cch1 +-encoding putative subunits of a Ca2+ channel, which is reported to regulate Ca2+ influx in S. pombe [27]. In single and double knockout cells of Yam8 and/or Cch1, the basal cytoplasmic Ca2+ level was higher than that in wt cells, consistent with the previous study (Figure 5A) [27,28]. Notably, FTY720 failed to increase the cytoplasmic Ca2+ level in these Ca2+ channel mutant cells (Figure 5A).
Figure 5. FTY720 stimulates Ca2+/calcineurin signaling via the Yam8/Cch1 channel.
(A) The wild-type (wt), Δyam8, Δcch1, or Δyam8Δcch1 cells harboring pKB6892 (adh1-GFP-19-AEQ) were treated with 10 μM FTY720 or vehicle (basal), and the experiments were performed as described in Figure 4 (A). The histogram was calculated as described in Figure 4 (A). Bars, SD. (B) Effects of GdCl3 on the FTY720-induced increase in the cytoplasmic Ca2+ level. The experiments were performed as described in Figure 4 (A), except that 1 mM or 10 mM of GdCl3 were also added to the EMM medium. The histogram was calculated as described in Figure 4A. (C) Deletion of Yam8 or Cch1 reduced markedly calcineurin activation induced by FTY720. The wild-type (wt), Δyam8, Δcch1, or Δyam8Δcch1 cells harboring the reporter plasmid (wt 3×CDRE::luc(R2.2)) were monitored as described in the legend of Figure 3(B). (D) Deletion of Yam8 or Cch1 enhanced the sensitivity to FTY720. A serial dilution assay of the wild-type (wt), Δyam8, Δcch1, and Δyam8Δcch1 mutant cells grown in rich YPD medium containing the indicated concentrations of FTY720.
In addition, the effect of a Ca2+ channel blocker, Gadolinium (III) chloride (GdCl3), on Ca2+ influx induced by FTY720 was examined. GdCl3 reduced the peak response of Ca2+ levels upon FTY720 treatment in a dose-dependent manner (Figure 5B), consistent with a previous report that Ca2+ currents mediated by Mid1, a homologue of Yam8 in budding yeast, expressed in Chinese hamster ovary cells were inhibited by Gd3+ [33]. Because Gd3+ is not efficiently transported across the plasma membrane, these data suggest that the increase in cytoplasmic Ca2+ levels induced by FTY720 depends on Ca2+ influx from the extracellular medium.
Furthermore, FTY720 failed to activate calcineurin in these Ca2+ channel mutant cells because the response of the 3×CDRE reporter stimulated by the addition of FTY720 was reduced markedly in these mutant cells (Figure 5C). These results suggest that the effect of FTY720 on calcineurin signaling is mediated at least in part by the activation of the Yam8-Cch1 channel complex and the resultant Ca2+ influx. Interestingly, single and double knockout cells of Yam8 and/or Cch1 exhibited enhanced sensitivity to FTY720 because these mutant cells barely grew in the presence of 30 μM FTY720, whereas the wt cells grew well (Figure 5D). The hypersensitivity to FTY720 may reflect defective Ca2+ homeostasis in these mutant cells.
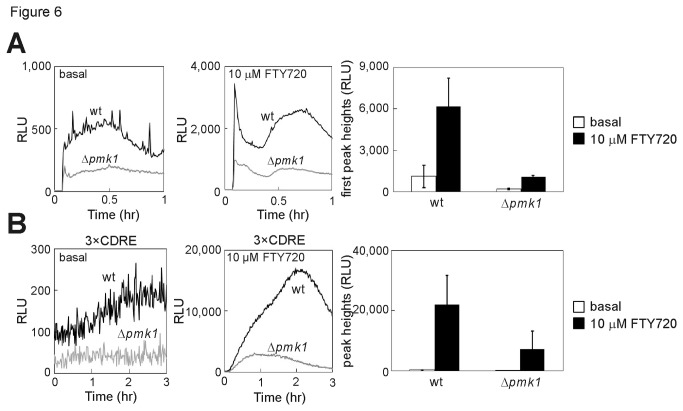
The effect of the Pmk1 MAPK pathway was also examined because this pathway has been reported to positively regulate the activation of the Yam8/Cch1 channel complex in both yeasts [27]. As shown in Figure 6A, in Pmk1-null cells, the basal cytoplasmic Ca2+ level was very low compared with wt cells, and the Ca2+ increase induced by the addition of FTY720 was reduced markedly (Figure 6A). In addition, the FTY720-mediated calcineurin activation was reduced markedly by the deletion of pmk1 + (Figure 6B).
Figure 6. Pmk1 MAP kinase activated the FTY720-induced Ca2+ influx. .
(A) Knockout of Pmk1 MAP kinase suppressed the FTY720-induced Ca2+ influx. WT and Δpmk1 cells were monitored as described in the legend of Figure 4 (A). (B) Knockout of Pmk1 MAP kinase suppressed the FTY720-induced calcineurin signaling. WT and Δpmk1 cells were monitored as described in the legend of Figure 3 (B).
The effect of FTY720-P on Ca2+/calcineurin signaling.
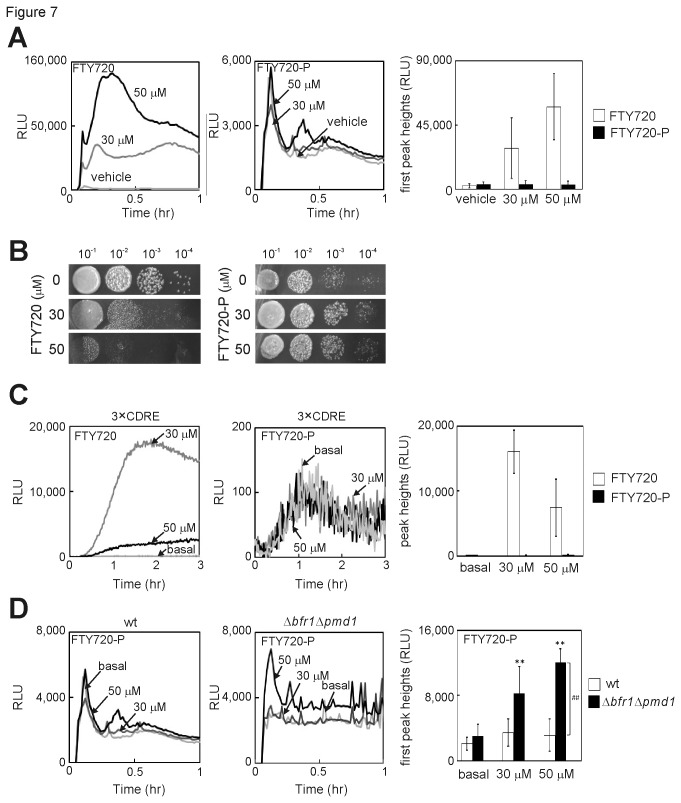
We wanted to determine whether the phosphorylated form of FTY720 (FTY720-P) also stimulates Ca2+ signaling. Notably, unlike FTY720, FTY720-P failed to induce Ca2+ influx because the addition of up to 50 μM FTY720-P did not affect the intracellular Ca2+ concentration (Figure 7A, FTY720-P). In contrast, nonphosphorylated FTY720 effectively stimulated Ca2+ influx in a dose-dependent manner (Figure 7A, FTY720). It should be noted that the pattern of the first sharp peak of Figure 7A (left panel) and Figure 4A was reproducibly different and the possible reasons for the different pattern may be due to the difference in the vehicles used in each experiment (Materials and Methods). This was further confirmed by the experiments comparing the pattern obtained with the vehicle used in Figure 4A (water) and the vehicle used in Figure 7A (ethanol containing NaOH for the insolubility of FTY720-P in water) (Figure S1). In addition, the inhibitory effect on cell growth was not observed in the medium containing 50 μM of FTY720-P, whereas no colonies were formed in the presence of the same concentration of nonphosphorylated FTY720 (Figure 7B). Finally, the addition of up to 50 μM FTY720-P did not affect the CDRE responses (Figure 7C). In addition, FTY720-P did not affect the intracellular localization of Prz1 as compared with vehicle alone (Figure S2).
Figure 7. Phosphorylated FTY720 (FTY720-P) failed to stimulate.
Ca2+/calcineurin signaling. (A) Effect of FTY720 and FTY720-P on intracellular Ca2+ levels. The wild-type cells harboring adh1-GFP-19-AEQ were treated with indicated concentrations of FTY720 or FTY720-P, and experiments were performed as described in Figure 4(A). Ethanol containing NaOH was used as vehicle (Materials and Methods). The histogram was calculated as described in Figure 4 (A). Bars, SD. (B) A serial dilution assay of the wild-type (wt) cells grown in rich YPD medium containing the indicated concentrations of FTY720 or FTY720-P. (C) Left: Real-time monitoring of calcineurin activity in living cells stimulated by FTY720 or FTY720-P. Wild-type cells harboring the multicopy plasmid (wt 3xCDRE::luc(R2.2)) reporter vector, pKB5723 (wt 3xCDRE) were treated as described in Figure 3(B). The data shown are representative of multiple experiments. Right: Graph shows the peak heights of 3xCDRE::luc(R2.2) reporter activity. The data were averaged from three independent experiments. Bars, SD. (D) The effect of FTY720-P induced rise of intracellular Ca2+ levels in ABC transporter knockout cells. The wt or Δbfr1Δpmd1 cells harboring adh1-GFP-19-AEQ were treated with indicated concentrations of FTY720-P, and experiments were performed as described in Figure 4(A). ** p<0.01; Significantly different from vehicle (using two-way ANOVA). ## p<0.01; Significantly different from wild-type cells (using Williams’ test).
We sought to take into account the possibility that the cellular penetrance of FTY720-P might be limited by the S. pombe cell wall as well as the possibility that the compound might be more rapidly metabolized in S. pombe cells than in animal cells. Because FTY720 was reported to be transported across cellular membranes with ATP-binding cassette (ABC) transporter family members [34-36], the observed lack of effect of FTY720-P on Ca2+ signaling could be due to rapid export of the compound from the cells. We then tested the intracellular Ca2+ levels induced by FTY720-P in cells lacking both bfr1 + and pmd1 +, encoding two major S. pombe drug-efflux transporters, which were involved in multidrug resistance [37,38]. As shown above, the addition of FTY720-P did not affect the intracellular Ca2+ levels in wild-type cells (Figure 7A). In contrast, in Δbfr1Δpmd1 null cells, the intracellular Ca2+ levels were significantly higher than wt cells in the absence and the presence of FTY720-P (Figure 7D). Data were also statistically analyzed using Williams’ test and the analysis indicated that the increase in intracellular Ca2+ concentrations upon FTY720 treatment in Δbfr1Δpmd1 null cells were statistically significant, with a P-value of < 0.01 (**) and that the difference in intracellular Ca2+ levels upon FTY720 stimuli (50 μM) between wt and Δbfr1Δpmd1 null cells were significant, with a P-value of < 0.01 (##) (Figure 7D; right panel). This result suggested the possibility that FTY720-P may poorly enter the cells and due to subsequent export of the drug by Bfr1 and Pmd1 resulted in the apparent loss of biological activity with respect to its effect on the intracellular Ca2+ concentration and calcineurin-mediated transcriptional activity.
Discussion
Here, we used the fission yeast model system to analyze the effect of FTY720 on Ca2+/calcineurin signaling and presented several lines of evidence that FTY720 can stimulate Ca2+ influx largely via Cch1/Yam8, resulting in the elevation of cytoplasmic Ca2+ levels and activation of calcineurin signaling pathway.
Because the fission yeast genome does not express structural homologues of S1P receptors in mammals, the observed responses induced by FTY720 could be mediated independently of S1P receptors. A number of recent studies suggest that FTY720 exerts various biological functions, including anti-cancer activities, and these effects other than immune modulation can be mediated independently of S1P receptors. Furthermore, while the functions of FTY720-P as a immunomodulator are carried out at nanomolar concentrations, a higher concentration of non-phosphorylated FTY720 were required to exert its biological activities such as inducing apoptosis[4,9,10,29-32,39]. The candidate cellular targets of FTY720 include cPLA2 [12], 14-3-3 [40], PKC [41], and PP2A [42]. Notably, FTY720 also increases the intracellular concentration of calcium ions and induces apoptosis in HL-60 [10], and although the involvement of PLC was suggested, cellular targets and the intracellular action mechanism of FTY720 regarding these effects remain to be fully elucidated. Here, we showed that the biological effects of FTY720 can be partly explained by Yam8/Cch1 channel and Ca2+/calcineurin signaling activation. Our previous study suggested that the persistent increase in the cytoplasmic Ca2+ level causes cell death due to apoptotic process in S. pombe [28]. Therefore, it would be intriguing if pro-apoptotic properties of FTY720 in mammals may also involve hyperactivation of Ca2+/calcineurin signaling pathway in higher eucaryotes, which is consistent with the notion that Ca2+/calcineurin signaling induces apoptosis.
Fission yeast cells also have the homolog of sphingoid long-chain base (LCB) kinase, pSPHK1 (accession no. T38776) that catalyzes the phosphorylation of LCBs to form LCB 1-phosphate in mammals [43]. However, pSPHK1 knockout cells were not severely sensitive to FTY720 in S. pombe (data not shown), as previously reported in Saccharomyces cerevisiae [19,20], which suggests that FTY720-P may play a role in FTY720 mediated effects. Therefore, our observation that FTY720-P does not stimulate calcium flux and calcineurin activation in fission yeast may only be true of exogenously applied FTY720-P. Intracellularly produced FTY720-P would not be subject to the same transport barriers as exogenous FTY720-P and may have biological activity.
The involvement of the Ca2+ channel protein Yam8/Cch1 underscores the existence of a specific mechanism for FTY720-dependent calcium signaling in fission yeast. Whether FTY720 can traverse the yeast cell wall and plasma membrane to act intracellularly, or affects cell integrity, thereby activating Yam8/Cch1 channel remains unknown. However, we favor the former possibility based on the lipophilic nature of FTY720, as well as our findings that an anti-fungal agent such as miconazole, or detergent such as 1% Tween 20, or an ER-stress-inducing compound DTT, failed to induce Ca2+ influx as efficiently as FTY720 did (our unpublished results). In addition, in the presence of extracellular Ca2+-chelators such as EGTA, FTY720 was unable to stimulate Ca2+ influx. Therefore, no evidence for FTY720-stimulated Ca2+ release from the internal store has been obtained in fission yeast thus far. It should be noted however, that EGTA addition failed to rescue the growth inhibition of wild-type cells by FTY720 (Figure S3). In addition, the addition of CaCl2 (up to 100 mM) failed to rescue the growth inhibition of Δyam8, Δcch1, Δyam8Δcch1 cells, thus suggesting that FTY720-induced cell growth defect may not be explained only by activation of Ca2+/calcineurin signaling, and may instead represent a broader effect. We therefore hypothesized that although the disturbance of appropriate regulation of the Ca2+/calcineurin signaling resulted in increased sensitivity of fission yeast proliferation to adapt to physiological responses induced by FTY720, and that Yam8/Cch1 plays an important role on calcineurin activation induced by FTY720, it may not support a direct connection between calcineurin activity and inhibition of proliferation. Because fission yeast cells also have the homolog of sphingoid long-chain base (LCB) kinase, pSPHK1 (accession no. T38776) that catalyzes the phosphorylation of LCBs to form LCB 1-phosphate in mammals [43], it would be intriguing if these kinases may be involved in the FTY720-mdiated Ca2+/calcineurin signaling in fission yeast.
The Ca2+/calcineurin signaling pathway plays an important role in various cellular functions, including immune regulation, cardiac hypertrophy and apoptosis, and FK506 and CsA are specific inhibitors of calcineurin both in humans and yeasts. Notably, the findings presented in this study suggested the cross-talk between FTY720-mediated and FK506-regulated signaling pathway, both of which are involved in immune regulation. In clinical organ transplantations, FK506-based combination therapy with other immunosuppressants is widely used to reduce the side effects of individual drugs. Notably, the combination therapy of FTY720 with CsA or FK506 has a marked prolonging effect on allograft survival as compared with the monotherapy of FTY720, CsA, or FK506 [2]. Our findings of FTY720-stimulated calcineurin activation may underlie the mechanism of synergistic effect of FTY720 in combination with calcineurin inhibitors. Because FTY720-stimulated calcineurin hyperactivation could result in the activation of the immune system, and may thus counteract the immune modulation of FTY720, FK506 treatment can inhibit calcineurin hyperactivation, thus inducing synergistic effect. A future chemical biology screen to search for mutants with altered sensitivity to FTY720 using fission yeast model system may be applicable to identify new factors required for FTY720-mediated Ca2+ signaling pathway.
Supporting Information
Effect of vehicle on the basal intracellular Ca2+ levels. Left panel: The wild-type cells harboring adh1-GFP-19-AEQ were treated with water and ethanol containing NaOH, and experiments were performed as described in Figure 4(A). Right panel: The histogram was calculated as described in Figure 4 (A). Bars, SD.
(TIF)
Effect of FTY720-P on the intracellular localization of GFP-Prz1. Translocation of GFP-Prz1 to the nucleus is induced by FTY720 addition, but not by FTY720-P. Wild-type cells expressing GFP-Prz1 were grown in EMM medium at 27°C and analyzed by fluorescence microscopy as described in Figure 3(A). The bar indicates 10 μm.
(TIF)
The effect of Ca2+ or EGTA on inhibition of proliferation by FTY720 in various strains. A serial dilution assay of the wild-type (wt), Δyam8, Δcch1, and Δyam8Δcch1 cells grown in rich YPD medium containing the indicated concentrations of FTY720, CaCl2 and EGTA.
(TIF)
Acknowledgments
We thank Dr. Takashi Toda, Dr. Mitsuhiro Yanagida, Dr. Yan Ma, Dr. Minoru Yoshida and the National Bio-Resource Project for providing strains and plasmids; Mayumi Fujioka for critical reading of the manuscript. We are grateful to the members of the Laboratory of Molecular Pharmacogenomics for their support.
Funding Statement
This work was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to R.S.). This work was also supported in part by the “Antiaging” Project for Private Universities, with a matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Adachi K, Kohara T, Naka N, Arit M, Chiba K et al. (1995) Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: discovery of a novel immunosuppressant, FTY720. Biomed - Chem Lett 5: 853-856. doi: 10.1016/0960-894X(95)00127-F. [DOI] [Google Scholar]
- 2. Chiba K (2005) FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther 108: 308-319. doi: 10.1016/j.pharmthera.2005.05.002. PubMed: 15951022. [DOI] [PubMed] [Google Scholar]
- 3. Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N et al. (2003) Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem 278: 47408-47415. doi: 10.1074/jbc.M307687200. PubMed: 13129923. [DOI] [PubMed] [Google Scholar]
- 4. Ho JW, Man K, Sun CK, Lee TK, Poon RT et al. (2005) Effects of a novel immunomodulating agent, FTY720, on tumor growth and angiogenesis in hepatocellular carcinoma. Mol Cancer Ther 4: 1430-1438. doi: 10.1158/1535-7163.MCT-05-0021. PubMed: 16170036. [DOI] [PubMed] [Google Scholar]
- 5. Lee TK, Man K, Ho JW, Wang XH, Poon RT et al. (2005) FTY720: a promising agent for treatment of metastatic hepatocellular carcinoma. Clin Cancer Res 11: 8458-8466. doi: 10.1158/1078-0432.CCR-05-0447. PubMed: 16322309. [DOI] [PubMed] [Google Scholar]
- 6. Nagahara Y, Ikekita M, Shinomiya T (2000) Immunosuppressant FTY720 induces apoptosis by direct induction of permeability transition and release of cytochrome c from mitochondria. J Immunol (Baltimore, Md. : 1950) 165: 3250-3259. PubMed: 10975841. [DOI] [PubMed] [Google Scholar]
- 7. Nagahara Y, Ikekita M, Shinomiya T (2002) T cell selective apoptosis by a novel immunosuppressant, FTY720, is closely regulated with Bcl-2. Br J Pharmacol 137: 953-962. doi: 10.1038/sj.bjp.0704970. PubMed: 12429567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Permpongkosol S, Wang JD, Takahara S, Matsumiya K, Nonomura N et al. (2002) Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int J Cancer 98: 167-172. doi: 10.1002/ijc.10178. PubMed: 11857403. [DOI] [PubMed] [Google Scholar]
- 9. Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T (2003) A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol 138: 1303-1312. doi: 10.1038/sj.bjp.0705182. PubMed: 12711631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shinomiya T, Li XK, Amemiya H, Suzuki S (1997) An immunosuppressive agent, FTY720, increases intracellular concentration of calcium ion and induces apoptosis in HL-60. Immunology 91: 594-600. doi: 10.1046/j.1365-2567.1997.d01-2281.x. PubMed: 9378500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pitman MR, Woodcock JM, Lopez AF, Pitson SM (2012) Molecular targets of FTY720 (fingolimod). Curr Mol Med 12: 1207-1219. doi: 10.2174/156652412803833599. PubMed: 22834825. [DOI] [PubMed] [Google Scholar]
- 12. Payne SG, Oskeritzian CA, Griffiths R, Subramanian P, Barbour SE et al. (2007) The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1-phosphate receptors. Blood 109: 1077-1085. PubMed: 17008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gould KL, Moreno S, Tonks NK, Nurse P (1990) Complementation of the mitotic activator, p80cdc25, by a human protein-tyrosine phosphatase. Science 250: 1573-1576. doi: 10.1126/science.1703321. PubMed: 1703321. [DOI] [PubMed] [Google Scholar]
- 14. Babst M, Odorizzi G, Estepa EJ, Emr SD (2000) Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. (Copenhagen, Denmark) 1, 248-258. [DOI] [PubMed]
- 15. Perego P, Jimenez GS, Gatti L, Howell SB, Zunino F (2000) Yeast mutants as a model system for identification of determinants of chemosensitivity. Pharmacol Rev 52: 477-492. PubMed: 11121507. [PubMed] [Google Scholar]
- 16. Perego P, Zunino F, Carenini N, Giuliani F, Spinelli S et al. (1998) Sensitivity to cisplatin and platinum-containing compounds of Schizosaccharomyces pombe rad mutants. Mol Pharmacol 54: 213-219. PubMed: 9658208. [DOI] [PubMed] [Google Scholar]
- 17. Sugiura R, Sio SO, Shuntoh H, Kuno T (2002) Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells 7: 619-627. doi: 10.1046/j.1365-2443.2002.00557.x. PubMed: 12081640. [DOI] [PubMed] [Google Scholar]
- 18. Simon JA, Bedalov A (2004) Yeast as a model system for anticancer drug discovery. Nat Rev Cancer 4: 481-492. doi: 10.1038/nrc1372. PubMed: 15170450. [DOI] [PubMed] [Google Scholar]
- 19. Welsch CA, Hagiwara S, Goetschy JF, Movva NR (2003) Ubiquitin pathway proteins influence the mechanism of action of the novel immunosuppressive drug FTY720 in Saccharomyces cerevisiae . J Biol Chem 278: 26976-26982. doi: 10.1074/jbc.M213144200. PubMed: 12709439. [DOI] [PubMed] [Google Scholar]
- 20. Welsch CA, Roth LW, Goetschy JF, Movva NR (2004) Genetic, biochemical, and transcriptional responses of Saccharomyces cerevisiae to the novel immunomodulator FTY720 largely mimic those of the natural sphingolipid phytosphingosine. J Biol Chem 279: 36720-36731. doi: 10.1074/jbc.M406179200. PubMed: 15190065. [DOI] [PubMed] [Google Scholar]
- 21. Rusnak F, Mertz P (2000) Calcineurin: form and function. Physiol Rev 80: 1483-1521. PubMed: 11015619. [DOI] [PubMed] [Google Scholar]
- 22. Cheng H, Sugiura R, Wu W, Fujita M, Lu Y et al. (2002) Role of the Rab GTP-binding protein Ypt3 in the fission yeast exocytic pathway and its connection to calcineurin function. Mol Biol Cell 13: 2963-2976. doi: 10.1091/mbc.01-09-0463. PubMed: 12181359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toda T, Dhut S, Superti-Furga G, Gotoh Y, Nishida E et al. (1996) The fission yeast pmk1 + gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol Cell Biol 16: 6752-6764. PubMed: 8943330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe . Methods Enzymol 194: 795-823. doi: 10.1016/0076-6879(91)94059-L. PubMed: 2005825. [DOI] [PubMed] [Google Scholar]
- 25. Doi Y, Takeuchi H, Horiuchi H, Hanyu T, Kawanokuchi J et al. (2013) Fingolimod phosphate attenuates oligomeric amyloid beta-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PLOS ONE 8: e61988. doi: 10.1371/journal.pone.0061988. PubMed: 23593505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirayama S, Sugiura R, Lu Y, Maeda T, Kawagishi K et al. (2003) Zinc finger protein Prz1 regulates Ca2+ but not Cl- homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J Biol Chem 278: 18078-18084. doi: 10.1074/jbc.M212900200. PubMed: 12637524. [DOI] [PubMed] [Google Scholar]
- 27. Deng L, Sugiura R, Takeuchi M, Suzuki M, Ebina H et al. (2006) Real-time monitoring of calcineurin activity in living cells: evidence for two distinct Ca2+-dependent pathways in fission yeast. Mol Biol Cell 17: 4790-4800. doi: 10.1091/mbc.E06-06-0526. PubMed: 16928959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma Y, Sugiura R, Koike A, Ebina H, Sio SO et al. (2011) Transient receptor potential (TRP) and Cch1-Yam8 channels play key roles in the regulation of cytoplasmic Ca2+ in fission yeast. PLOS. ONE. 6: e22421. doi: 10.1371/journal.pone.0022421. PubMed: 21811607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y et al. (2002) Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res, 62: 621410-621419. PubMed: 11888913. [PubMed] [Google Scholar]
- 30. Yasui H, Hideshima T, Raje N, Roccaro AM, Shiraishi N et al. (2005) FTY720 induces apoptosis in multiple myeloma cells and overcomes drug resistance. Cancer Res, 65: 657478-657484. PubMed: 16103102. [DOI] [PubMed] [Google Scholar]
- 31. Nagaoka Y, Otsuki K, Fujita T, Uesato S (2008) Effects of phosphorylation of immunomodulatory agent FTY720 (fingolimod) on antiproliferative activity against breast and colon cancer cells. Biol Pharm Bull 31: 1177-1181. doi: 10.1248/bpb.31.1177. PubMed: 18520051. [DOI] [PubMed] [Google Scholar]
- 32. Estrada-Bernal A, Palanichamy K, Ray Chaudhury A, Van Brocklyn JR (2012) Induction of brain tumor stem cell apoptosis by FTY720: a potential therapeutic agent for glioblastoma. Neuro Oncol 14: 405-415. doi: 10.1093/neuonc/nos005. PubMed: 22351749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K et al. (1999) Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285: 882-886. doi: 10.1126/science.285.5429.882. PubMed: 10436155. [DOI] [PubMed] [Google Scholar]
- 34. Honig SM, Fu S, Mao X, Yopp A, Gunn MD et al. (2003) FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest 111: 627-637. doi: 10.1172/JCI16200. PubMed: 12618517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nijnik A, Clare S, Hale C, Chen J, Raisen C et al. (2012) The role of sphingosine-1-phosphate transporter Spns2 in immune system function. J Immunol (Baltimore, Md. : 1950) 189: 102-111. PubMed: 22664872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hisano Y, Nishi T, Kawahara A (2012) The functional roles of S1P in immunity. J Biochem 152: 305-311. doi: 10.1093/jb/mvs090. PubMed: 22923732. [DOI] [PubMed] [Google Scholar]
- 37. Nagao K, Taguchi Y, Arioka M, Kadokura H, Takatsuki A et al. (1995) bfr1+, a novel gene of Schizosaccharomyces pombe which confers brefeldin A resistance, is structurally related to the ATP-binding cassette superfamily. J Bacteriol 177: 1536-1543. PubMed: 7883711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toone WM, Kuge S, Samuels M, Morgan BA, Toda T et al. (1998) Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev 12: 1453-1463. doi: 10.1101/gad.12.10.1453. PubMed: 9585505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshino T, Tabunoki H, Sugiyama S, Ishii K, Kim SU et al. (2011) Non-phosphorylated FTY720 induces apoptosis of human microglia by activating SREBP2. Cell Mol Neurobiol 31: 1009-1020. doi: 10.1007/s10571-011-9698-x. PubMed: 21519925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woodcock JM, Ma Y, Coolen C, Pham D, Jones C et al. (2010) Sphingosine and FTY720 directly bind pro-survival 14-3-3 proteins to regulate their function. Cell Signal 22: 1291-1299. doi: 10.1016/j.cellsig.2010.04.004. PubMed: 20403428. [DOI] [PubMed] [Google Scholar]
- 41. Sensken SC, Gräler MH (2010) Down-regulation of S1P1 receptor surface expression by protein kinase C inhibition. J Biol Chem 285: 6298-6307. doi: 10.1074/jbc.M109.049692. PubMed: 20032465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roberts KG, Smith AM, McDougall F, Carpenter H, Horan M et al. (2010) Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res 70: 5438-5447. doi: 10.1158/1538-7445.AM10-5438. PubMed: 20551067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Imai H, Nishiura H (2005) Phosphorylation of sphingoid long-chain bases in Arabidopsis: functional characterization and expression of the first sphingoid long-chain base Kinase gene in plants. Plant Cell Physiol 46: 375-380. doi: 10.1093/pcp/pci023. PubMed: 15695468. [DOI] [PubMed] [Google Scholar]
- 44. Bimbó A, Jia Y, Poh SL, Karuturi RK, den Elzen N et al. (2005) Systematic deletion analysis of fission yeast protein kinases. Eukaryot Cell 4: 799-813. doi: 10.1128/EC.4.4.799-813.2005. PubMed: 15821139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of vehicle on the basal intracellular Ca2+ levels. Left panel: The wild-type cells harboring adh1-GFP-19-AEQ were treated with water and ethanol containing NaOH, and experiments were performed as described in Figure 4(A). Right panel: The histogram was calculated as described in Figure 4 (A). Bars, SD.
(TIF)
Effect of FTY720-P on the intracellular localization of GFP-Prz1. Translocation of GFP-Prz1 to the nucleus is induced by FTY720 addition, but not by FTY720-P. Wild-type cells expressing GFP-Prz1 were grown in EMM medium at 27°C and analyzed by fluorescence microscopy as described in Figure 3(A). The bar indicates 10 μm.
(TIF)
The effect of Ca2+ or EGTA on inhibition of proliferation by FTY720 in various strains. A serial dilution assay of the wild-type (wt), Δyam8, Δcch1, and Δyam8Δcch1 cells grown in rich YPD medium containing the indicated concentrations of FTY720, CaCl2 and EGTA.
(TIF)